Abstract
Deer keds are hematophagous ectoparasites (Diptera: Hippoboscidae) that mainly parasitize Cervidae. These flies are particularly important for animal health due to the occurrence of numerous pathogenic microorganisms. They may also attack humans and their bites may cause allergenic symptoms. The aim of the study was to identify the molecular characteristics of Borrelia burgdorferi sensu lato and Bartonella spp. pathogens detected in Lipoptena spp. sampled both from the hosts and from the environment. For identification of Bartonella spp and B. burgdorferi s. l., the primers specific to the rpoB and flaB gene fragments were used, respectively. The overall prevalence of B. burgdorferi s.l. DNA in Lipoptena cervi was 14.04%, including 14.8% infection in the tested group of winged specimens. The overall prevalence of Bartonella spp. was 57.02%. The presence of these bacteria was detected in 53.5% of specimens of L. cervi and 75.7% of L. fortisetosa. The phylogenetic analysis showed five new haplotypes of the rpoB gene of Bartonella sp. isolated from L. cervi/Lipoptena fortisetosa. We also identified one new haplotype of B. afzelii and three haplotypes of B. burgdorferi isolated from winged specimens of L. cervi. This is the first study to detect the genetic material of B. burgdorferi s.l. in L. cervi in Poland and the first report on the identification of these bacteria in host-seeking specimens in the environment.
1. Introduction
The family Hippoboscidae (Diptera) is a group of obligate hematophagous ectoparasites of mammals and birds, including more than 213 species and 21 genera [1]. In Europe, five Lipoptena species have been described, while in Poland, two of them, Lipoptena cervi and Lipoptena fortisetosa, have been reported so far [2]. Deer keds are common ectoparasites of cervids, but they can attack a wide range of animals, including European bison, horses, cattle, badgers, dogs, and red foxes [2,3]. Deer keds directly affect the condition of the host. Their blood feeding causes anemia, weight loss, itching, and secondary infections resulting from dermatitis lesions [4]. They may also threaten foresters, hunters, and people who visit forest areas, causing skin lesions that evolve after deer ked bites which are painful, often lead to the development of inflammation of the skin, and often also cause allergic reactions [5,6]. Moreover, in deer keds, the presence of the DNA of several pathogens has been described, including bacteria, such as Bartonella spp., Borrelia burgdorferi sensu lato, Anaplasma spp., Coxiella spp., Mycoplasma spp. Francisella tularensis, and Ehrlichia spp., protozoa such as Trypanosoma spp., and apicomplexan parasites, such as Babesia spp. or Theileria spp. [7,8,9,10,11].
Bartonellae are small, intracellular Gram-negative bacteria distributed in a wide range of hematophagous arthropods and vertebrates worldwide [12]. About 53 of the Bartonella species and three subspecies have been described (https://lpsn.dsmz.de/genus/bartonella; accessed on 12 November 2021). Some of these species have been recognized as potentially zoonotic agents causing human disease with various cardiovascular, neurological, and rheumatological conditions [13]. Blood-feeding arthropods, such as human lice (Pediculus humanus), cat fleas (Ctenocephalides felis), sand flies (Lutzomyia verrucarum), and various hard tick species (Ixodes spp., Dermacentor spp., as well as Haemaphysalis spp.), may be involved in the transmission of Bartonella pathogens [7].
In turn, an ethological agent of Lyme borreliosis, the spirochaete B. burgdorferi s.l., is a complex of 22 genospecies, of which 11 occur in Europe. Five of them: Borrelia afzelii, Borrelia garinii, Borrelia burgdorferii sensu stricto, Borrelia spielmanii and Borrelia bavariensis, are associated with human Lyme disease [14,15]. In Europe, the primary vector of Borrelia spirochetes is the Ixodes spp. tick [16].
This study aimed to determine the presence of Bartonella spp. and B. bugdorferii s.l. DNA in Lipoptena spp. collected from cervids and winged specimens from the environment. Moreover, the study presents the molecular characteristics and phylogenetic analyses of these bacterial pathogens.
2. Results
A total of 235 individuals of Lipoptena spp. were collected, from which two species were identified: 198 belonging to L. cervi (including 27 adults winged) and 37 of L. fortisetosa (Table 1).

Table 1.
Specimens of Lipoptena cervi and Lipoptena fortisetosa analysed for Bartonella spp. and Borrelia burgdorferi s.l. infection.
The overall prevalence of B. burgdorferi s.l DNA in L. cervi was 14.04% (33/235), including 14.8% (4/27) infection in the tested group of winged specimens. Borrelia burgdorferi s.l. DNA was not detected in L. fortisetosa. In turn, Bartonella spp. was detected in both Lipoptena species. The overall prevalence of Bartonella spp. was 57.02% (134/235). The presence of these bacteria was detected in 53.5% (106/198) specimens of L. cervi and in 75.7% (28/37) of L. fortisetosa. Co-infection with two pathogens, B. burgdorferi s.l. and Bartonella spp., was detected in 23 from a total of 198 (11.61%) L. cervi.
