Abstract
Medroxyprogesterone acetate (MPA) is one of the most widely used contraceptives in the world. Epidemiologic studies have uncovered a possible link between the use of MPA and an increased risk of HIV-1 transmission. However, the understanding of the mechanism is still limited. Our previous publication demonstrated that the lysosomal activity in human vaginal epithelial cells attenuated the trafficking of viral particles during HIV-1 transcytosis. In this study, we show that treating human primary cervical epithelial cells with MPA led to a reduction in lysosomal activity. This reduction caused an increase in the intracellular HIV-1 accumulation and, consequently, an increase in viral release. Our study uncovers a novel mechanism by which MPA enhances HIV-1 release in primary cervical epithelial cells, thus providing vital information for HIV intervention and prevention.
1. Introduction
HIV-1 is transmitted predominantly through mucosal epithelial exposure [1]. Approximately 40% of new HIV-1 infections originate from exposure in the female genital tract [2]. During sexual intercourse, HIV-1 transmission from male to female is more efficient than from female to male. As a result, the lower female reproductive tract has become the predominant site for HIV-1 entry in most new HIV-1 infections worldwide. This also means that HIV-1 infection disproportionately affects women [3].
The use of medroxyprogesterone acetate (MPA) is one of the most popular contraception methods in areas of high HIV seroprevalence. Interestingly, MPA is also one of the most widely used contraceptives in the world, especially in areas endemic for HIV-1 [4]. Epidemiologic reports have uncovered a possible link between the use of MPA and an increased risk of HIV-1 acquisition and transmission. This link has been shown by numerous groups and succinctly summarized [4,5,6,7]. More recent meta-analysis of high-quality studies showed a significant risk of HIV acquisition associated with MPA use [8,9,10]. However, because a recent open-label randomized Evidence for Contraceptive Options and HIV Outcomes (ECHO) trial did not find a substantial difference in HIV risk among the use of MPA, a copper intrauterine device (IUD), and a levonorgestrel (LNG) implant among African women, the association between MPA use and increased HIV risk has become controversial [11,12,13].
Studies using animal models have corroborated the findings of epidemiological studies that identified the link between MPA use and higher HIV-1 acquisition. For instance, macaques that were given subcutaneous MPA implants had a higher rate of contracting SIV than those that were given a placebo [14]. The link between physiological elevation of progesterone level and viral susceptibility was also apparent in studies where macaques were challenged with viral doses during their undisturbed menstrual cycles. Specifically, virus transmission occurred most frequently during the high-progesterone, late luteal phase of their menstrual cycle, underscoring the critical role of hormonal regulation during HIV-1 mucosal transmission [15].
Several mechanisms have been proposed to explain why MPA may lead to enhanced HIV-1 transmission. Both endogenous and exogenous progesterone have been shown to thin or impair the integrity of the cervicovaginal mucosa, consequently enhancing HIV acquisition [14,16,17,18,19,20]. MPA use has also been reported to elevate levels of inflammatory mediators that activate HIV targets cells and promote their recruitment to the cervicovaginal mucosa [17,21,22,23,24,25,26], suppress innate or adaptive immunity against HIV [27,28], modulate the composition of vaginal microbiota [29], and enhance transcytosis of HIV [30], among other effects. However, further studies are warranted to comprehensively understand the action of MPA on HIV transmission.
Several studies using cervical, intestinal, and other epithelial cell types show that HIV-1 traffics through an intact epithelial cell layer via transcytosis [31,32,33,34,35]. Using confocal microscopy and 3D surface reconstruction, findings from Maher et al. demonstrated that HIV-1 binds and penetrates the external surface of cervicovaginal epithelial cells [36]. This critical finding supports the idea that HIV-1 can cross the vaginal epithelial layer through either transcytosis or tight junctions between the epithelial cells. Carias et al. also reported that vaginal epithelial cells may play a bigger role in HIV-1 acquisition than previously believed and argued that the virus is passing between the cells through a diffusive percolation mechanism, penetrating through areas where junctions are absent [37]. In our previous publication, we defined the negative effect of lysosomal activity on HIV-1 transcytosis [38]. Here, we show that MPA treatment of human primary cervical/vaginal epithelial cells led to a reduction in lysosomal activity, consequently resulting in increased intracellular HIV-1 accumulation and virus release with implications of higher viral transmission rates. These data suggest a novel mechanism by which MPA influences HIV susceptibility.
2. Results
2.1. MPA Treatment Enhances Intracellular HIV-1 Accumulation
It has been shown that MPA can enhance HIV-1 uptake and transcytosis [30]. In our previous publication, we established a method for measuring HIV accumulation in human vaginal epithelial cell line VK2/E6E7 cells (termed VK2 thereafter) using Western blot [38]. Here, we used the same method to investigate the influence of MPA on HIV accumulation in VK2 cells. We treated VK2 cells with 1 nM MPA, a physiologically relevant concentration [39,40], for 1 h. Then, we exposed the cells to 60 ng p24 protein content of HIV-1 IIIB virus for 15, 60, 120, and 360 min. After trypsinization and wash, we analyzed the samples by Western blot. As shown in Figure 1A, MPA pretreatment did not significantly affect HIV virion uptake/accumulation in VK2 cells within the 6 h timeframe. Conversely, exposure to MPA for a longer period markedly changed the level of intracellular HIV. As shown in Figure 1B, the levels of virus remained significantly elevated within the cells after a three-day incubation period. Therefore, in our experimental system, MPA treatment can modulate intracellular viral accumulation upon prolonged exposure. Despite these findings, our results show the efficiency in viral uptake seemed to be limited in VK2 cells. In our system, about 0.1% of input HIV-1 IIIB virus was up taken by the VK2 cells in a 6-hour incubation (Figure S1).
