A Unique Case of Fatal Coinfection Caused by Leptospira spp. and Hepatozoon canis in a Red Fox Cub (Vulpes vulpes)
Abstract
:1. Introduction
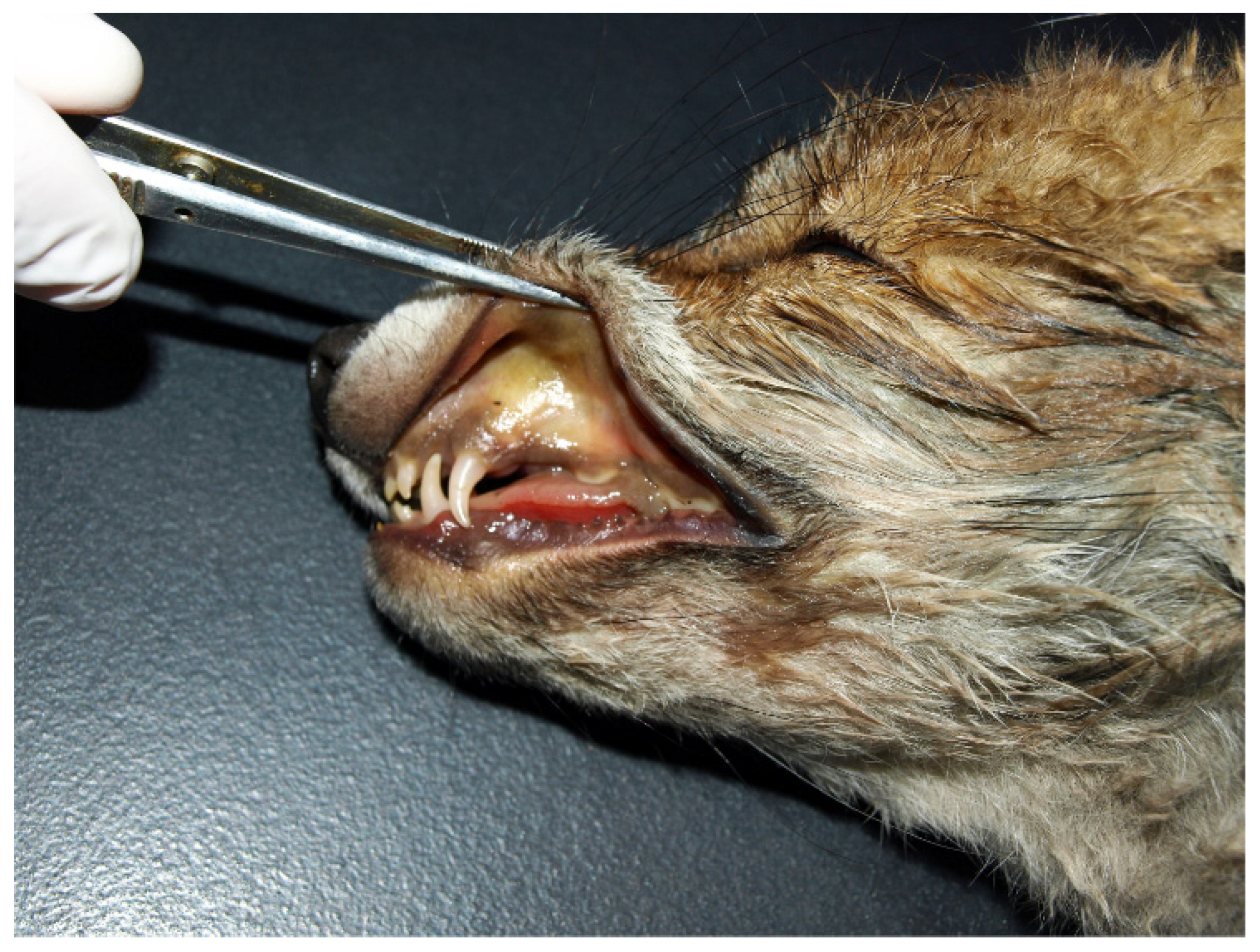
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Żmudzki, J.; Arent, Z.; Jabłoński, A.; Nowak, A.; Zębek, S.; Stolarek, A.; Łukasz, B.; Brzana, A.; Pejsak, Z. Seroprevalence of 12 serovars of pathogenic Leptospira in red foxes (Vulpes vulpes) in Poland. Acta Veter. Scand. 2018, 60, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanszki, Z.; Kurucz, K.; Zeghbib, S.; Kemenesi, G.; Lanszki, J.; Jakab, F. Identification of Hepatitis E Virus in the Feces of Red Foxes (Vulpes vulpes). Animals 2020, 10, 1841. [Google Scholar] [CrossRef]
- Waindok, P.; Raue, K.; Grilo, M.L.; Siebert, U.; Strube, C. Predators in northern Germany are reservoirs for parasites of One Health concern. Parasitol. Res. 2021, 120, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Candela, M.G.; López-Bao, J.V.; Pereira, M.; Jiménez, M.Á.; León-Vizcaíno, L. Leptospirosis in Wild and Domestic Carnivores in Natural Areas in Andalusia, Spain. Vector-Borne Zoonotic Dis. 2009, 9, 549–554. [Google Scholar] [CrossRef]
- Åkerstedt, J.; Lillehaug, A.; Larsen, I.-L.; Eide, N.E.; Arnemo, J.M.; Handeland, K. Serosurvey for canine distemper virus, canine adenovirus, leptospira interrogans, and toxoplasma gondii in free-ranging canids in scandinavia and svalbard. J. Wildl. Dis. 2010, 46, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Slavica, A.; Deždek, D.; Konjević, D.; Cvetnić, Ž.; Sindicic, M.; Stanin, D.; Habus, J.; Turk, N. Prevalence of leptospiral antibodies in the red fox (Vulpes vulpes) population of Croatia. Veter. Med. 2011, 56, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Žele-Vengušt, D.; Lindtner-Knific, R.; Mlakar-Hrženjak, N.; Jerina, K.; Vengušt, G. Exposure of Free-Ranging Wild Animals to Zoonotic Leptospira interrogans Sensu Stricto in Slovenia. Animals 2021, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, C.; Casado, N.; Criado-Fornelio, A.; Alvarez de Miguel, F.; Dominguez-Penafiel, G. A molecular survey of Piro-plasmida and Hepatozoon isolated from domestic and wild animals in Burgos (northern Spain). Vet. Parasitol. 2009, 162, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Dežđek, D.; Vojta, L.; Ćurković, S.; Lipej, Z.; Mihaljević, Ž.; Cvetnić, Ž.; Beck, R. Molecular detection of Theileria annae and Hepatozoon canis in foxes (Vulpes vulpes) in Croatia. Veter. Parasitol. 2010, 172, 333–336. [Google Scholar] [CrossRef]
- Cardoso, L.; Cortes, H.C.E.; Eyal, O.; Reis, A.; Lopes, A.P.; Vila-Viçosa, M.J.; Rodrigues, P.A.; Baneth, G. Molecular and his-topathological detection of Hepatozoon canis in red foxes (Vulpes vulpes) from Portugal. Parasit Vectors 2014, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Duscher, G.G.; Fuehrer, H.P.; Kübber-Heiss, A. Fox on the run—molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasit Vectors 2014, 7, 521. [Google Scholar] [PubMed] [Green Version]