The derived sequences of Bartonella (rpoB gene) and Borreliella (flaB gene) species were submitted to the GenBank database (Acc. No. ON016083—ON016087 for Bartonella sp., ON016088 for Borrelia afzelii, and ON016089—ON016091 for Borrelia burgdorferi). The maximum likelihood of phylogenetic reconstructions produced a strong topology (Figure 1 and Figure 2).

Figure 1.
Maximum-likelihood tree computed with the GTR+I+G model of sequence evolution, representing phylogenetic relationships among the sequences of rpoB gene for RNA polymerase beta—subunit of Bartonella sp. found in Poland (H1–H5, marked in green) and downloaded (H6–H36) from GenBank. Numbers listed at nodes represent percent support for that node from 1000 bootstrap replicates. The tree has been rooted with sequences of Brucella melitensis. Lineages B, C, D, E, according to Sato et al. [17,18].
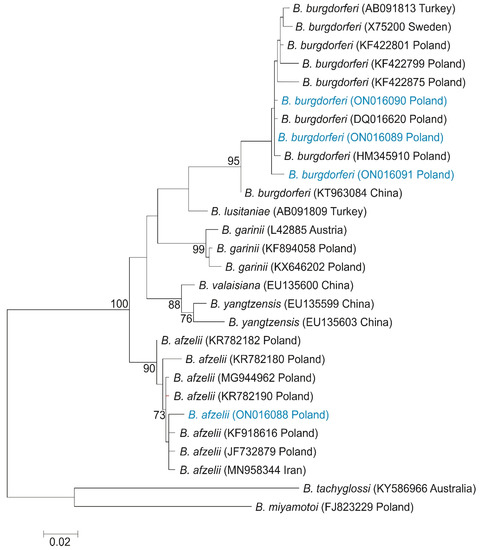
Figure 2.
Maximum-likelihood tree computed with the GTR + G model of sequence evolution, representing phylogenetic relationships among the sequences of flagellin gene of Borreliella sp. found in Poland (H1–H4, marked in blue) and downloaded (H5–H28) from GenBank. Numbers listed at nodes represent percent support for that node from 1000 bootstrap replicates. The tree has been rooted with sequences of B. tachyglossi and. B. miyamotoi.
Among three individuals of L. cervi and three individuals of L. fortisetosa, there were found five haplotypes of the rpoB gene of Bartonella sp., defined by 53 polymorphic sites, 46 transitions, and 7 transversions. The rpoB sequences obtained in the current study were classified into a distinct Bartonella phylogenetic lineage named C, D, and E [18], and represented a novel Bartonella species. The rpoB haplotype H1 belongs to a distinct phylogenetic branch within lineage D, while the phylogenetic lineage C is represented in the current study by haplotypes: H2 (host L. fortisetosa), H3 (host L. cervi), and H4 (host L. fortisetosa). The haplotype H5 belongs to the distinct phylogenetic lineage E. Phylogenetic analyses corroborated the results obtained from the nucleotide network (Figure 3) and confirmed that haplotypes of Bartonella derived in this study are divided into three phylogenetic lineages: C (haplotypes H2, H3, and H4), D (haplotype H1), and E (haplotype H5). Haplotype H1 differed by eight mutation steps from haplotypes H15 (GenBank Acc. No. LC485119) found in Japan and H20 (GenBank Acc. No. MF580657) described in Poland. Three and five mutation steps separated haplotypes H2 and H4 in the current study from the Japanese haplotype H19 (GenBank Acc. No. MF580656). Haplotype H3 differed by nine substitutions from H17 (GenBank Acc. No. LC485121) obtained in Japan. Haplotypes H5 and H23 from Lithuania (GenBank Acc. No. MT876361) differed in only one mutation step.
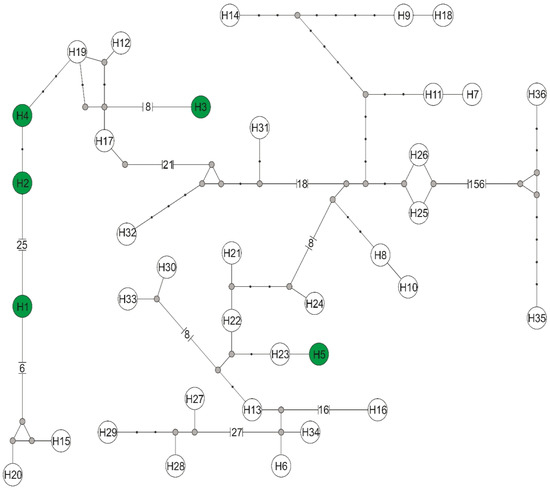
Figure 3.