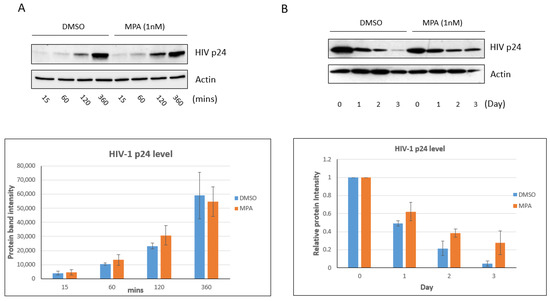
Figure 1.
MPA enhances intracellular HIV accumulation in VK2/E6E7 cells. (A) VK2 cells were treated with 1 nM MPA for 1 h. Control cells were treated with DMSO alone. After the 1 h treatment, the cells were exposed to 60 ng p24 content of HIV-1 IIIB virus for 15, 60, 120, or 360 min, respectively. After trypsinization to remove viral particles attached to the cell surface, the samples were analyzed by Western blot. The bar graph shows the densitometry analysis of the Western blot result. The results shown are representative of three independent experiments. (B) VK2 cells were treated with 1 nM MPA for 1 h. Control cells were treated with DMSO alone. After the 1 h treatment, the cells were exposed to 60 ng p24 content of HIV-1 IIIB virus for 3 h. The viral particles on the cell surface were removed by trypsinization and PBS wash. The cells were then cultured in fresh medium containing 1 nM MPA for the indicated days. The Day 0 samples were harvested immediately after the addition of fresh medium. The harvested samples were analyzed by Western blot. The bar graph shows the densitometry analysis of the Western blot result. Day 0 was set as 1. The value of Days 1, 2 and 3 represent their ratio to Day 0. The results shown are representative of three independent experiments.
Next, we wanted to determine if the same can occur in primary cervical epithelial cells. To do this, we examined primary cervical epithelial cells from 12 different donors.
Figure 2A shows representative p24 Western blots of samples from two donors. We observed that cells treated with MPA showed significantly higher HIV accumulation, as indicated by higher p24 accumulation, three days after infection, compared to untreated cells (Figure 2B; p = 0.0016, paired t-test). Our data from primary cells support the notion that MPA-induced enhancement of viral accumulation is physiologically relevant.
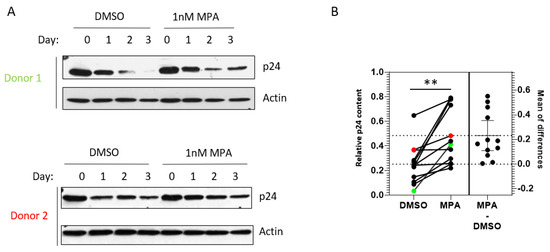
Figure 2.
MPA enhances intracellular HIV-1 accumulation in primary cervical epithelial cells. (A) Primary cervical epithelial cells from 12 donors were treated with 1 nM MPA for 1 h. Control cells were treated with DMSO alone. After the 1 h treatment, the cells were exposed to HIV-1 IIIB and processed as described in Figure 1B. Representative data from two donor samples are shown. (B) A chart depicting the densitometry analysis of p24 accumulation, indicative of HIV-1 accumulation, in primary cervical epithelial cell samples from 12 different donors as described in Figure 2A. The relative p24 content (Y-axis) represents the ratio of p24 level on Day 3 over that on Day 0 (p = 0.0016, paired t-test). The data from Donors 1 and 2 in Figure 2A are labeled as green and red dots, respectively.
2.2. MPA Reduces Lysosomal Activity
In our previous study, we found that the lysosomal degradation pathway was involved in HIV trafficking in VK2 cells [38]. When we used a lysosomal inhibitor cocktail of pepstatin A, leupeptin, and E-64 to block the lysosomal degradation pathway, higher levels of HIV accumulated in VK2 cells. Here, we postulate that MPA inhibits lysosomal activity, consequently enhancing intracellular HIV accumulation. To determine whether MPA directly affects lysosomal activity in primary cervical epithelial cells, we treated these cells with 1 nM MPA and assessed lysosomal activity using LysoSensorTM by measuring changes in mean fluorescent intensity (MFI). Figure 3A depicts representative flow cytometry data of cells from two donors. Our examination of primary cervical epithelial cells from 21 donors showed that MPA treatment significantly reduced the LysoSensor fluorescence staining intensity in all samples compared to their DMSO-treated controls (Figure 3B; p = 0.0016, paired t-test). Even though the reduction in fluorescence intensity seemed marginal, these minor changes may represent a dramatic shift in pH value due to the pH indicator feature of LysoSensor. Such pH shifts can have a big influence on lysosomal activity [41]. These results indicate that MPA treatment reduced lysosomal activity in these cells.
