The Domestic Dog as a Laboratory Host for Brugia malayi
Abstract
1. Introduction
2. Results
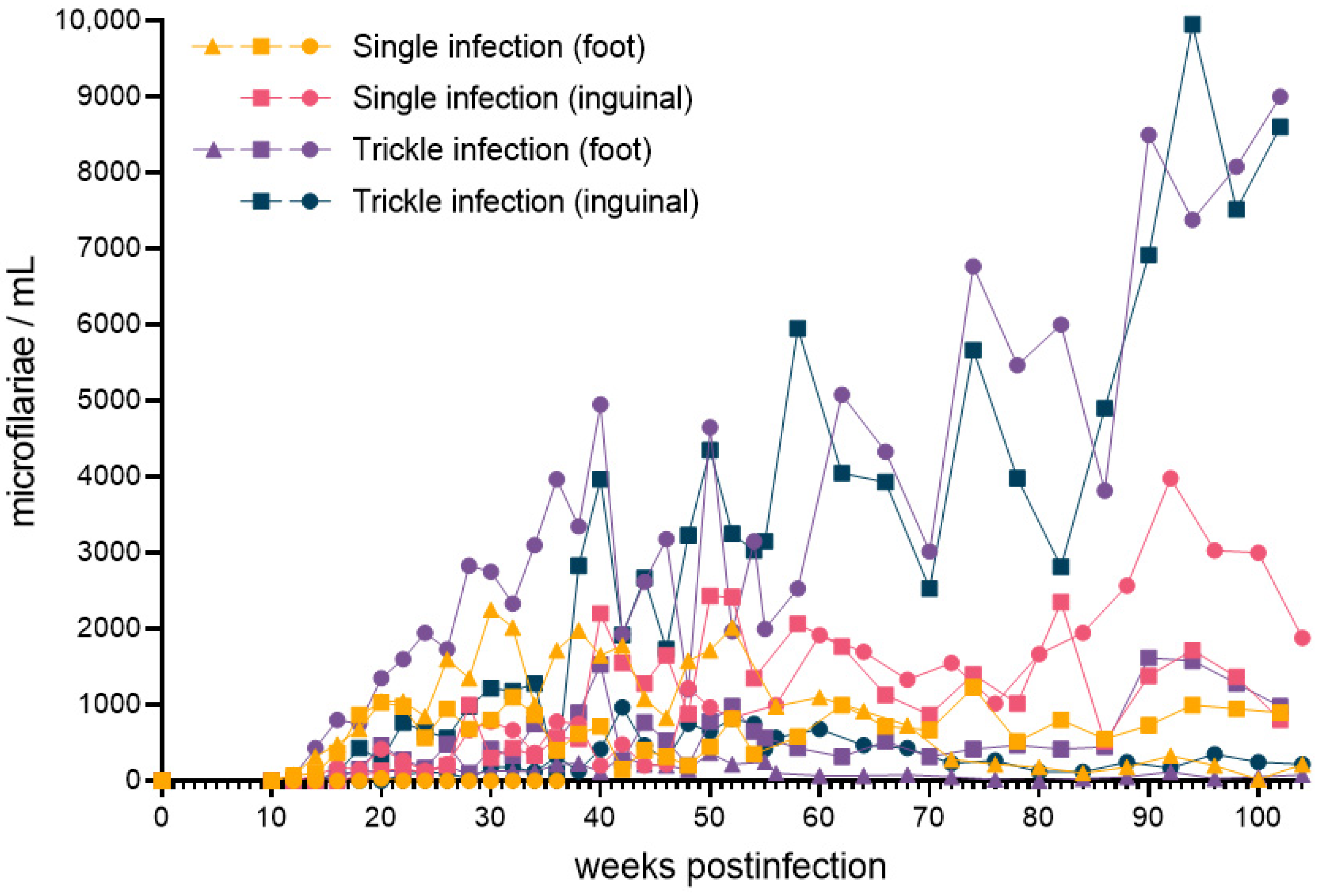
2.1. Microfilaremia
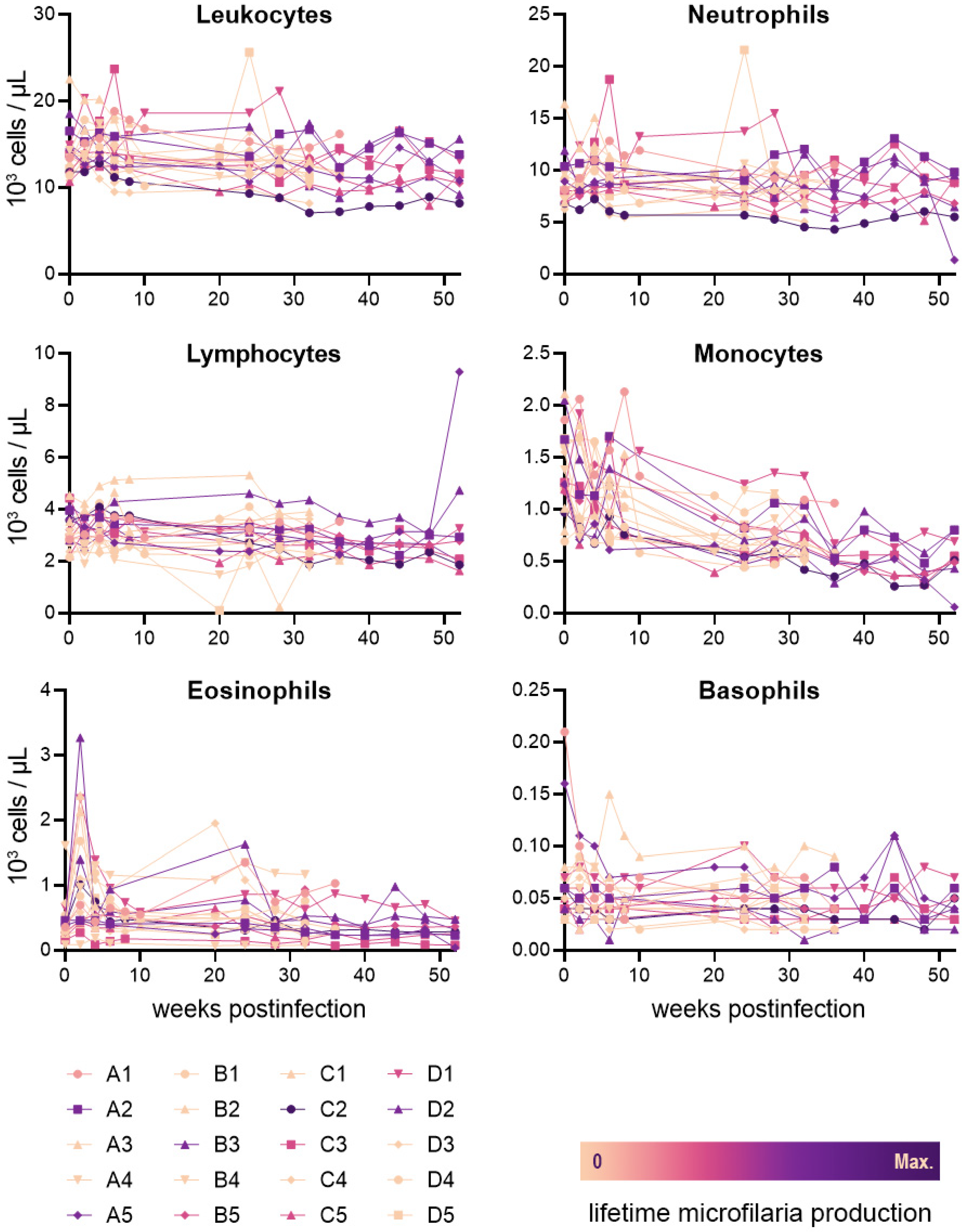
2.2. Leukocyte Values
2.3. Clinical Signs
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Experimental Infections
4.3. Microfilaria Counts by Giemsa Staining
4.4. CBC Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Lymphatic Filariasis. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 1 April 2022).
