Seasonality of Coxiella burnetii among Wild Rabbits (Oryctolagus cuniculus) and the Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem
Abstract
:1. Introduction
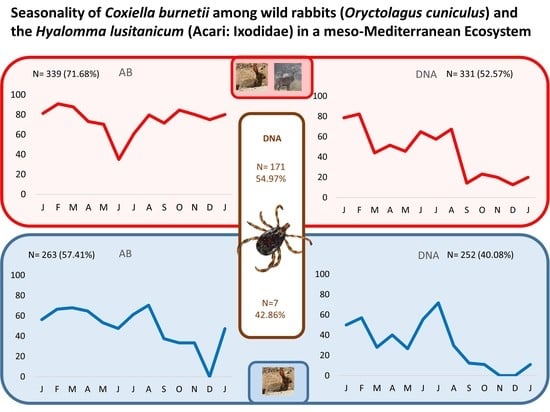
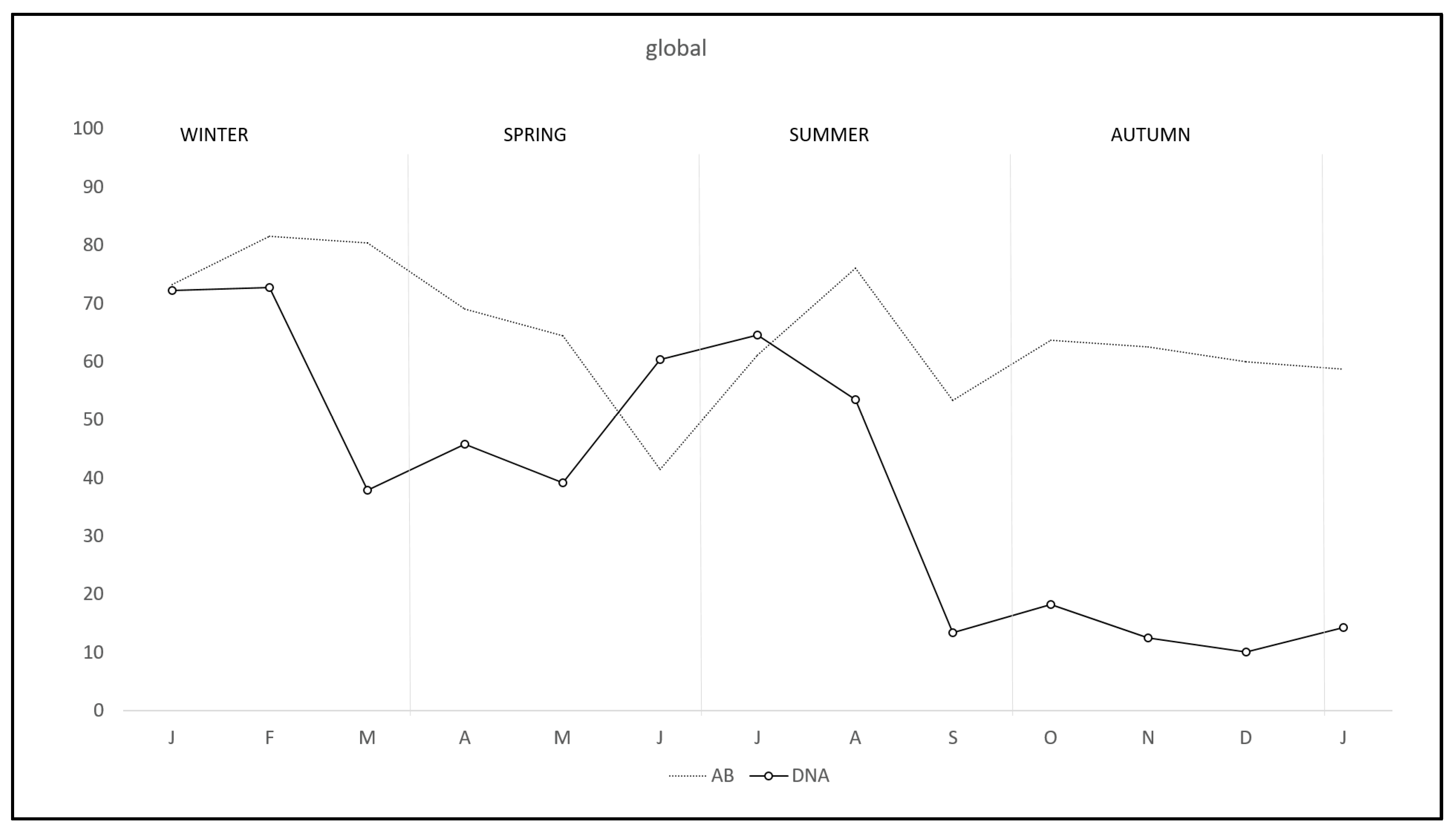
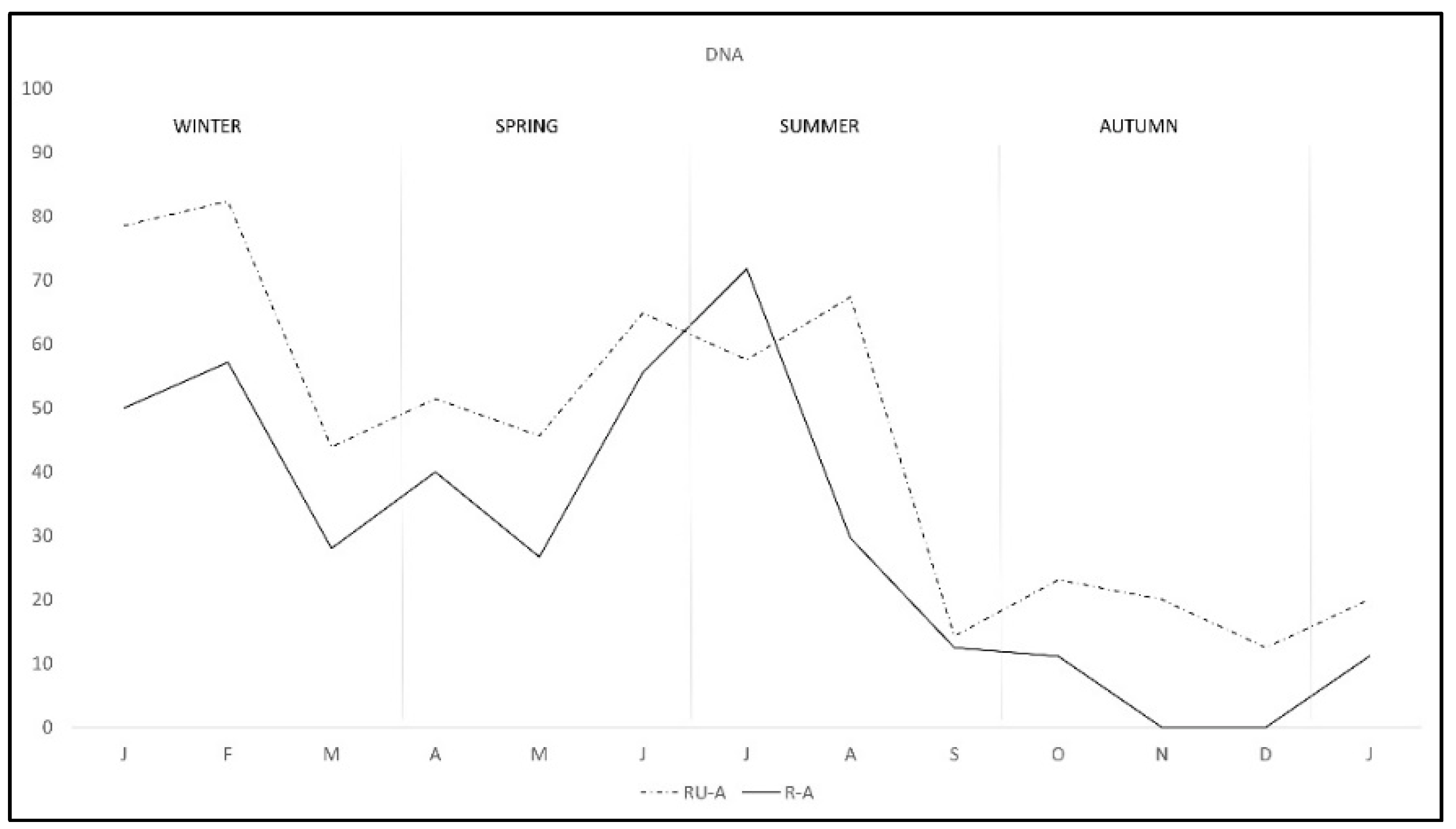
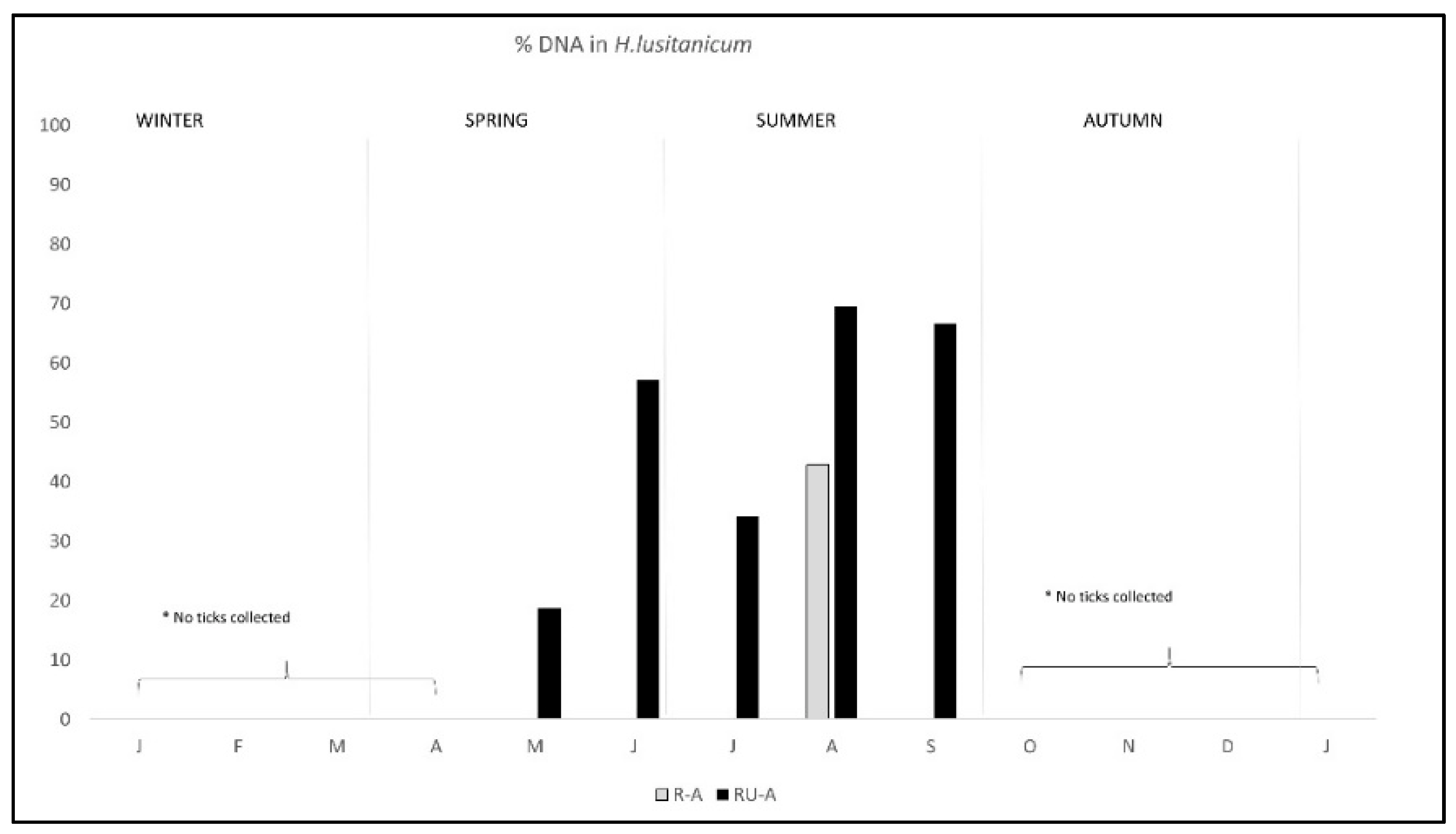
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Host Sampling and Management
4.3. Preparation of Samples for PCR Analysis
4.4. PCR Analysis
4.5. ELISA Analyses
4.6. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derrick, E.H. Q fever, a new fever entity: Clinical features, diagnosis and laboratory investigation. Med. J. Aust. 1937, 2, 281–299. [Google Scholar] [CrossRef]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella Burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [Green Version]
- CDC. Q Fever. Available online: https://www.cdc.gov/qfever/transmission/index.html (accessed on 31 January 2019).