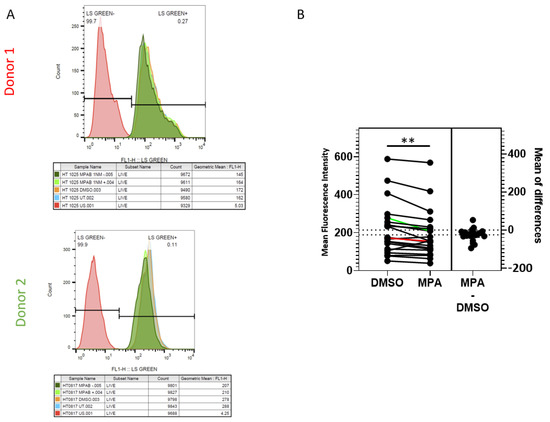
Figure 3.
MPA reduces lysosomal activity as revealed by LysoSensorTM staining. (A) Primary cervical epithelial cells from 21 donors were pretreated as described in the methods section. After two PBS washes, the samples were stained with 0.5 µM LysoSensorTM Green DND-189 for 15 min at 37 °C. After two PBS washes, the cells were analyzed using BD FACSCalibur. Representative data from two donor samples are shown. (B) A chart depicting the analysis of LysoSensorTM staining of primary cervical epithelial cell samples from 21 donors as described in Figure 3A (p = 0.0016, paired t-test). The Y-axis shows the mean fluorescence intensity of each sample. The results of Donors 1 and 2 in Figure 3A were labeled in red and green lines, respectively.
Lysosomal activity has been shown to inversely affect the intracellular human microtubule-associated protein 1 light chain 3 (LC3) pool [42]. Therefore, to test the lysosomal activity functionally, we evaluated the effect of MPA on the stability of LC3 in primary cervical epithelial cells. To do this, we tested the effect of MPA on LC3 levels using pEX-GFP-hLC3WT, an expression plasmid for the LC3-GFP fusion protein. First, we transfected primary cervical epithelial cells from seven donors with pEX-GFP-hLC3WT. Then, we treated these cells with MPA and measured their fluorescence by flow cytometry. Figure 4A depicts representative flow cytometry data of cells from two donors. The MFI of the GFP-positive cells increased significantly after MPA treatment for all seven primary cell samples examined, indicative of higher LC3 levels, compared to their DMSO-treated controls (Figure 4B; p = 0.0049, paired t-test). Tanida et al. showed that the lysosome slightly degraded the LC3 protein under normal conditions, with degradation becoming more extensive under starvation conditions [43]. These findings are consistent with our observations in Figure 4. Taken together, these findings further support the notion that MPA reduces lysosomal activity.
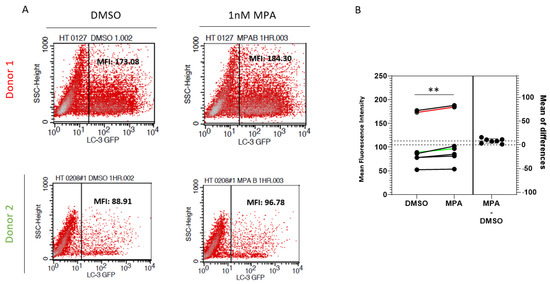
Figure 4.
MPA enhances the stability of the LC-3 protein. (A) pEX-GFP-hLC3WT, an LC3-GFP fusion protein expression plasmid, was transfected into primary cervical epithelial cell samples from seven donors. The transfected cells were treated with 1 nM MPA as described in the methods section. Control cells were treated with DMSO alone. GFP intensity was measured by flow cytometry using BD FACSCaliburTM. (B) A chart depicting the analysis of GFP intensity of samples from seven donors as described in Figure 4A. The Y-axis shows the mean fluorescence intensity of each sample. The result of Donors 1 and 2 was labeled as red and green, respectively.
2.3. MPA Enhances HIV-1 Release from Human Primary Cervical Epithelial Cells
Most HIV-1 transmission events worldwide result from heterosexual sex with an infected partner. Based on analyses of viral complexity in the blood during the first several weeks of infection, approximately 80% of heterosexual transmission events and infections are established from a single HIV-1 variant known as the transmitted/founder virus (T/F virus) (Reviewed [44]). To test if MPA-induced enhancement of HIV-1 is physiologically relevant, we used human primary cervical epithelial cells and transmitted/founder virus HIV-1 CH040. First, we examined the accumulation of CH040 in VK2 cells. Surprisingly, the turnaround of HIV-1 CH040 in VK2 cells was dramatically faster than that of HIV IIIB. As shown in Figure 5A, the level of HIV p24 dramatically decreased at the 6 h time point. Both MPA and the cocktail of lysosomal inhibitors conferred enhancement of HIV-1 p24 accumulation at the 4 and 6 h time point. Interestingly, the combination of MPA and the cocktail of lysosomal inhibitors showed additive effects on the enhancement of p24 accumulation with almost 100% effectiveness. This finding sheds light on the future investigation of the molecular mechanism of MPA regulating lysosome activity. Finally, we tested the effect of MPA on viral release from the human primary cervical cells. Human primary cervical cells from 13 donors were treated with either 1 nM MPA or DMSO only. Then, we exposed the treated cells to HIV-1 CH040 for 3 h. After treatment with low-concentration trypsin and PBS wash, we cultured the cells for 4 h in fresh medium containing either 1 nM MPA dissolved in DMSO or DMSO only. Then, we harvested the viral samples to measure viral release using qRT-PCR. As shown in Figure 5B, MPA treatment significantly enhanced viral release compared to treatment with DMSO alone (p = 0.0078, paired t-test) even though viral released from six donors kept unchanged. Our data suggest that MPA enhances viral release from human primary cervical epithelial cells.