Median-joining network of rpoB gene haplotypes for RNA polymerase beta—subunit of the genus Bartonella. Haplotypes obtained in this study with the number from H1 to H5 are marked with a green background, while haplotypes downloaded from GenBank have symbols H6–H36 (symbols according to Supplementary files Table S1). Missing haplotypes are indicated by a grey dot.
Among four individuals of L. cervi, four haplotypes were found of the flagellin gene of Borrelia sp. Haplotype H1 belongs to B. afzelii, while haplotypes H2, H3, and H4 represented B. burgdorferi, and were defined by six polymorphic sites, four transitions, and two transversions. The haplotype network based on the flaB sequences revealed the presence of the same Borrelia sp. phylogenetic lineages (Figure 4).
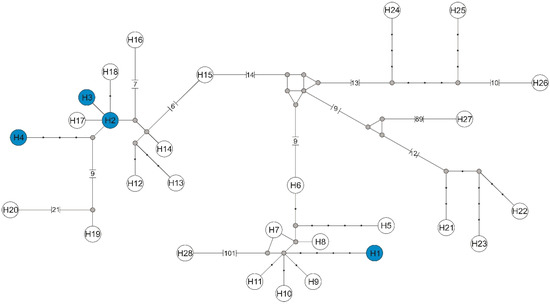
Figure 4.
Median-joining network of flagellin gene haplotypes of the genus Borrelia. Haplotypes found in this study with the number from H1 to H4 are marked with a blue background, while haplotypes downloaded from GenBank have symbols H5–H28 (symbols according to Supplementary files Table S2). Missing haplotypes are indicated by a grey dot.
Haplotype H1 belonging to B. afzelii differed by seven substitutions from haplotypes H7 (GenBank Acc. No. MG944962), H8 (GenBank Acc. No. KR782190), H9 (GenBank Acc. No. JF732879), and H10 (GenBank Acc. No. KF918616) described in Poland, and haplotype H11 (GenBank Accession No. MN958344) found in Iran (Supplementary files Table S2).
Nine and 12 mutation steps separated the H1 haplotypes from H6 (GenBank Acc. No. KR782182) and H5 (GenBank Acc. No. KR782180), also obtained in Poland. Haplotypes H2, H3, and H4 belonging to the B. burgdorferi differed by at least one substitution from haplotypes H17 (GenBank Acc. No. DQ016620) and H18 (GenBank Acc. No. HM345910), described in Poland
3. Discussion
The present study is the first report on the detection of B. burgdorferi s.l. DNA in L. cervi in Poland. Interestingly, these bacteria were detected in 4 out of 27 winged insects sampled from the environment and specimens collected from cervids. Deer keds have a specific development cycle. These flies are viviparous species, in which the offspring develop into mature third-stage larvae within the uterus of the female. They generate fully-grown larvae that pupate immediately after falling to the ground. When the flies find a suitable host, they shed their wings (then take a blood meal) and remain in the wingless form for the rest of their lives [2,19]. The results obtained in this study may suggest that this bacteria is transferred during the embryonic development of the larvae. Further detailed studies on the parasite-host system are needed to confirm or exclude this hypothesis. The existence of this type of transmission in Lipoptena spp., has been shown by De Bruin et al. [20] in the case of Bartonella schoenbuchensis and A. phagocytophilum in wingless females, developing larvae, and fully developed pupae. The authors indicated vertical transmission of these pathogens from female L. cervi to their offspring. In turn, Gałęcki et. al. [11] identified the genetic material of Bartonella spp., Mycoplasma spp. and Rickettsia spp. in winged specimens of L. fortisetosa sampled from the environment. The results obtained indicated that L. fortisetosa carries the DNA of pathogens, which might be collected through bloodmeal and transferred during the embryonic development of the larvae. Similarly, Korhonen et al. [21] detected Bartonella spp. DNA in an unfed adult deer ked. In the future, it would be useful to perform molecular analyzes on several genes, including nuclear and mitochondrial markers, to link the deer ked’s life cycle to the genetic variant.
It is also interesting that B. burgdorferi DNA was also detected in deer keds collected from cervids which are incompetent hosts for these pathogens [22]. Buss et al. [8], using PCR, confirmed the presence of B. burgdorferi and A. phagocytophilum in L. cervi removed from white-tailed deer. The prevalence of infection by these pathogens in the tested specimens was 39.50% for B. burgdorferi and 29.12% for A. phagocytophilum. In results obtained in the current study, B. burgdorferi was detected in 13.94% of L. cervi collected from red deer. In a similar study, Gałęcki et al. [11] identified the genetic material of Babesia spp., Borrelia spp., and Theileria spp. in L. fortisetosa, which had had direct contact with cervids. These results may suggest that these insects took blood from the host at an early stage of infection with this spirochete. However, based on the results obtained in this study, we were unable to make such a conclusion.