- Subramanian, S.; Stolk, W.A.; Ramaiah, K.D.; Plaisier, A.P.; Krishnamoorthy, K.; Van Oortmarssen, G.J.; Dominic Amalraj, D.; Habbema, J.D.F.; Das, P.K. The Dynamics of Wuchereria bancrofti Infection: A Model-Based Analysis of Longitudinal Data from Pondicherry, India. Parasitology 2004, 128, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Vanamail, P.; Subramanian, S.; Das, P.K.; Pani, S.P.; Rajagopalan, P.K.; Bundy, D.A.; Grenfell, B.T. Estimation of Age-Specific Rates of Acquisition and Loss of Wuchereria bancrofti Infection. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 689–693. [Google Scholar] [CrossRef]
- Critchley, J.; Addiss, D.; Ejere, H.; Gamble, C.; Garner, P.; Gelband, H. Albendazole for the Control and Elimination of Lymphatic Filariasis: Systematic Review. Trop. Med. Int. Health 2005, 10, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Addiss, D.; Santos, A.; Figueredo-Silva, J.; Norões, J. Direct Assessment in Vivo of the Efficacy of Combined Single-Dose Ivermectin and Diethylcarbamazine against Adult Wuchereria bancrofti. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 219–222. [Google Scholar] [CrossRef]
- Norões, J.; Dreyer, G.; Santos, A.; Mendes, V.G.; Medeiros, Z.; Addiss, D. Assessment of the Efficacy of Diethylcarbamazine on Adult Wuchereria bancrofti in Vivo. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 78–81. [Google Scholar] [CrossRef]
- Geary, T.G.; Woo, K.; McCarthy, J.S.; Mackenzie, C.D.; Horton, J.; Prichard, R.K.; de Silva, N.R.; Olliaro, P.L.; Lazdins-Helds, J.K.; Engels, D.A.; et al. Unresolved Issues in Anthelmintic Pharmacology for Helminthiases of Humans. Int. J. Parasitol. 2010, 40, 1–13. [Google Scholar] [CrossRef]
- Ash, L.R.; Riley, J.M. Development of Subperiodic Brugia malayi in the Jird, Meriones unguiculatus, with Notes on Infections in Other Rodents. J. Parasitol. 1970, 56, 969–973. [Google Scholar] [CrossRef]
- Edeson, J.F.B.; Wharton, E.H. The Transmission of Wuchereria malayi from Man to the Domestic Cat. Trans. R. Soc. Trop. Med. Hyg. 1957, 51, 366–370. [Google Scholar] [CrossRef]
- Burren, C.H. The Behaviour of Brugia malayi Microfilariae in Experimentally Infected Domestic Cats. Ann. Trop. Med. Parasitol. 1972, 66, 235–242. [Google Scholar] [CrossRef]
- Michalski, M.L.; Griffiths, K.G.; Williams, S.A.; Kaplan, R.M.; Moorhead, A.R. The NIH-NIAID Filariasis Research Reagent Resource Center. PLoS Negl. Trop. Dis. 2011, 5, e1261. [Google Scholar] [CrossRef]
- Cross, J.H.; Partono, F.; Hsu, M.Y.; Ash, L.R.; Oemijati, S. Experimental Transmission of Wuchereria bancrofti to Monkeys. Am. J. Trop. Med. Hyg. 1979, 28, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, J.R.; Connor, D.H.; Purnomo; Marwoto, H.A. Bancroftian Filariasis. Wuchereria bancrofti Infection in the Silvered Leaf Monkey (Presbytis cristatus). Am. J. Pathol. 1983, 112, 383–386. [Google Scholar]