- Farkas, R.; Solymosi, N.; Takács, N.; Hornyák, Á.; Hornok, S.; Nachum-Biala, Y.; Baneth, G. First molecular evidence of Hepatozoon canis infection in red foxes and golden jackals from Hungary. Parasites Vectors 2014, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Najm, N.-A.; Meyer-Kayser, E.; Hoffmann, L.; Pfister, K.; Silaghi, C. Hepatozoon canis in German red foxes (Vulpes vulpes) and their ticks: Molecular characterization and the phylogenetic relationship to other Hepatozoon spp. Parasitol. Res. 2014, 113, 2679–2685. [Google Scholar] [CrossRef]
- Hodžić, A.; Alić, A.; Fuehrer, H.-P.; Harl, J.; Wille-Piazzai, W.; Duscher, G.G. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasites Vectors 2015, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Imre, M.; Dudu, A.; Ilie, M.S.; Morariu, S.; Imre, K.; Dărăbuş, G. Molecular Survey of Hepatozoon canis in Red Foxes (Vulpes vulpes) from Romania. J. Parasitol. 2015, 101, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Mitková, B.; Hrazdilová, K.; Steinbauer, V.; D’Amico, G.; Mihalca, A.D.; Modrý, D. Autochthonous Hepatozoon infection in hunting dogs and foxes from the Czech Republic. Parasitol. Res. 2016, 115, 4167–4171. [Google Scholar] [CrossRef]
- Major, A.; Schweighauser, A.; Francey, T. Increasing Incidence of Canine Leptospirosis in Switzerland. Int. J. Environ. Res. Public Health 2014, 11, 7242–7260. [Google Scholar] [CrossRef] [Green Version]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European consensus statement on lep-tospirosis in dogs and cats. J. Small. Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.; Steinicke, K.; Arndt, G.; Gruber, A.D.; Guerra, B.; Jansen, A.; Kaser-Hotz, B.; Klopfleisch, R.; Lotz, F.; Luge, E.; et al. Pulmonary Abnormalities in Dogs with Leptospirosis. J. Vet. Intern. Med. 2010, 24, 1277–1282. [Google Scholar] [CrossRef]
- Baneth, G.; Shkap, V.; Presentej, B.Z.; Pipane, E. Hepatozoon canis: The prevalence of antibodies and gametocytes in dogs in Israel. Vet. Res. Commun. 1996, 20, 41–46. [Google Scholar] [CrossRef]
- Baneth, G.; Weigler, B. Retrospective Case-Control Study of Hepatozoonosis in Dogs in Israel. J. Veter. Intern. Med. 1997, 11, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Karagenc, T.I.; Pasa, S.; Kirli, G.; Hosgor, M.; Bilgic, H.B.; Ozon, Y.H.; Atasoy, A.; Eren, H. A parasitological, molecular and serological survey of Hepatozoon canis infection in dogs around the Aegean coast of Turkey. Veter. Parasitol. 2006, 135, 113–119. [Google Scholar] [CrossRef]
- Baneth, G.; Samish, M.; Shkap, V. Life cycle of Hepatozoon canis (apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris). J. Parasitol. 2007, 93, 283–299. [Google Scholar] [CrossRef]
- Conceicao-Silva, F.M.; Abranches, P.; Silva-Pereira, M.C.D.; Janz, J.G. Hepatozoonosis in foxes from portugal. J. Wildl. Dis. 1988, 24, 344–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lempp, C.; Jungwirth, N.; Grilo, M.L.; Reckendorf, A.; Ulrich, A.; van Neer, A.; Bodewes, R.; Pfankuche, V.M.; Bauer, C.; Osterhaus, A.D.M.E.; et al. Pathological findings in the red fox (Vulpes vulpes), stone marten (Martes foina) and raccoon dog (Nyctereutes procyonoides), with special emphasis on infectious and zoonotic agents in Northern Germany. PLoS ONE 2017, 12, e0175469. [Google Scholar] [CrossRef]