The phylogenetic analysis based on flagellin gene (flaB) sequences of different species of Borrelia genus showed the presence of one new haplotype of B. afzelii and three haplotypes of B. burgdorferi, of which two are newly described. A haplotype of B. afzelii grouped with haplotypes described in Poland by Wodecka et al. [23] (GenBank Acc. Nos. KF918616 and JF732879), and in Iran by Naddaf et al. [24] (GenBank Acc. No. MN958344), showed a 98.84% similarity with these sequences (Figure 2). On the ML tree, haplotypes of B. burgdorferi were grouped with sequences described in Poland by Wodecka et al. [23] (GenBank Acc. No. DQ016620 and HM345910).
In turn, the presence of Bartonella spp. in both species, L. cervi and L. fortisetosa, was confirmed in the current study. The bacteria DNA was found in 57.02% of deer keds collected from cervids. A similarly high percentage (33.3%) of L. fortisetosa infected by this bacteria collected from a different animal host in the area of eastern Poland, was shown by Bartosik et al. [25]. Szewczyk et al. [26] showed that the overall prevalence of infection with Bartonella spp. was 75.12% among L. cervi collected from red deer. Gałęcki et al. [11] detected the DNA of these pathogens in 63.2% of L. fortisetosa.
Molecular analysis of an RNA polymerase beta subunit (rpoB) gene fragment revealed five new haplotypes of Bartonella sp., represented as C, D, and E phylogenetic lineage of this species [17,18]. Haplotype H1 of Bartonella sp. (host L. fortisetosa), representing lineage D, grouped with haplotypes described in Poland by Szewczyk et al. [26] (GenBank Acc. No. MF580657), and in Japan by Sato et al. [18] (GenBank Acc. No. LC485119), revealed a 98.8% similarity (Figure 1). The rpoB sequences H2–H4, belonging to the phylogenetics group C, are also grouped with haplotypes described in Japan [17,18] (GenBank Acc. Nos. AB703149 and LC485121, respectively) and in Poland [26] (GenBank Acc. No. MF580656). The haplotype H5 of Bartonella sp. found in two different individuals of L. cervi shared a 99.85% similarity with Bartonella sp. from moose blood in Lithuania (GenBank Acc. No. MT876361; unpublished). Haplotype H5 and sequences obtained in Lithuania grouped together with haplotype of Bartonella sp., were found in blood from white-tailed deer in the USA [27] (GenBank Acc. No. AY805112). The grouping of the haplotypes identified in our survey with the genetic variants described in the GenBank indicates their close relationship and common origin.
Co-infections of Lipotena spp. are also known, but they are rare. Busset et al. [8], in their study, showed co-infection with B. burgdorferi s.l. and A. phagocytophilum in 6.25% of L. cervi. In addition, the genetic material of Coxiella spp., Trypanosoma spp., Theileria luwenshuni, and T. ovis have been identified in L. fortisetosa [9,10,28]. The results obtained in the present study can confirm the possibility of the occurrence of the presence of more than one pathogen in these insects.
In conclusion, both the results of the present study and the literature data indicate the possibility of the occurrence of various pathogens in Lipotena spp. The obtained data indicate that deer keds may potentially harbor both the studied pathogens, and might be an important biological marker for research on their circulation in the environment. However, further detailed studies are necessary to confirm that these bloodsucking insects could be treated as their potential biological vector and/or reservoir, and their potential transmission by deer keds should be assessed in accordance with Koch’s postulates [29,30].
4. Materials and Method
4.1. Field Work
The study was carried out in the Piska Forest (53°46′ N, 21°27′ E) and the Białowieża Primeval Forest (52°42′ N, 23°52′ E) located in north-eastern Poland. Deer keds were collected manually using tweezers from the fur of red deer during the autumn hunting season. In total, 208 Lipoptena spp. were collected from five hunted red deer in the Białowieża Primeval Forest and from 12 hunted red deer in the Piska Forest. The animals were culled in accordance with the Annual Hunting Plans in selected hunting circles operating in the studied macroregion, during hunting periods indicated in the Regulation of the Minister of the Environment of 16 March 2005 on the determination of hunting periods for game animals (Journal of Laws 2005, No. 48, item 459). Insects (27 specimens) were also collected from vegetation in autumn using an entomological net and after landing on clothing in the Białowieża Primeval Forest. The collected material was preserved in plastic sample tubes containing 70% ethyl alcohol. Species identification of the collected specimens was carried out using taxonomic keys according to Borowiec [2], Andreani et al. [31], and Salvetti et al. [32] under a stereoscopic microscope (OPTA—TECH, Warsaw, Poland).