- Dissanaike, A.S.; Mak, J.W. A Description of Adult Wuchereria bancrofti (Rural Strain) from an Experimental Infection in the Long-Tailed Macaque, Macaca Fascicularis (Sny. M. Irus). J. Helminthol. 1980, 54, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Edeson, J.F.; Buckley, J.J. Studies on Filariasis in Malaya: On the Migration and Rate of Growth of Wuchereria malayi in Experimentally Infected Cats. Ann. Trop. Med. Parasitol. 1959, 53, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ewert, A. Distribution of Developing and Mature Brugia malayi in Cats at Various Times after a Single Inoculation. J. Parasitol. 1971, 57, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Ewert, A.; Folse, D.; Hillman, G.; Wang, Y.-X. Effect of Diethylcarbamazine Citrate on Brugia malayi Infections in Cats Following Daily, Weekly, or Monthly Administration. Am. J. Trop. Med. Hyg. 1983, 32, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Chansiri, K.; Yodmek, S. Periodicity of Brugia malayi Appearance in Blood of Domestic Thai Cats in Surat Thani Province. J. Trop. Med. Parasitol. 2004, 27, 15–20. [Google Scholar]
- Laing, A.B.G. Influence of the Animal Host on the Microfilarial Periodicity of Brugia malayi. Trans. R. Soc. Trop. Med. Hyg. 1961, 55, 558. [Google Scholar] [CrossRef]
- Michael, E.; Grenfell, B.T.; Bundy, D.A.P. The Association between Microfilaraemia and Disease in Lymphatic Filariasis. Proc. R. Soc. London. Ser. B Biol. Sci. 1994, 256, 33–40. [Google Scholar]
- King, C.L.; Nutman, T.B. Regulation of the Immune Response in Lymphatic Filariasis and Onchocerciasis. Immunol. Today 1991, 12, A54–A58. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Debrah, A.Y.; Specht, S.; Hoerauf, A. Filariasis and Lymphoedema. Parasite Immunol. 2009, 31, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Kwarteng, A.; Ahuno, S.T. Immunity in Filarial Infections: Lessons from Animal Models and Human Studies. Scand. J. Immunol. 2017, 85, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Lawrence, R.A. Immunological Tolerance: The Key Feature in Human Filariasis? Parasitol. Today 1991, 7, 271–276. [Google Scholar] [CrossRef]
- Ottesen, E.A. Immunological Aspects of Lymphatic Filariasis and Onchocerciasis in Man. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 9–18. [Google Scholar] [CrossRef]
- Freedman, D.O. Immune Dynamics in the Pathogenesis of Human Lymphatic Filariasis. Parasitol. Today 1998, 14, 229–234. [Google Scholar] [CrossRef]
- Nicolas, L.; Langy, S.; Plichart, C.; Deparis, X. Filarial Antibody Responses in Wuchereria bancrofti Transmission Area Are Related to Parasitological but Not Clinical Status. Parasite Immunol. 1999, 21, 73–80. [Google Scholar] [CrossRef]
- Jaoko, W.G.; Simonsen, P.E.; Meyrowitsch, D.W.; Estambale, B.B.A.; Malecela-Lazaro, M.N.; Michael, E. Filarial-Specific Antibody Response in East African Bancroftian Filariasis: Effects of Host Infection, Clinical Disease, and Filarial Endemicity. Am. J. Trop. Med. Hyg. 2006, 75, 97–107. [Google Scholar] [CrossRef][Green Version]
- Turner, P.; Copeman, B.; Gerisi, D.; Speare, R. A Comparison of the Og4C3 Antigen Capture ELISA, the Knott Test, an IgG4 Assay and Clinical Signs, in the Diagnosis of Bancroftian Filariasis. Trop. Med. Parasitol. Off. Organ Dtsch. Trop. Ges. Dtsch. Ges. Tech. Zs. 1993, 44, 45. [Google Scholar]