- Kubo, M.; Miyoshi, N.; Yasuda, N. Hepatozoonosis in Two Species of Japanese Wild Cat. J. Veter. Med. Sci. 2006, 68, 833–837. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Engelberts, M.F.M.; Boer, K.R.; Ahmed, N.; Hartskeerl, R.A. Development and Validation of a Real-Time PCR for Detection of Pathogenic Leptospira Species in Clinical Materials. PLoS ONE 2009, 4, e7093. [Google Scholar] [CrossRef]
- Hodžić, A.; Alić, A.; Duscher, G.G. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: A molecular study. Ticks Tick-Borne Dis. 2018, 9, 589–593. [Google Scholar] [CrossRef]
- Lopez, A.; Martinson, S.A. Respiratory system, mediastinum and Pleurae Chapter 9. In Pathologic Basis of Veterinary Diseases, 6th ed.; Zachary, J.F., Ed.; Elsevier: St. Louis, MO, USA, 2017; pp. 471–561. [Google Scholar]
- Omeragić, J.; Šerić Haračić, S.; Klarić Soldo, D.; Kapo, N.; Fejzić, N.; Škapur, V.; Medlock, J. Distribution of ticks in Bosnia and Herzegovina. Ticks Tick Borne Dis. 2022, 13, 101870. [Google Scholar] [CrossRef]
- Hodžić, A.; Mrowietz, N.; Cézanne, R.; Bruckschwaiger, P.; Punz, S.; Habler, V.E.; Tomsik, V.; Lazar, J.; Duscher, G.G.; Glawischnig, W.; et al. Occurrence and diversity of arthropod-transmitted pathogens in red foxes (Vulpes vulpes) in western Austria, and possible vertical (transplacental) transmission of Hepatozoon canis. Parasitology 2018, 145, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; O’Keeffe, A.M.; Lewis, J.C.M.; Stocker, L.R.; Laurenson, M.K.; Philbey, A.W. Infectious canine hepatitis in red foxes (Vulpes vulpes) in the United Kingdom. Veter. Rec. 2010, 166, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Abbondati, E.; Cox, A.L.; Mitchell, G.B.B.; Pizzi, R.; Sharp, C.P.; Philbey, A.W. Infectious canine hepatitis in red foxes (Vulpes vulpes) in wildlife rescue centres in the UK. Veter. Rec. 2016, 178, 421. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alić, A.; Šupić, J.; Goletić, T.; Rešidbegović, E.; Lutvikadić, I.; Hodžić, A. A Unique Case of Fatal Coinfection Caused by Leptospira spp. and Hepatozoon canis in a Red Fox Cub (Vulpes vulpes). Pathogens 2022, 11, 11. https://doi.org/10.3390/pathogens11010011
Alić A, Šupić J, Goletić T, Rešidbegović E, Lutvikadić I, Hodžić A. A Unique Case of Fatal Coinfection Caused by Leptospira spp. and Hepatozoon canis in a Red Fox Cub (Vulpes vulpes). Pathogens. 2022; 11(1):11. https://doi.org/10.3390/pathogens11010011
Chicago/Turabian StyleAlić, Amer, Jovana Šupić, Teufik Goletić, Emina Rešidbegović, Ismar Lutvikadić, and Adnan Hodžić. 2022. "A Unique Case of Fatal Coinfection Caused by Leptospira spp. and Hepatozoon canis in a Red Fox Cub (Vulpes vulpes)" Pathogens 11, no. 1: 11. https://doi.org/10.3390/pathogens11010011
APA StyleAlić, A., Šupić, J., Goletić, T., Rešidbegović, E., Lutvikadić, I., & Hodžić, A. (2022). A Unique Case of Fatal Coinfection Caused by Leptospira spp. and Hepatozoon canis in a Red Fox Cub (Vulpes vulpes). Pathogens, 11(1), 11. https://doi.org/10.3390/pathogens11010011