- EFSA; ECDC. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. EFSA J. 2018, 16, 188–192. [Google Scholar] [CrossRef]
- Wiebe, M.E.; Burton, I.P.R.; Shankel, D.M. Isolation and Characterization of Two Cell Types of Coxiella Burnetii Phase I. J. Bacteriol. 1972, 110, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [Green Version]
- González-Barrio, D.; Almería, S.; Caro, M.R.; Salinas, J.; Ortiz, J.A.; Gortázar, C.; Ruiz-Fons, F. Coxiella burnetii shedding by farmed red deer (Cervus elaphus). Transbound. Emerg. Dis. 2015, 62, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Oporto, B.; Barandika, J.F.; Hurtado, A.; Aduriz, G.; Moreno, B.; Garcia-Perez, A.L. Incidence of ovine abortion by Coxiella burnetii in Northern Spain. Ann. N. Y. Acad. Sci. 2006, 1078, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, L.; Bauerfeind, U.; Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Offergeld, R.; et al. Coxiella burnetii-pathogenic agent of Q (Query) fever. Transfus. Med. Hemother. 2014, 41, 60–72. [Google Scholar]
- Kim, S.G.; Kim, E.H.; Lafferty, C.J.; Dubovi, E. Coxiella burnetii in bulk tank milk samples, united states. Emerg. Infect. Dis. 2005, 11, 619–621. [Google Scholar] [CrossRef]
- González-Barrio, D.; Ruiz-Fons, F. Coxiella Burnetii in Wild Mammals: A Systematic Review. Transbound. Emerg. Dis. 2019, 66, 662–671. [Google Scholar] [CrossRef]
- Barandika, J.F.; Hurtado, A.; Garcia-Esteban, C.; Gil, H.; Escudero, R.; Barral, M.; Jado, I.; Juste, R.A.; Anda, P.; García-Pérez, A.L. Tick-borne zoonotic bacteria in wild and domestic small mammals in Northern Spain. Appl. Environ. Microbiol. 2007, 73, 6166–6171. [Google Scholar]
- González-Barrio, D.; Maio, E.; Vieira-Pinto, M.; Ruiz-Fons, F. European rabbits as reservoir for Coxiella burnetii. Emerg. Infect. Dis. 2015, 21, 1055–1058. [Google Scholar]
- González-Barrio, D.; Velasco Ávila, A.L.; Boadella, M.; Beltrán-Beck, B.; Barasona, J.Á.; Santos, J.P.V.; Queirós, J.; García-Pérez, A.L.; Barral, M.; Ruiz-Fons, F. Host and environmental factors modulate the exposure of free-ranging and farmed red deer (Cervus elaphus) to Coxiella burnetii. Appl. Environ. Microbiol. 2015, 81, 6223–6231. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Fons, F.; Rodríguez, Ó.; Torina, A.; Naranjo, V.; Gortázar, C.; de la Fuente, J. Prevalence of Coxiella burnetti Infection in wild and farmed ungulates. Vet. Microbiol. 2008, 126, 282–286. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Report of WHO Workshop on Q Fever, Giessen, 2–5 September 1986; WHO: Geneva, Switzerland.