- Tefferi, A. Blood Eosinophilia: A New Paradigm in Disease Classification, Diagnosis, and Treatment. Mayo Clin. Proc. 2005, 80, 75–83. [Google Scholar] [CrossRef]
- Musso, D. Relevance of the Eosinophil Blood Count in Bancroftian Filariasis as a Screening Tool for the Treatment. Pathog. Glob. Health 2013, 107, 96–102. [Google Scholar] [CrossRef]
- Palmieri, J.R.; Masbar, S.; Marwoto, H.A.; Tirtokusumo, S.; Darwis, F. The Domestic Cat as a Host for Brugian Filariasis in South Kalimantan (Borneo), Indonesia. J. Helminthol. 1985, 59, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Dondero, T.J., Jr.; Menon, V.V. Clinical Epidemiology of Filariasis Due to Brugia malayi on a Rubber Estate in West Malaysia. Southeast Asian J. Trop. Med. Public Health 1972, 3, 355–365. [Google Scholar] [PubMed]
- Ravindran, R.; Varghese, S.; Nair, S.N.; Balan, V.M.; Lakshmanan, B.; Ashruf, R.M.; Kumar, S.S.; Gopalan, A.K.K.; Nair, A.S.; Malayil, A.; et al. Canine Filarial Infections in a Human Brugia malayi Endemic Area of India. Biomed. Res. Int. 2014, 2014, 630160. [Google Scholar] [CrossRef] [PubMed]
- Ambily, V.R.; Pillai, U.N.; Arun, R.; Pramod, S.; Jayakumar, K.M. Detection of Human Filarial Parasite Brugia malayi in Dogs by Histochemical Staining and Molecular Techniques. Vet. Parasitol. 2011, 181, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Chirayath, D.; Alex, P.C.; George, S.; Ajithkumar, S.; Panicker, V.P. Identification of Brugia malayi in Dogs in Kerala, India. Trop Biomed. 2017, 34, 804–814. [Google Scholar]
- Satjawongvanit, H.; Phumee, A.; Tiawsirisup, S.; Sungpradit, S.; Brownell, N.; Siriyasatien, P.; Preativatanyou, K. Molecular Analysis of Canine Filaria and Its Wolbachia Endosymbionts in Domestic Dogs Collected from Two Animal University Hospitals in Bangkok Metropolitan Region, Thailand. Pathogens 2019, 8, 114. [Google Scholar] [CrossRef]
- Mallawarachchi, C.H.; Chandrasena, N.T.G.A.; Wickramasinghe, S.; Premaratna, R.; Gunawardane, N.Y.I.S.; Mallawarachchi, N.S.; de Silva, N.R. A Preliminary Survey of Filarial Parasites in Dogs and Cats in Sri Lanka. PLoS ONE 2018, 13, e0206633. [Google Scholar]
- Rathnayake, S.; Chandrasena, N.; Wijerathna, T.; Mallawarachchi, H.; Gunathilaka, N. Canine Filaria Species in Selected Lymphatic Filariasis Endemic and Non-Endemic Areas in Sri Lanka. Parasitol. Res. 2022, 121, 2187–2191. [Google Scholar] [CrossRef]
- Simón, F.; González-Miguel, J.; Diosdado, A.; Gómez, P.J.; Morchón, R.; Kartashev, V. The Complexity of Zoonotic Filariasis Episystem and Its Consequences: A Multidisciplinary View. Biomed. Res. Int. 2017, 2017, 6436130. [Google Scholar] [CrossRef]
- Hines, S.A.; Crandall, R.B.; Crandall, C.A.; Thompson, J.P. Lymphatic Filariasis. Brugia malayi Infection in the Ferret (Mustela Putorius Furo). Am. J. Pathol. 1989, 134, 1373–1376. [Google Scholar]