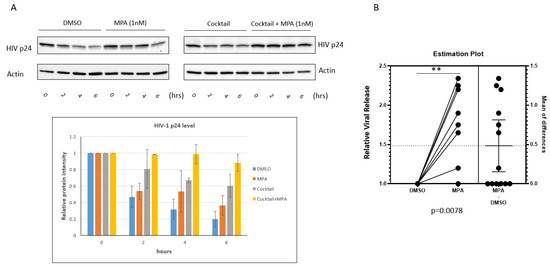
Figure 5.
MPA enhances HIV-1 release from primary cervical epithelial cells. (A) DMSO, MPA, lysosomal inhibitor cocktail, or the combination of MPA and lysosomal inhibitor cocktail treated VK2 cells were exposed to the CH040 virus for 3 h. Trypsin (0.03%) was used to treat the cells at room temperature for 1 min to remove viral particles attached to the cell surface. Cells were cultured for the indicated periods of time. After the incubation, cells were harvested and subjected to Western blot analysis. The MPA treatment experiment (left panel of Figure 5A) was performed three times independently. The cocktail treatment experiment (right panel of Figure 5A) was independently performed twice. The bar graph shows the densitometry analysis of the Western blot result. (B) A box and whisker plot showing relative viral release from human primary cervical epithelial cells pretreated with 1 nM MPA for 1 h. Control samples were treated with DMSO alone. The pretreated cells were exposed to HIV-1 CH040 for 3 h. Then, the cells were treated with a low concentration of trypsin (0.03%) at room temperature. After a thorough wash, the cells were cultured for 4 h in fresh medium containing either 1 nM MPA or DMSO. Finally, the viral samples were harvested and analyzed by qRT-PCR to measure viral release. Samples from 13 donors were analyzed together as one group (p = 0.0109, paired t-test). The value for the DMSO samples was set as 1.
3. Discussion
The safety of contraception is an important public health issue. Contraception benefits women directly by allowing them to control their reproductive health and reduce the number of unintended pregnancies. It also has several indirect benefits, such as reducing the number of abortions, decreasing maternal and infant morbidity and mortality, and lowering the risk of vertical HIV-1 transmission. MPA is the most commonly used injectable contraceptive. While MPA is highly effective and long-lasting, there have been concerns regarding the association of MPA use with higher incidence of HIV-1 transmission. However, a recent large open-label randomized ECHO trial did not find a significant difference in HIV risk among the use of MPA, a copper IUD, and an LNG implant among African women [11]. Based on the result of this ECHO trial, the WHO took quick action to change the Medical Eligibility Criteria for MPA use among women at high risk for HIV infection from category 2 to category 1; the latter recommends the use of MPA in any circumstances. This action drew criticism due to concerns with the ECHO trial in trial design and data interpretation [12,13,45,46,47]. As MPA is widely used in areas endemic for HIV-1, it is critical to understand how MPA influences HIV-1 transmission so that appropriate measures can be instituted to prevent HIV-1 transmission.
In our previous study, we established a cell-based model to study the transcytosis of HIV in VK2 cells [38]. In that study, we found the transcytosis of HIV-1 in vaginal epithelial cells to be inversely related to lysosomal activity. Here, we used this same model to investigate whether MPA can influence HIV transcytosis in primary cervical epithelial cells. Indeed, we found that MPA enhanced both intracellular HIV-1 accumulation and extracellular viral release. Interestingly, our data also showed that MPA inhibited lysosomal activity of these cells. Taken together, our findings suggest that MPA enhances HIV intracellular accumulation and extracellular release by inhibiting lysosomal activity. Several studies have revealed the association of lysosomal activity with HIV-1 pathogenesis [48,49,50,51,52,53,54,55,56,57,58,59]. While these reports agree that inhibiting lysosomal activity would increase HIV-1 infectivity, whether lysosomal activity would affect viral release remains controversial. In this regard, Fredericksen et al. showed that lysosomotropic agents do not alter HIV-1 release [57].
In contrast, Schaeffer et al. showed that inhibition of lysosomal activity increases the extracellular release of HIV-1 [48]. Our findings are consistent with those of Schaeffer et al. in that we observed an increase in viral accumulation and release when we inhibited the lysosomal activity of HIV-infected cells. It is well accepted that lysosomes use their hydrolytic enzymes to destroy pathogens that enter the cell. Logically, for epithelial cells that exhibit strong endocytosis activity, it is not surprising that viral accumulation and release increase when lysosomal activity is inhibited.
At this time, we need to interpret our data cautiously due to the use of an ex vivo assay, a small sample size, and factors that are beyond our control, such as menstruation status. Additionally, it is worth mentioning that MPA treatment induces various immunological changes in the female genital tract [60]. The altered immune landscape might also provide a possible explanation for the increased HIV-1 p24 level in the MPA-treated cells. This interesting hypothesis warrants further investigation. Nonetheless, our study uncovers a plausible explanation as to how MPA can enhance HIV transmission. Importantly, it provides vital preliminary evidence to policy experts responsible for disseminating information about the link between progesterone use in contraception and HIV-1 acquisition/transmission.