- Tigertt, W.D.; Benenson, A.S.; Gochenour, W.S. Airborne Q fever. Microbiol. Mol. Biol. Rev. 1961, 25, 285–293. [Google Scholar] [CrossRef]
- Toledo, A.; Jado, I.; Olmeda, A.S.; Casado-Nistal, M.A.; Gil, H.; Escudero, R.; Anda, P. Detection of Coxiella Burnetii in ticks collected from Central Spain. Vector-Borne Zoonotic Dis. 2009, 9, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Sidi-Boumedine, K.; Rousset, E.; Moutailler, S.; Jourdain, E. The importance of ticks in Q fever transmission: What has (and has not) been demonstrated? Trends Parasitol. 2015, 31, 536–552. [Google Scholar] [CrossRef]
- González, J.; González, M.G.; Valcárcel, F.; Sánchez, M.; Martín-Hernández, R.; Tercero, J.M.; Olmeda, A.S. Transstadial transmission from nymph to adult of Coxiella Burnetii by naturally infected Hyalomma Lusitanicum. Pathogens 2020, 9, 884. [Google Scholar]
- González, J.; Valcárcel, F.; Pérez-Sánchez, J.L.; Tercero-Jaime, J.M.; Olmeda, A.S. Seasonal dynamics of Ixodid ticks on wild rabbits Oryctolagus Cuniculus (Leporidae) from Central Spain. Exp. Appl. Acarol. 2016, 70, 369–380. [Google Scholar] [CrossRef]
- González, J.; González, M.G.; Valcárcel, F.; Sánchez, M.; Martín-Hernández, R.; Tercero, J.M.; Olmeda, A.S. Prevalence of Coxiella Burnetii (Legionellales: Coxiellaceae) Infection Among Wildlife Species and the Tick Hyalomma Lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem. J. Med. Entomol. 2019, 57, 551–556. [Google Scholar] [CrossRef]
- Valcárcel, F.; González, J.; Sánchez, J.L.P.; Jaime, J.M.T.; Olmeda, A.S. Long-term ecological study of host-seeking adults of Hyalomma Lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Climate. J. Med. Entomol. 2016, 53, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, F.; González, J.; Tercero, J.M.; Olmeda, A.S. Long term study of Ixodid Ticks feeding on red deer (Cervus Elaphus) in a Meso-Mediterranean climate. Exp. Appl. Acarol. 2016, 69, 61–72. [Google Scholar]
- Camps, J. Desde el «Alilepus» hasta el «Cuniculus». Bol. Cunicult. 1996, 48–53. Available online: http://www.historiaveterinaria.org/update/origconi-1456742015.pdf (accessed on 14 December 2021).
- Delibes-Mateos, M.; Redpath, S.M.; Angulo, E.; Ferreras, P.; Villafuerte, R. Rabbits as a keystone species in Southern Europe. Biol. Conserv. 2007, 137, 149–156. [Google Scholar] [CrossRef]
- Conejo de Monte—Especies Cinegéticas | Ciencia y Caza: Tu Web de Caza, Investigación y Formación Cinegética. Available online: https://www.cienciaycaza.org/especies-cinegeticas/conejo-de-monte/51 (accessed on 14 December 2021).
- Soriguer, R.C. Biología y Dinámica de Una Población de Conejos En Andalucía Occidental. CSIC-EBD 1981, 8-3, 4–367. [Google Scholar]
- Villafuerte, R.; Lazo, A.; Moreno, S. Influence of food abundance and quality on rabbit fluctuations: Conservation and management implications in Donana National Park (SW Spain). Rev. Ecol. (Terre vie). 1997, 52, 345–356. [Google Scholar]
- Gonçalves, H.; Alves, P.C.; Rocha, A. Seasonal Variation in the Reproductive Activity of the Wild Rabbit (Oryctolagus Cuniculus Algirus) in a Mediterranean Ecosystem. Wildl. Res. 2002, 29, 165. [Google Scholar] [CrossRef]
- Calvete, C. Epidemiología de Enfermedad Hemorrágica (VHD) y Mixomatosis en el conejo silvestre (Oryctolagus Cuniculus L. 1758) en el Valle Medio del Ebro- modelización de VHD y herramientas de gestión 1999. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, Spain, 1999. [Google Scholar]
- Contreras, P. Epidemiología de Enfermedades Víricas Del Conejo Silvestre. Trabajo de Fin de Grado, UCM. 2018. Available online: https://docplayer.es/85324765-Facultad-de-veterinaria.html (accessed on 14 December 2021).