4.2. PCR Detection of Bartonella spp. and B. burgdorferi s.l.
The DNA from each fly was extracted using the AX Tissue Mini kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s protocol. The DNA was measured spectrophotometrically in a nanospectrophotometer PEARL (Implen, Germany) and then frozen to −20 °C for further molecular study. To detect Bartonella sp., a 200 ng DNA template and a pair of primers—1400F/2300R—were used to amplify an 850 base pairs (bp) fragment of the rpoB gene [33]. PCR reactions were conducted according to Paziewska et al. [34]. For the reaction mixture, RUN Taq polymerase (A&A Biotechnology, Gdynia, Poland) was used. In turn, B. burgdorferi s.l. was detected in insects with the use of two pairs of primers specific to the flaB gene fragment, as previously described [23,35]. For amplification, a 200 ng DNA template was used. In turn, for re—amplification, 1 µL of the amplification product was used. DFS—Plus DNA Taq Polymerase (GeneOn, Ludwigshafen am Rhein, Germany) was used for both reactions. The presence of 824 bp (rpoB gene) and 605 bp (flaB gene) reaction products was considered positive. PCR and nested PCR products were visualized on 1% and 2% ethidium—bromide—stained agarose gels. Gels were visualized using ChemiDoc, MP Lab software (Imagine, BioRad, Hercules, Clearwater, FL, USA) or Omega 10 (UltraLum, Berlin, CT, USA) and TotalLab software (TotalLab, Newcastle upon Tyne, UK). The positive products of PCR and nested PCR were purified using a QIAEX II Gel extraction kit (Qiagen, Hilden, Germany) or Agarose—Out DNA Purification Kit (EURx, Gdańsk, Poland), and sequenced by Genomed (Warsaw, Poland).
4.3. Phylogenetic Analysis
The resulting six sequences of the rpoB gene for the RNA polymerase beta subunit of Bartonella sp. and four sequences of flagellin gene (flaB) of Borrelia genus were aligned and revised manually in BioEdit v 7.0.4 [36]. To determine bacteria species, the DNA sequences were compared with the GenBank references (Supplementary files) by BLAST (http://www.ncbi.nlm.nih.gov/; accessed on 14 November 2021).
To test the phylogenetic relationships among the obtained haplotypes of rpoB and flaB genes and sequences downloaded from GenBank (Supplementary files), phylogenetic trees were constructed using a maximum-likelihood (ML) algorithm in MEGA6 v.06 [37] with 1000 bootstrap replicates to assess the tree node support. Also used were additional sequences of Brucella melitensis (GenBank Acc. No. MK629659 and MK629660 for rpoB gene) and Borrelia species (B. tachyglossi (GenBank Acc. No. KY586966) and B. miyamotoi (GenBank Acc. No. FJ823229), both for flaB gene) downloaded as trees outgroups. In the phylogenetic analyses, a nucleotide substitution model was used, determined under the Akaike information criterion [38] implemented in jModelTest v. 0.1.1 [39]. The GTR+I+G model was selected as the best—fitting model for rpoB gene sequences, while for flagellin gene sequences, the GTR+G model was chosen. Also calculated and visualized were the relationships among haplotypes and sequences of rpoB and flaB gene downloaded from GenBank, by constructing a haplotype network using the median—joining method available in Network v. 10.2.0.0 (http://www.fluxus—engineering.com; accessed on 10 December 2021).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens11101111/s1, Table S1: List of species and GenBank accession numbers of their RNA polymerase beta subunit (rpoB) gene sequences used in the network phylogenetic analysis (Figure 1 and Figure 3). Table S2: List of species and GenBank accession numbers of their flagellin gene (fla B) sequences used in the network phylogenetic analysis (Figure 2 and Figure 4). References [40,41,42,43,44,45,46,47,48,49,50] are cited in the supplementary materials.
Author Contributions
J.W. (Joanna Werszko)—research concepts, preparation and submission of the manuscript, participation in molecular study, corresponding author; M.Ś.—phylogenetic analysis; T.S. and J.W. (Joanna Witecka)—molecular analysis; Ż.S.-B. and K.W.—collection of insects; M.A.—processing of data, participation in the interpretation of results. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the MINIATURA 2 grant nr. 2018/02/X/NZ8/00037 Research Project, funded by the National Science Centre, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw sequencing data are available on the National Center for Biotechnology Information under projectaccession number ON016083—ON016087 (for Bartonella sp.) ON016088 (for Borrelia afzelii), and ON016089—ON016091 (for Borrelia burgdorferi) (https://www.ncbi.nlm.nih.gov/nuccore/, accessed on 22 September 2022).