4. Materials and Methods
4.1. Cell Lines, HIV-1 Virus, Plasmids, Reagents, and Antibodies
Human vaginal epithelial cell line VK2/E6E7 cells (ATCC CRL-2616) were cultured in defined keratinocyte serum-free medium (SFM) (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C and 5% CO2. HIV-1 IIIB-infected H9 cell line [61,62,63] was obtained from the NIH AIDS Research and Reference Reagent Program (NIH-ARRRP). The HIV-1 IIIB virus was harvested from these H9 cells. pCH040.c/2625, a full-length transmitted/founder (T/F) HIV-1 subtype B infectious molecular clone [64] and HEK 293T cells were obtained from NIH-ARRRP. The HIV-1 CH040 virus was produced by transfecting HEK 293T cells with the pCH040.c/2625 plasmid using polyethylenimine (PEI) as described previously [65]. Filipin, mouse monoclonal anti-actin antibody, DMSO, and MPA were purchased from Sigma (St. Louis, MO, USA). LysoSensor Green DND-189 was purchased from Thermo Fisher Scientific. LC3B antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). Pepstatin A, leupeptin and E-64 were purchased from AG Scientific (San Diego, CA, USA).
4.2. Source of Tissues as Well as Cervical Epithelial Cell Isolation and Culture
Female genital tract tissues used in this study were provided by the Cooperative Human Tissue Network (CHTN)-Western Division at Vanderbilt University Medical Center and by the Meharry Medical College Translational Pathology/Tissue Acquisition Shared Resource. Identifying details of the samples were removed. The sample collection protocol was approved by the Meharry Medical College IRB. The protocol for isolating primary cervical epithelial cells was adapted from the method established by Fichorova et al. [66]. Briefly, the specimen tissues were first washed three times with Hank’s balanced salt solution containing 10% penicillin-streptomycin (Thermo Fisher Scientific) and then cut into 0.5 × 0.5 cm blocks with a sterile scalpel. The tissue blocks were then placed with the cervical surface down onto a six-well plate with 200 µL of EpiGROTM Human Epidermal Keratinocyte Complete Culture Medium (Millipore, Burlington, MA, USA) and cultured at 37 °C with 5% CO2 for 2 h. After the 2 h incubation, fresh EpiGROTM medium (2 mL) was added for long-term culture. The medium was replaced every three days. The adherent cells were usually visible in 7–10 days. Under normal circumstances, the cells would become confluent in 14 days. Cells were passaged when the confluency reached 80–90%.
4.3. MPA, Lysosomal Inhibitors Cocktail Treatment and Viral Inoculation
Physiologically relevant concentration of MPA (1 nM) [39,40] was used to pretreat VK2 cells or primary cervical epithelial cells for 1 h. Control cells were treated with DMSO alone. The cells, the VK2 cells were exposed to either 60 ng p24 protein content of HIV-1 IIIB or 200 ng p24 protein content of the CH040 virus for the indicated time periods. The primary cervical epithelial cells were exposed to 200 ng p24 protein content of the CH040 virus for the indicated time periods. After viral exposure, the virus-containing medium was removed. Trypsin (0.03%) was used to treat the cells at room temperature for 1 min to remove viral particles attached to the cell surface. After three washes with PBS, the cells were cultured in fresh medium containing 1 nM MPA for the indicated time periods. The culture medium and cells were collected at the indicated time points and subjected to qRT-PCR and Western blot analysis, respectively.
Lysosomal inhibitors cocktail containing pepstain A, leupeptin, and E-64 was used to pretreat VK2 cells overnight at the final concentration of 29 µM pepstatin A, 52 mM leupeptin and 69 mM E-64. One-hour 1 nM MPA treatment was added into the overnight treated samples for the combination treatment of MPA and lysosomal inhibitors cocktail. After the pretreatment, the cells were exposed to CH040 virus as described above.
4.4. SDS-PAGE and Western Blot
The harvested cells were lysed using RIPA buffer (10 mM Tris-Cl [pH 8.0] containing 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, and fresh 1 mM PMSF). The cell lysates were subjected to a 21-kg spin at 4 °C for 1 h. The supernatant was mixed with 4×SDS-PAGE sample buffer (Bio-Rad, Hercules, CA, USA) and subjected to SDS-PAGE. The resultant protein bands were transferred to Amersham Protran Premium 0.2 µm NC (Sigma) using the Bio-Rad Trans-Blot Turbo Transfer System. Anti-HIV-1 p24 monoclonal antibody (NIH-ARRRP, 183-H12-5C) [67], Polyclonal Anti-Human Immunodeficiency Virus Immune Globulin (NIH-ARRRP, ARP-395), and anti-actin (Sigma) were used for staining IIIB p24, CH040 p24 and action respectively in Western blotting as previously described [68]. Bio-Rad ChemiDoc MP was used to visualize the protein bands.
4.5. LysoSensorTM Staining
Primary cervical epithelial cells were treated as described earlier. After the 1 h MPA treatment, the cells were washed twice with PBS. LysoSensor Green DND-189 (0.5 µM) (Thermo Fisher Scientific) was then used to stain the cells at 37 °C for 15 min following manufacturer’s instructions. After staining, the cells were washed twice with PBS and then analyzed using BD FACSCalibur (BD Biosciences, San Jose, CA, USA). The LysoSensor Green DND-189 staining of the primary cervical epithelial cell samples was duplicated, and the mean of the MFI was used for the data processing.