- Parer, I. The Population Ecology of the Wild Rabbit (Oryctolagus Cuniculus (L)), in a Mediterranean-Type Climate in New South Wales. Wildl. Res. 1977, 4, 171. [Google Scholar] [CrossRef]
- Wood, D.H. The demography of a rabbit population in an Arid region of New South Wales Australia. J. Anim. Ecol. 1980, 49, 55. [Google Scholar]
- Richardson, B.; Wood, D. Experimental ecological studies on a Subalpine rabbit population I. Mortality factors acting on emergent kittens. Wildl. Res. 1982, 9, 443. [Google Scholar] [CrossRef]
- Basco, P.I.; Carballedo, A.D.; Cota, S.; Olmeda, A.S.; Valcárcel, F. Estudio de control biológico de garrapatas en la finca “La Garganta”. Revista Complut Ciencias Vet. 2008, 2, 1–12. [Google Scholar]
- Valcárcel, F.; González, J.; Tercero-Jaime, J.M.; Olmeda, A.S. The effect of excluding ungulates on the abundance of Ixodid ticks on wild rabbit (Oryctolagus Cuniculus). Exp. Appl. Acarol. 2017, 72, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, F.; González, J.; González, M.; Sánchez, M.; Tercero, J.; Elhachimi, L.; Carbonell, J.D.; Olmeda, A.S. Comparative ecology of Hyalomma Lusitanicum and Hyalomma Marginatum Koch, 1844 (Acarina: Ixodidae). Insects 2020, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, D.; Hagen, F.; Tilburg, J.J.H.C.; Ruiz-Fons, F. Coxiella Burnetii genotypes in Iberian wildlife. Microb. Ecol. 2016, 72, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Balashov, Y.; Daiter, A. Bloodsucking arthropods and rickettsiae. Sci. Leningr. 1973, 251, 1. [Google Scholar]
- Körner, S.; Makert, G.R.; Ulbert, S.; Pfeffer, M.; Mertens-Scholz, K. The prevalence of Coxiella Burnetii in hard ticks in Europe and their role in Q fever transmission revisited—A systematic review. Front. Vet. Sci. 2021, 8, 655715. [Google Scholar] [CrossRef]
- Babudieri, B. Q fever: A zoonosis. Advances Vet. Sci. 1959, 5, 81–182. [Google Scholar]
- Astobiza, I.; Barral, M.; Ruiz-Fons, F.; Barandika, J.F.; Gerrikagoitia, X.; Hurtado, A.; García-Pérez, A.L. Molecular investigation of the occurrence of Coxiella Burnetii in wildlife and ticks in an endemic area. Vet. Microbiol. 2011, 147, 190–194. [Google Scholar] [CrossRef]
- Barandika, J.F.; Hurtado, A.; García-Sanmartín, J.; Juste, R.A.; Anda, P.; García-Pérez, A.L. Prevalence of tick-borne zoonotic bacteria in questing adult ticks from Northern Spain. Vector Borne Zoonotic Dis. 2008, 8, 829–835. [Google Scholar] [CrossRef]
- Varela-Castro, L.; Zuddas, C.; Ortega, N.; Serrano, E.; Salinas, J.; Castellà, J.; Castillo-Contreras, R.; Carvalho, J.; Lavín, S.; Mentaberre, G. On the possible role of ticks in the eco-epidemiology of Coxiella Burnetii in a Mediterranean Ecosystem. Ticks Tick-Borne Dis. 2018, 9, 687–694. [Google Scholar] [CrossRef]
- Smith, D.; Derrick, E. Studies in the epidemiology of Q fever: The isolation of six strains of Riquettsia Burnetii from the tick Haemaphysalis Humerosa. Aust. J. Exp. Biol. Med. 1940, 18, 1–8. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Evstigneeva, A.S.; Ul’yanova, T.Y.; Tarasevich, I.V. The survival of Coxiella Burnetii in soils. Eurasian Soil Sci. 2007, 40, 565–568. [Google Scholar] [CrossRef]