Acknowledgments
The authors thank Piotr Rode for his assistance in preparing the Figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahola, N.; Goodman, S.M.; Robert, V. The Hippoboscidae (Insecta: Diptera) from Madagascar, with new records from the “Parc National de Midongy Befotaka”. Parasite 2011, 18, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, L. Wpleszczowate-Hippoboscidae. In Klucze do Oznaczania Owadów Polski Cz. 28, z. 21; Wydawnictwo PWN: Warszawa, Poland, 1984. [Google Scholar]
- Sokół, R.; Gałęcki, R. Prevalence of keds on city dogs in central Poland. Med. Vet. Entomol. 2017, 31, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Madslien, K.; Ytrehus, B.; Vikøren, T.; Malmsten, J.; Isacsen, K.; Hygen, H.O.; Solberg, E.J. Hair-loss epizootic in moose (Alces alces) associated with massive deer ked (Lipoptena cervi) infestation. J. Wildl. Dis. 2011, 47, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Buczek, W.; Buczek, A.M.; Bartosik, K.; Buczek, A. Comparison of Skin Lesions Caused by Ixodes ricinus Ticks and Lipoptena cervi Deer Keds Infesting Humans in the Natural Environment. Int. J. Env. Res. Public Health 2020, 17, 3316. [Google Scholar] [CrossRef]
- Maślanko, W.; Bartosik, K.; Raszewska-Famielec, M.; Ewelina-Szwaj, E.; Asman, M. Exposure of Humans to Attacks by Deer Keds and Consequences of Their Bites-A Case Report with Environmental Background. Insects 2020, 11, 859. [Google Scholar] [CrossRef]
- Halos, L.; Jamal, T.; Maillard, R.; Girard, B.; Guillot, J.; Chomel, B.; Vayssier-Taussat, M.; Boulouis, H.-J. Role of Hippoboscidae flies as potential vector of Bartonella spp. infecting wild domestic ruminants. Appl. Environ. Microbiol. 2004, 70, 6302–6305. [Google Scholar] [CrossRef]
- Buss, M.; Case, L.; Kearney, B.; Coleman, C.; Henning, J.D. Detection of Lyme disease and anaplasmosis pathogens via PCR in Pennsylvania deer ked. J. Vector Ecol. 2016, 41, 292–294. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, K.-T.; Kwon, O.-D.; Ock, Y.; Kim, T.; Choi, D.; Kwak, D. Novel Detection of Coxiella spp., Theileria luwenshuni, and T. ovis Endosymbionts in Deer Keds (Lipoptena fortisetosa). PLoS ONE 2016, 11, e0156727. [Google Scholar] [CrossRef]
- Werszko, J.; Steiner-Bogdaszewska, Ż.; Jeżewski, W.; Szewczyk, T.; Kuryło, G.; Wołkowycki, M.; Wróblewski, P.; Karbowiak, G. Molecular detection of Trypanosoma spp. in Lipoptena cervi and Lipoptena fortisetosa (Diptera: Hippoboscidae) and their potential role in the transmission of pathogens. Parasitology 2020, 147, 1629–1635. [Google Scholar] [CrossRef]
- Gałęcki, R.; Jaroszewski, J.; Bakuła, T.; Galon, E.M.; Xuan, X. Molecular Detection of Selected Pathogens with Zoonotic Potential in Deer Keds (Lipoptena fortisetosa). Pathogens 2021, 10, 324. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Chang, C.C.; Chuang, S.T.; Chomel, B.B. Bartonella species and their ectoparasites: Selective host adaptation or strain selection between the vector and the mammalian host? Comp. Immunol Microbiol. Infect. Dis. 2011, 34, 299–314. [Google Scholar] [CrossRef]
- Maggi, R.G.; Mozayeni, B.R.; Pultorak, E.L.; Hegarty, B.C.; Bradley, J.M.; Correa, M.; Breitschwerdt, E.B. Bartonella spp. bacteremia and rheumatic symptoms in patient from Lyme disease-endemic region. Emerg. Infect. Dis. 2012, 18, 783–791. [Google Scholar] [CrossRef]
- Karbowiak, G.; Biernat, B.; Stańczak, J.; Werszko, J.; Szewczyk, T.; Sytykiewicz, H. The role of particular ticks developmental stages in the circulation of tick—borne pathogens in Central Europe. 5. Borreliaceae. Ann. Parasitol. 2018, 64, 151–171. [Google Scholar] [CrossRef]
- Răileanu, C.; Tauchmann, O.; Vasić, A.; Wöhnke, E.; Silaghi, C. Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identifcation and survey of tick—borne encephalitis virus in ticks from north-eastern Germany. Parasit. Vectors 2020, 13, 106. [Google Scholar] [CrossRef]