4.6. Transfection and Flow Cytometry Analysis
The LC30-GFP fusion protein expression plasmid pEX-GFP-hLC3WT was transfected into the primary cervical epithelial cells using Lipofectamine 2000 (Themo Fisher Scientific) following manufacturer’s instructions. pEX-GFP-hLC3WT [43] was a gift from Dr. Isei Tanida (Addgene plasmid # 24987). Twenty-four hours after transfection, the cells were treated with 1 nM MPA dissolved in DMSO for 1 h. Control cells were treated with DMSO alone. After the treatment, the cells were detached using trypsin, washed twice with PBS, and subjected to flow cytometry analysis using BD FACSCaliburTM.
4.7. qRT-PCR
qRT-PCR was used to measure relative viral release. Total RNA from the viral samples was isolated using the Aurum total RNA Mini Kit (Bio-Rad). cDNA was produced using the High Capacity cDNA Reverse Transcription Kit (Themo Fisher Scientific). qPCR was performed on a Bio-Rad CFX96 Touch Real-Time PCR Detection System using the following program: 95 °C (3 min); 40 cycles of 95 °C (15 s); 60 °C using SYBR Green Supermix (Bio-Rad) (1 min). The primers used were LTR S4 (5’-AAGCCTCAATAAAGCTTGCCTTGA) and LTR AS3 (5’-GTTCGGGCGCCACTGCTAG), as described previously [69]. Each pair of DMSO and MPA samples was analyzed in the same qPCR run. Relative viral release was calculated using Bio-Rad CFX Maestro based on the 2−ΔΔCT method. Paired t-test was used to calculate the p-value.
4.8. Statistical Analysis
Paired t-test was performed using GraphPad Prism. A p-value of ≤0.05 was considered statistically significant (*). A p-value of ≤0.01 was considered statistically very significant (**).
5. Conclusions
In this study, we show that MPA treatment of human primary cervical/vaginal epithelial cells led to a reduction in lysosomal activity, consequently resulting in increased intracellular HIV-1 accumulation and virus release with implications of higher viral transmission rates. These data suggest a novel mechanism by which MPA influences HIV susceptibility.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10091192/s1.
Author Contributions
Q.S., A.R.C., B.L.K., F.V., J.E.K.H., and B.L. conceived and designed the experiments. X.J., Q.S., A.R.C., B.L.K., M.G.I., H.-Y.Z., and B.L. performed the experiments. Q.S., A.R.C., M.G.I., and B.L. contributed reagents/materials/analysis tools. X.J., Q.S., B.L.K., F.V., and B.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the NIH-funded Meharry Clinical and Translational Research Center (MeTRC) (NIH-U54MD007593). It is also partially supported by the RCMI Program in Health Disparities Research at Meharry Medical College (U54MD007586), NIH grant SC1GM089269, the Tennessee Center for AIDS Research (TN-CFAR) (P30AI110527), the Vanderbilt Institute for Clinical and Translational Research (CTSA) (UL1RR024975), and the MMC, VICC, TSU: Partners in Eliminating Cancer Disparities grant (U54CA163069). B.L.K was supported by NIH grant (2T32HL007737). The funders had no role in study de-sign, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of Meharry Medical College (Protocol Number: 14-12-321; Date of Approval: 21 April 2015).
Informed Consent Statement
Not applicable since only de-identified samples were used for this study.
Data Availability Statement
The data presented in this study are available in the article and its Supplementary Material.
Acknowledgments
We thank Waldemar Popik for reagents, James Mungin for helpful discussions, and Olga Korolkova, Tanu Ran, and Shawn Goodwin for technical assistance. We also thank Sheila Yong from the Meharry Office for Scientific Editing and Publications for scientific editing support (S21MD000104). The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 IIIB-infected H9 cells from Robert Gallo; pCH040.c/2625 from John Kappes and Christina Ochsenbauer; HEK-293 cells from Andrew Rice; anti-HIV-1 p24 monoclonal antibody (183-H12-5C) from Bruce Chesebro and Kathy Wehrly; Polyclonal Anti-Human Immunodeficiency Virus Immune Globulin, Pooled Inactivated Human Sera, ARP-3957, contributed by NABI and National Heart Lung and Blood Institute (Luiz Barbosa). Some of the tissue samples were provided by the Cooperative Human Tissue Network, a National Cancer Institute supported resource. Other investigators may have received samples from these same tissue specimens.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tebit, D.M.; Ndembi, N.; Weinberg, A.; Quiñones-Mateu, M.E. Mucosal transmission of human immunodeficiency virus. Curr. HIV Res. 2012, 10, 3–8. [Google Scholar] [CrossRef]
- Hladik, F.; McElrath, M.J. Setting the stage: Host invasion by HIV. Nat. Rev. Immunol. 2008, 8, 447–457. [Google Scholar] [CrossRef]
- Nicolosi, A.; Correa Leite, M.L.; Musicco, M.; Arici, C.; Gavazzeni, G.; Lazzarin, A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: A study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology 1994, 5, 570–575. [Google Scholar] [CrossRef]