- Tissot-Dupont, H.; Torres, S.; Nezri, M.; Raoult, D. Hyperendemic focus of Q fever related to sheep and wind. Am. J. Epidemiol. 1999, 150, 67–74. [Google Scholar] [CrossRef]
- Blanc, G.; Ascione, L.; Bésiat, P. Experimental Rickettsiaemia in Tortoises (Testudo mauritanica) by inoculation with R. burneti and Infection of the tick Hyalomma aegyptium. Bull. Soc. Pathol. Exotique. 1959, 52, 564–567. [Google Scholar]
- Davis, G.E. Ticks and relapsing fever in the United States. Public Health Rep. (1896–1970) 1940, 55, 2347. [Google Scholar] [CrossRef]
- Montoya, M.; Mesón, M. El Conejo de Monte. Vida, Costumbres y Fomento; Fundacción Conde del Valle de Salazar; Editorial Solitario S.L.: Madrid, Spain, 2010; ISBN 978-84-936836-7-2. [Google Scholar]
- Apanaskevich, D.A.; Santos-Silva, M.M.; Horak, I.G. The genus Hyalomma Koch, 1844. IV. Redescription of all parasitic stages of H. (Euhyalomma) Lusitanicum Koch, 1844 and the Adults of H. (E.) Franchinii Tonelli Rondelli, 1932 (Acari: Ixodidae) with a first description of Its immature stages. Folia Parasitol. 2008, 55, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004; ISBN 84-96214-18-4. [Google Scholar]
- Gil-Collado, J.; Guillén, J.L.; Zapatero, L.M. Claves para la identificación de los Ixodoidea Españoles (adultos). Rev. Ibérica De Parasitol. 1979, 39, 107–118. [Google Scholar]
- Hoogstraal, H.; Morel, P.C. Haemaphysalis (Rhipistoma) Hispanica gil Collado, a parasite of the European rabbit, redescription of adults, and description of immature stages (Ixodoidea: Ixodidae). J. Parasitol. 1970, 56, 813–822. [Google Scholar] [CrossRef]
- Manilla, G. Fauna d’Italia. Acari:Ixodida; Fauna d’Italia; Calderini: Bologna, Italy, 1998; ISBN 88-8219-162-1. [Google Scholar]

| Area (Rabbits) | EPI | Rhipicephalus | Hyalomma | Haemaphysalis | Ixodes | Ticks |
|---|---|---|---|---|---|---|
| R-A (265) | n | 5.027 | 15 | 53 | 12 | 5.107 |
| Mean | 18.97 | 0.06 | 0.20 | 0.05 | 19.27 | |
| Variance | 640.84 | 0.26 | 0.42 | 0.12 | 644.88 | |
| Median | 7 | 0 | 0 | 0 | 8 | |
| % | 98.43 | 0.29 | 1.04 | 0.23 | 100.00 | |
| RU-A (344) | n | 3.496 | 1.270 | 43 | 8 | 4.817 |
| Mean | 7.21 | 3.69 | 0.09 | 0.02 | 9.93 | |
| Variance | 138.01 | 141.58 | 0.23 | 0.06 | 257.08 | |
| Median | 3 | 0 | 0 | 0 | 4 | |
| % | 72.58 | 26.36 | 0.89 | 0.17 | 100.00 | |
| Overall (609) | n | 8.523 | 1.285 | 96 | 20 | 9.924 |
| Mean | 10.46 | 2.11 | 0.12 | 0.02 | 12.18 | |
| Variance | 375.16 | 83.24 | 0.31 | 0.09 | 431.83 | |
| Median | 4 | 0 | 0 | 0 | 6 | |
| % | 85.88 | 12.95 | 0.97 | 0.20 | 100.00 |
| Sex and Age | Global | R-A | RU-A |
|---|---|---|---|
| % | % | % | |
| Female | 49.09 (162/330) | 43.15 (63/146) | 53.80 (99/184) |
| Young | 66.67 (20/30) | 66.67 (8/12) | 66.67 (12/18) |
| Subadult | 45.95 (34/74) | 44.00 (11/25) | 46.94 (23/49) |
| Adult | 47.79 (108/226) | 40.37 (44/109) | 54.70 (64/117) |
| Male | 44.66 (113/253) | 35.85 (38/106) | 51.02 (75/147) |
| Young | 45.71 (16/35) | 33.33 (4/12) | 52.17 (12/23) |
| Subadult | 42.65 (29/68) | 18.75 (3/16) | 50.00 (26/52) |
| Adult | 45.33 (68/150) | 39.74 (31/78) | 51.39 (37/72) |