- Jackson-Thompson, B.M.; Kim, S.Y.; Jaiswal, S.; Scott, J.R.; Jones, S.R.; Morris, C.P.; Fite, J.J.; Laurie, K.; Hoy, A.R.; Dardzinski, B.J.; et al. Brugia malayi Infection in Ferrets—A Small Mammal Model of Lymphatic Filariasis. PLoS Negl. Trop. Dis. 2018, 12, e0006334. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.C.; Blair, L.S.; McCall, J.W. Brugia pahangi and Dirofilaria immitis: Experimental Infections in the Ferret, Mustela Putorius Furo. Exp. Parasitol. 1979, 47, 327–332. [Google Scholar] [CrossRef]
- Ash, L.R.; Riley, J.M. Development of Brugia pahangi in the Jird, Meriones unguiculatus, with Notes on Infections in Other Rodents. J. Parasitol. 1970, 56, 962–968. [Google Scholar] [CrossRef]
- Kowalski, J.C.; Ash, L.R. Repeated Infections of Brugia pahangi in the Jird, Meriones unguiculatus. Southeast Asian J. Trop. Med. Public Health 1975, 6, 195–198. [Google Scholar]
- Grenfell, B.T.; Michael, E.; Denham, D.A. A Model for the Dynamics of Human Lymphatic Filariasis. Parasitol. Today 1991, 7, 318–323. [Google Scholar] [CrossRef]
- Evans, C.C.; Burkman, E.J.; Dzimianski, M.T.; Garner, B.; Moorhead, A.R. The Course of Brugia malayi Microfilaremia in Experimentally Infected Cats. Vector-Borne Zoonotic Dis. 2021, 21, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karnam, A.; Das, M.; Babu, S.P.S.; Bayry, J. Wuchereria bancrofti Filaria Activates Human Dendritic Cells and Polarizes T Helper 1 and Regulatory T Cells via Toll-like Receptor 4. Commun. Biol. 2019, 2, 169. [Google Scholar] [CrossRef]
- Babu, S.; Bhat, S.Q.; Kumar, N.P.; Lipira, A.B.; Kumar, S.; Karthik, C.; Kumaraswami, V.; Nutman, T.B. Filarial Lymphedema Is Characterized by Antigen-Specific Th1 and Th17 Proinflammatory Responses and a Lack of Regulatory T Cells. PLoS Negl. Trop Dis. 2009, 3, e420. [Google Scholar] [CrossRef]
- Maizels, R.M.; Hewitson, J.P.; Smith, K.A. Susceptibility and Immunity to Helminth Parasites. Curr. Opin. Immunol. 2012, 24, 459–466. [Google Scholar] [CrossRef]
- King, C.L.; Mahanty, S.; Kumaraswami, V.; Abrams, J.S.; Regunathan, J.; Jayaraman, K.; Ottesen, E.A.; Nutman, T.B. Cytokine Control of Parasite-Specific Anergy in Human Lymphatic Filariasis. Preferential Induction of a Regulatory T Helper Type 2 Lymphocyte Subset. J. Clin. Investig. 1993, 92, 1667–1673. [Google Scholar] [CrossRef]
- Torrero, M.N.; Hübner, M.P.; Larson, D.; Karasuyama, H.; Mitre, E. Basophils Amplify Type 2 Immune Responses, but Do Not Serve a Protective Role, during Chronic Infection of Mice with the Filarial Nematode Litomosoides sigmodontis. J. Immunol. 2010, 185, 7426–7434. [Google Scholar] [CrossRef] [PubMed]
- Mitre, E.; Taylor, R.T.; Kubofcik, J.; Nutman, T.B. Parasite Antigen-Driven Basophils Are a Major Source of IL-4 in Human Filarial Infections. J. Immunol. 2004, 172, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, T.; Porte, P.; Lee, J.; Rajan, T.V. Eosinophils, but Not Eosinophil Peroxidase or Major Basic Protein, Are Important for Host Protection in Experimental Brugia pahangi Infection. Infect. Immun. 2005, 73, 8442–8443. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.E.; Rothenberg, M.E.; Lawrence, R.A. Eotaxin-1-Regulated Eosinophils Have a Critical Role in Innate Immunity against Experimental Brugia malayi Infection. Eur. J. Immunol. 