- Hel, Z.; Stringer, E.; Mestecky, J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr. Rev. 2010, 31, 79–97. [Google Scholar] [CrossRef]
- Wand, H.; Ramjee, G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. Aids 2012, 26, 375–380. [Google Scholar] [CrossRef]
- Martin, H.L., Jr.; Nyange, P.M.; Richardson, B.A.; Lavreys, L.; Mandaliya, K.; Jackson, D.J.; Ndinya-Achola, J.O.; Kreiss, J. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 1998, 178, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kiddugavu, M.; Makumbi, F.; Wawer, M.J.; Serwadda, D.; Sewankambo, N.K.; Wabwire-Mangen, F.; Lutalo, T.; Meehan, M.; Xianbin; Gray, R.H. Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda. Aids 2003, 17, 233–240. [Google Scholar] [CrossRef]
- Polis, C.B.; Curtis, K.M.; Hannaford, P.C.; Phillips, S.J.; Chipato, T.; Kiarie, J.N.; Westreich, D.J.; Steyn, P.S. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. Aids 2016, 30, 2665–2683. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.S.; Chen, P.L.; Kwok, C.; Baeten, J.M.; Brown, J.; Crook, A.M.; van Damme, L.; Delany-Moretlwe, S.; Francis, S.C.; Friedland, B.A.; et al. Hormonal contraception and the risk of HIV acquisition: An individual participant data meta-analysis. PLoS Med. 2015, 12, e1001778. [Google Scholar] [CrossRef] [PubMed]
- Ralph, L.J.; McCoy, S.I.; Shiu, K.; Padian, N.S. Hormonal contraceptive use and women’s risk of HIV acquisition: A meta-analysis of observational studies. Lancet Infect. Dis. 2015, 15, 181–189. [Google Scholar] [CrossRef]
- Evidence for Contraceptive O, Consortium HIVOT: HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019, 394, 303–313. [CrossRef]
- Hapgood, J.P. Is the Injectable Contraceptive Depo-Medroxyprogesterone Acetate (DMPA-IM) Associated with an Increased Risk for HIV Acquisition? The Jury Is Still Out. AIDS Res. Hum. Retrovir. 2020, 36, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sathyamala, C. Depot contraception and HIV: An exercise in obfuscation. BMJ 2019, 367, l5768. [Google Scholar] [CrossRef] [PubMed]
- Marx, P.A.; Spira, A.I.; Gettie, A.; Dailey, P.J.; Veazey, R.S.; Lackner, A.A.; Mahoney, C.J.; Miller, C.J.; Claypool, L.E.; Ho, D.D.; et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 1996, 2, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, S.A.; Guenthner, P.C.; Lin, C.Y.; Dobard, C.; Sharma, S.; Adams, D.R.; Otten, R.A.; Heneine, W.; Hendry, R.M.; McNicholl, J.M.; et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J. Acquir. Immune Defic. Syndr. 2011, 57, 261–264. [Google Scholar] [CrossRef]
- Zalenskaya, I.A.; Chandra, N.; Yousefieh, N.; Fang, X.; Adedipe, O.E.; Jackson, S.S.; Anderson, S.M.; Mauck, C.K.; Schwartz, J.L.; Thurman, A.R.; et al. Use of contraceptive depot medroxyprogesterone acetate is associated with impaired cervicovaginal mucosal integrity. J. Clin. Investig. 2018, 128, 4622–4638. [Google Scholar] [CrossRef]
- Chandra, N.; Thurman, A.R.; Anderson, S.; Cunningham, T.D.; Yousefieh, N.; Mauck, C.; Doncel, G.F. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res. Hum. Retrovir. 2013, 29, 592–601. [Google Scholar] [CrossRef]
- Poonia, B.; Walter, L.; Dufour, J.; Harrison, R.; Marx, P.A.; Veazey, R.S. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J. Endocrinol. 2006, 190, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Hild-Petito, S.; Veazey, R.S.; Larner, J.M.; Reel, J.R.; Blye, R.P. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res. Hum. Retrovir. 1998, 14 (Suppl. 1), S125–S130. [Google Scholar]
- Edfeldt, G.; Lajoie, J.; Röhl, M.; Oyugi, J.; Åhlberg, A.; Khalilzadeh-Binicy, B.; Bradley, F.; Mack, M.; Kimani, J.; Omollo, K.; et al. Regular use of depot medroxyprogesterone acetate causes thinning of the superficial lining and apical distribution of HIV target cells in the human ectocervix. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Byrne, E.H.; Anahtar, M.N.; Cohen, K.E.; Moodley, A.; Padavattan, N.; Ismail, N.; Bowman, B.A.; Olson, G.S.; Mabhula, A.; Leslie, A.; et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: A prospective cohort study. Lancet Infect. Dis. 2016, 16, 441–448. [Google Scholar] [CrossRef]
- Guthrie, B.L.; Introini, A.; Roxby, A.C.; Choi, R.Y.; Bosire, R.; Lohman-Payne, B.; Hirbod, T.; Farquhar, C.; Broliden, K. Depot Medroxyprogesterone Acetate Use Is Associated With Elevated Innate Immune Effector Molecules in Cervicovaginal Secretions of HIV-1-Uninfected Women. J. Acquir. Immune Defic. Syndr. 2015, 69, 1–10. [Google Scholar] [CrossRef]
- Deese, J.; Masson, L.; Miller, W.; Cohen, M.; Morrison, C.; Wang, M.; Ahmed, K.; Agot, K.; Crucitti, T.; Abdellati, S.; et al. Injectable Progestin-Only Contraception is Associated With Increased Levels of Pro-Inflammatory Cytokines in the Female Genital Tract. Am. J. Reprod. Immunol. 2015, 74, 357–367. [Google Scholar] [CrossRef]