- Asman, M.; Witecka, J.; Korbecki, J.; Solarz, K. The potential risk of exposure to Borrelia garinii, Anaplasma phagocytophilum and Babesia microti in the Wolinski National Park (north-western Poland). Sci. Rep. 2021, 11, 4860. [Google Scholar] [CrossRef]
- Sato, S.; Kabeya, H.; Yamazaki, M.; Takeno, S.; Suzuki, K.; Kobayashi, S.; Souma, K.; Masuko, T.; Chomel, B.B.; Maruyama, S. Prevalence and genetic diversity of Bartonella species in sika deer (Cervus nippon) in Japan. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 575–581. [Google Scholar] [CrossRef]
- Sato, S.; Kabeya, H.; Ishiguro, S.; Shibasaki, Y.; Maruyama, S. Lipoptena fortisetosa as a vector of Bartonella bacteria in Japanese sika deer (Cervus nippon). Parasites Vectors 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Haarløv, N. Life cycle and distribution pattern of Lipoptena cervi (L.) (Dipt., Hippobosc.) on Danish deer. Oikos 1964, 15, 93–129. [Google Scholar] [CrossRef]
- De Bruin, A.; Van Leeuwen, A.D.; Jahfari, S.; Takken, W.; Földvári, M.; Dremmel, L.; Sprong, H.; Földvári, G. Vertical transmission of Bartonella schoenbuchensis in Lipoptena cervi. Parasites Vectors 2015, 8, 176. [Google Scholar] [CrossRef]
- Korhonen, E.M.; Pérez Vera, C.; Pulliainen, A.T.; Sironen, T.; Aaltonen, K.; Kortet, R.; Härkönen, L.; Härkönen, S.; Paakkonen, T.; Nieminen, P.; et al. Molecular detection of Bartonella spp. in deer ked pupae, adult keds and moose blood in Finland. Epidemiol. Infect. 2015, 143, 578–585. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Tälleklint, L. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J. Med. Entomol. 1992, 29, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B. flaB gene as a molecular marker for distinct identification of Borrelia species in environmental samples by the PCR-restriction fragment length polymorphism method. Appl. Environ. Microbiol. 2011, 77, 7088–7092. [Google Scholar] [CrossRef]
- Naddaf, S.R.; Mahmoudi, A.; Ghasemi, A.; Rohani, M.; Mohammadi, A.; Ziapour, S.P.; Nemati, A.H.; Mostafavi, E. Infection of hard ticks in the Caspian Sea littoral of Iran with Lyme borreliosis and relapsing fever borreliae. Ticks Tick Borne Dis. 2020, 11, 101500. [Google Scholar] [CrossRef]
- Bartosik, K.; Maślanko, W.; Buczek, A.; Asman, M.; Witecka, J.; Szwaj, E.; Błaszkiewicz, P.S.; Świsłocka, M. Two New Haplotypes of Bartonella sp. Isolated from Lipoptena fortisetosa (Diptera: Hippoboscidae) in SE Poland. Insects 2021, 24, 485. [Google Scholar] [CrossRef]
- Szewczyk, T.; Werszko, J.; Steiner-Bogdaszewska, Ż.; Laskowski, Z.; Karbowiak, G. Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasites Vectors 2017, 10, 487. [Google Scholar] [CrossRef]
- Tate, C.M.; Mead, D.G.; Luttrell, M.P.; Howerth, E.W.; Dugan, V.G.; Munderloh, U.G.; Davidson, W.R. Experimental infection of white-tailed deer with Anaplasma phagocytophilum, etiologic agent of human granulocytic anaplasmosis. J. Clin. Microbiol. 2005, 43, 3595–3601. [Google Scholar] [CrossRef]
- Hornok, S.; De la Fuente, J.; Biro, N.; De Mera, I.G.F.; Meli, L.M.; Elek, V.; Gonczi, E.; Meili, T.; Tanczos, B.; Farkas, R.; et al. First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector—Borne Zoonotic Dis. 2011, 11, 1319–1321. [Google Scholar] [CrossRef]
- Evans, A.S. Causation and disease: The Henle-Koch postulates revisited. Yale J. Biol Med. 1976, 49, 175–195. [Google Scholar] [PubMed]
- Byrd, A.L.; Segre, J.A. Infectious disease. Adapting Koch’s postulates. Science 2016, 351, 224–226. [Google Scholar] [CrossRef]