2005, 35, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Blauvelt, C.P.; Kumaraswami, V.; Nutman, T.B. Cutting Edge: Diminished T Cell TLR Expression and Function Modulates the Immune Response in Human Filarial Infection. J. Immunol. 2006, 176, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Blauvelt, C.P.; Kumaraswami, V.; Nutman, T.B. Diminished Expression and Function of TLR in Lymphatic Filariasis: A Novel Mechanism of Immune Dysregulation. J. Immunol. 2005, 175, 1170–1176. [Google Scholar] [CrossRef]
- Semnani, R.T.; Venugopal, P.G.; Leifer, C.A.; Mostböck, S.; Sabzevari, H.; Nutman, T.B. Inhibition of TLR3 and TLR4 Function and Expression in Human Dendritic Cells by Helminth Parasites. Blood 2008, 112, 1290–1298. [Google Scholar] [CrossRef]
- Semnani, R.T.; Liu, A.Y.; Sabzevari, H.; Kubofcik, J.; Zhou, J.; Gilden, J.K.; Nutman, T.B. Brugia malayi Microfilariae Induce Cell Death in Human Dendritic Cells, Inhibit Their Ability to Make IL-12 and IL-10, and Reduce Their Capacity to Activate CD4+ T Cells. J. Immunol. 2003, 171, 1950–1960. [Google Scholar] [CrossRef]
- Crandall, R.B.; McGreevy, P.B.; Connor, D.H.; Crandall, C.A.; Neilson, J.T.; McCall, J.W. The Ferret (Mustela Putorius Furo) as an Experimental Host for Brugia malayi and Brugia pahangi. Am. J. Trop. Med. Hyg. 1982, 31, 752–759. [Google Scholar] [CrossRef]
| Infection Group | Microfilaremia Status | Total | |
|---|---|---|---|
| + | − | ||
| 1 | 8 | 2 | 10 |
| 2 | 8 | 2 | 10 |
| 3 | 1 | 13 | 14 |
| 4 | 8 | 2 | 10 |
| 5 | 7 | 3 | 10 |
| 6 | 8 | 2 | 10 |
| 7 | 6 | 4 | 10 |
| 8 | 3 | 7 | 10 |
| 9 | 4 | 5 | 9 |
| 10 | 3 | 7 | 10 |
| 11 | 5 | 4 | 9 |
| 12 | 6 | 4 | 10 |
| 13 | 1 | 1 | 2 |
| 14 | 5 | 5 | 10 |
| Total | 73 | 61 | 134 |
| Schedule of Infection | |||
|---|---|---|---|
| Single (1 injection of 500 L3) | Trickle (4 injections; 125 L3 each) | ||
| Route of Infection | Inguinal | n = 5 | n = 5 |
| Hind Paw | n = 5 | n = 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, C.C.; Greenway, K.E.; Campbell, E.J.; Dzimianski, M.T.; Mansour, A.; McCall, J.W.; Moorhead, A.R. The Domestic Dog as a Laboratory Host for Brugia malayi. Pathogens 2022, 11, 1073. https://doi.org/10.3390/pathogens11101073
Evans CC, Greenway KE, Campbell EJ, Dzimianski MT, Mansour A, McCall JW, Moorhead AR. The Domestic Dog as a Laboratory Host for Brugia malayi. Pathogens. 2022; 11(10):1073. https://doi.org/10.3390/pathogens11101073
Chicago/Turabian StyleEvans, Christopher C., Katelin E. Greenway, Elyssa J. Campbell, Michael T. Dzimianski, Abdelmoneim Mansour, John W. McCall, and Andrew R. Moorhead. 2022. "The Domestic Dog as a Laboratory Host for Brugia malayi" Pathogens 11, no. 10: 1073. https://doi.org/10.3390/pathogens11101073
APA StyleEvans, C. C., Greenway, K. E., Campbell, E. J., Dzimianski, M. T., Mansour, A., McCall, J. W., & Moorhead, A. R. (2022). The Domestic Dog as a Laboratory Host for Brugia malayi. Pathogens, 11(10), 1073. https://doi.org/10.3390/pathogens11101073