| Total | 47.17 (275/583) | 40.08 (101/252) | 52.57 (174/331) |
| Young | 55.38 (36/65) | 50.00 (12/24) | 58.54 (24/41) |
| Subadult | 44.37 (63/142) | 34.15 (14/41) | 48.51 (49/101) |
| Adult | 46.81 (176/376) | 40.11 (75/187) | 53.44 (101/189) |
| Sex and Age | Global | R-A | RU-A |
|---|---|---|---|
| % | % | % | |
| Female | 65.22 (225/345) | 57.05 (89/156) | 71.96 (136/189) |
| Young | 14.29 (2/14) | 14.29 (2/14) | 14.29 (3/21) |
| Subadult | 56.41 (7/25) | 28.00 (7/25) | 69.81 (37/53) |
| Adult | 75.86 (80/117) | 68.38 (80/117) | 83.48 (96/115) |
| Male | 65.76 (62/107) | 57.94 (62/107) | 71.33 (107/150) |
| Young | 8.57 (2/12) | 16.67 (2/12) | 4.35 (1/23) |
| Subadult | 68.06 (6/17) | 35.29 (6/17) | 78.18 (43/55) |
| Adult | 78.00 (54/78) | 69.23 (54/78) | 87.50 (63/72) |
| Total | 65.45 (151/263) | 57.41 (151/263) | 71.68 (243/339) |
| Young | 11.43 (4/26) | 15.38 (4/26) | 9.09 (4/44) |
| Subadult | 62.00 (13/42) | 30.95 (13/42) | 74.07 (80/108) |
| Adult | 76.70 (134/195) | 68.72 (134/195) | 85.03 (159/187) |
| Sex and Age Where H. lusitanicum Were Collected | Global | R-A | RU-A |
|---|---|---|---|
| % | % | % | |
| Female | 51.89 (55/106) | 20.00 (1/5) | 53.47 (54/101) |
| Young | - | - | - |
| Subadult | 40.00 (4/10) | - | 40.00 (4/10) |
| Adult | 53.13 (51/96) | 20.00 (1/5) | 54.95 (50/91) |
| Male | 58.33 (42/72) | 100.00 (2/2) | 57.14 (40/70) |
| Young | 50.00 (1/2) | - | 50.00 (1/2) |
| Subadult | 56.25 (9/16) | - | 56.25 (9/16) |
| Adult | 59.26 (32/54) | 100.00 (2/2) | 57.69 (30/52) |
| Total | 54.49 (97/178) | 42.86 (3/7) | 54.97(94/171) |
| Young | 50.00 (1/2) | - | 50.00 (1/2) |
| Subadult | 50.00 (13/26) | - | 50.00 (13/26) |
| Adult | 55.33 (83/150) | 42.86 (3/7) | 56.34 (80/142) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.; Valcárcel, F.; González, J.; González, M.G.; Martín-Hernández, R.; Tercero, J.M.; González-Jara, P.; Olmeda, A.S. Seasonality of Coxiella burnetii among Wild Rabbits (Oryctolagus cuniculus) and the Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem. Pathogens 2022, 11, 36. https://doi.org/10.3390/pathogens11010036
Sánchez M, Valcárcel F, González J, González MG, Martín-Hernández R, Tercero JM, González-Jara P, Olmeda AS. Seasonality of Coxiella burnetii among Wild Rabbits (Oryctolagus cuniculus) and the Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem. Pathogens. 2022; 11(1):36. https://doi.org/10.3390/pathogens11010036
Chicago/Turabian StyleSánchez, María, Félix Valcárcel, Julia González, Marta G. González, Raquel Martín-Hernández, José M. Tercero, Pablo González-Jara, and A. Sonia Olmeda. 2022. "Seasonality of Coxiella burnetii among Wild Rabbits (Oryctolagus cuniculus) and the Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem" Pathogens 11, no. 1: 36. https://doi.org/10.3390/pathogens11010036
APA StyleSánchez, M., Valcárcel, F., González, J., González, M. G., Martín-Hernández, R., Tercero, J. M., González-Jara, P., & Olmeda, A. S. (2022). Seasonality of Coxiella burnetii among Wild Rabbits (Oryctolagus cuniculus) and the Hyalomma lusitanicum (Acari: Ixodidae) in a Meso-Mediterranean Ecosystem. Pathogens, 11(1), 36. https://doi.org/10.3390/pathogens11010036