- Andreani, A.; Sacchetti, P.; Belcari, A. Comparative morphology of the deer keds Lipoptena fortisetosa first recorded from Italy. Med. Vet. Entomol. 2019, 33, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, M.; Bianchi, A.; Marangi, M.; Barlaam, A.; Giacomelli, S.; Bertoletti, I.; Roy, L.; Giangaspero, A. Deer keds on wild ungulates in northern Italy, with a taxonomic key for the identification of Lipoptena spp. of Europe. Med. Vet. Entomol. 2020, 34, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Renesto, P.; Gouvernet, J.; Drancourt, M.; Roux, V.; Raoult, D. Use of rpoB analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 2001, 3, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Paziewska, A.; Harris, P.D.; Zwolińska, L.; Bajer, A.; Siński, E. Recombination within and between species of the alpha proteobacterium Bartonella infecting rodents. Microb. Ecol. 2011, 61, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Rymaszewska, A.; Sawczuk, M.; Skotarczak, B. Detectability of tick-borne agents DNA in the blood of dogs undergoing treatment for borreliosis. Ann. Agric. Environ. Med. 2009, 16, 9–14. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Akaike, H. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 1973, 60, 255–265. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Bai, Y.; Malania, L.; Alvarez Castillo, D.; Moran, D.; Boonmar, S.; Chanlun, A.; Suksawa, F.; Maruyama, S.; Knobel, D.; Kosoy, M. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multi-locus sequence typing. PLoS ONE 2013, 21, e80894. [Google Scholar] [CrossRef]
- Antequera-Gómez, M.L.; Lozano-Almendral, L.; Barandika, J.F.; González-Martín-Niño, R.M.; Rodríguez-Moreno, I.; García-Pérez, A.L.; Gil, H. Bartonella chomelii is the most frequent species infecting cattle grazing in communal mountain pastures in Spain. Appl. Env. Microbiol. 2015, 81, 623–629. [Google Scholar] [CrossRef]
- Lozano-Sardaneta, Y.N.; Blum-Domínguez, S.; Huerta, H.; Tamay-Segovia, P.; Fernández-Figueroa, E.A.; Becker, I.; Sánchez-Montes, S. Detection of Candidatus Bartonella odocoilei n. sp. in Lipoptena mazamae associated with white-tailed deer in Campeche, Mexico. Med. Vet. Entomol. 2021, 35, 652–657. [Google Scholar] [CrossRef]
- Mediannikov, O.; Davoust, B.; Cabre, O.; Rolain, J.M.; Raoult, D. Bartonellae in animals and vectors in New Caledonia. Comp. Immunol. Microbiol. Infect Dis. 2011, 34, 497–501. [Google Scholar] [CrossRef]
- Bazrgari, N.; Garoosi, G.A.; Dadar, M. Genetic Diversity and Phylogenetic Relationship of Clinical Isolates of Brucella melitensis Based on Gene Polymorphism of β Subunit of RNA Polymerase (rpoB) Gene in Iran. Iran J. Med. Microbiol. 2020, 14, 425–440. [Google Scholar] [CrossRef]
- Güner, E.S.; Hashimoto, N.; Takada, N.; Kaneda, K.; Imai, Y.; Masuzawa, T. First isolation and characterization of Borrelia burgdorferi sensu lato strains from Ixodes ricinus ticks in Turkey. J. Med. Microbiol. 2003, 52, 807–813. [Google Scholar] [CrossRef]
- Noppa, L.; Burman, N.; Sadziene, A.; Barbour, A.G.; Bergström, S. Expression of the flagellin gene in Borrelia is controlled by an alternative sigma factor. Microbiology (Reading) 1995, 141, 85–93. [Google Scholar] [CrossRef][Green Version]
- Wodecka, B.; Skotarczak, B. Identification of host blood-meal sources and Borrelia in field-collected Ixodes ricinus ticks in north-western Poland. Ann. Agric. Environ. Med. 2016, 23, 59–63. [Google Scholar] [CrossRef]
- Livey, I.; Gibbs, C.P.; Schuster, R.; Dorner, F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol. Microbiol. 1995, 18, 257–269. [Google Scholar] [CrossRef]
- Chu, C.Y.; Liu, W.; Jiang, B.G.; Wang, D.M.; Jiang, W.J.; Zhao, Q.M.; Zhang, P.H.; Wang, Z.X.; Tang, G.P.; Yang, H.; et al. Novel genospecies of Borrelia burgdorferi sensu lato from rodents and ticks in southwestern China. J. Clin. Microbiol. 2008, 46, 3130–3133. [Google Scholar] [CrossRef]
- Loh, S.M.; Gillett, A.; Ryan, U.; Irwin, P.; Oskam, C. Molecular characterization of ‘Candidatus Borrelia tachyglossi’ (family Spirochaetaceae) in echidna ticks, Bothriocroton concolor. Int. J. Syst. Evol. Microbiol. 2017, 67, 1075–1080. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).