- Morrison, C.; Fichorova, R.N.; Mauck, C.; Chen, P.L.; Kwok, C.; Chipato, T.; Salata, R.; Doncel, G.F. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J. Acquir. Immune Defic. Syndr. 2014, 66, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, K.G.; Shah, N.; Klein, R.S.; Mayer, K.H.; Sobel, J.D.; Warren, D.L.; Jamieson, D.J.; Duerr, A.C.; Rompalo, A.M. Group HIVERS: Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J. Infect. Dis. 2005, 191, 358–366. [Google Scholar] [CrossRef]
- Wieser, F.; Hosmann, J.; Tschugguel, W.; Czerwenka, K.; Sedivy, R.; Huber, J.C. Progesterone increases the number of Langerhans cells in human vaginal epithelium. Fertil. Steril. 2001, 75, 1234–1235. [Google Scholar] [CrossRef]
- Huijbregts, R.P.; Helton, E.S.; Michel, K.G.; Sabbaj, S.; Richter, H.E.; Goepfert, P.A.; Hel, Z. Hormonal contraception and HIV-1 infection: Medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology 2013, 154, 1282–1295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trunova, N.; Tsai, L.; Tung, S.; Schneider, E.; Harouse, J.; Gettie, A.; Simon, V.; Blanchard, J.; Cheng-Mayer, C. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology 2006, 352, 169–177. [Google Scholar] [CrossRef]
- Mitchell, C.M.; McLemore, L.; Westerberg, K.; Astronomo, R.; Smythe, K.; Gardella, C.; Mack, M.; Magaret, A.; Patton, D.; Agnew, K.; et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J. Infect. Dis. 2014, 210, 651–655. [Google Scholar] [CrossRef]
- Ferreira, V.H.; Dizzell, S.; Nazli, A.; Kafka, J.K.; Mueller, K.; Nguyen, P.V.; Tremblay, M.J.; Cochrane, A.; Kaushic, C. Medroxyprogesterone Acetate Regulates HIV-1 Uptake and Transcytosis but Not Replication in Primary Genital Epithelial Cells, Resulting in Enhanced T-Cell Infection. J. Infect. Dis. 2015, 211, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gach, J.S.; Becerra, J.C.; Phan, T.B.; Pudney, J.; Moldoveanu, Z.; Joseph, S.B.; Landucci, G.; Supnet, M.J.; Ping, L.H.; et al. The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells. PLoS Pathog. 2013, 9, e1003776. [Google Scholar] [CrossRef]
- Bomsel, M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 1997, 3, 42–47. [Google Scholar] [CrossRef]
- Bobardt, M.D.; Chatterji, U.; Selvarajah, S.; van der Schueren, B.; David, G.; Kahn, B.; Gallay, P.A. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 2007, 81, 395–405. [Google Scholar] [CrossRef]
- Devito, C.; Broliden, K.; Kaul, R.; Svensson, L.; Johansen, K.; Kiama, P.; Kimani, J.; Lopalco, L.; Piconi, S.; Bwayo, J.J.; et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 2000, 165, 5170–5176. [Google Scholar] [CrossRef] [PubMed]
- Saidi, H.; Magri, G.; Nasreddine, N.; Requena, M.; Belec, L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: Implication for mechanisms of sexual transmission. Virology 2007, 358, 55–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maher, D.; Wu, X.; Schacker, T.; Horbul, J.; Southern, P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc. Natl. Acad. Sci. USA 2005, 102, 11504–11509. [Google Scholar] [CrossRef] [PubMed]
- Carias, A.M.; McCoombe, S.; McRaven, M.; Anderson, M.; Galloway, N.; Vandergrift, N.; Fought, A.J.; Lurain, J.; Duplantis, M.; Veazey, R.S.; et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J. Virol. 2013, 87, 11388–11400. [Google Scholar] [CrossRef]
- Kinlock, B.L.; Wang, Y.; Turner, T.M.; Wang, C.; Liu, B. Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. PLoS ONE 2014, 9, e96760. [Google Scholar] [CrossRef]
- Halpern, V.; Combes, S.L.; Dorflinger, L.J.; Weiner, D.H.; Archer, D.F. Pharmacokinetics of subcutaneous depot medroxyprogesterone acetate injected in the upper arm. Contraception 2014, 89, 31–35. [Google Scholar] [CrossRef]
- Ortiz, A.; Hirol, M.; Stanczyk, F.Z.; Goebelsmann, U.; Mishell, D.R. Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J. Clin. Endocrinol. Metab. 1977, 44, 32–38. [Google Scholar] [CrossRef]
- Manuals & Protocols: Lysotracker and Lysosensor Probes. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp07525.pdf (accessed on 16 August 2021).
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef]
- Tanida, I.; Yamaji, T.; Ueno, T.; Ishiura, S.; Kominami, E.; Hanada, K. Consideration about negative controls for LC3 and expression vectors for four colored fluorescent protein-LC3 negative controls. Autophagy 2008, 4, 131–134. [Google Scholar] [CrossRef]
- Joseph, S.B.; Swanstrom, R.; Kashuba, A.D.; Cohen, M.S. Bottlenecks in HIV-1 transmission: Insights from the study of founder viruses. Nat. Rev. Microbiol. 2015, 13, 414–425. [Google Scholar] [CrossRef]
- Helen, R.; Consortium, E. DMPA and HIV: Why we need a trial. Contraception 2014, 90, 354–356. [Google Scholar]
- Gollub, E.L.; Jones, H.E.; Ralph, L.J.; van de Wijgert, J.; Padian, N.; Stein, Z. The Need for Policy Change Regarding Progestin-Only Injectable Contraceptives. J. Women’s Health 2019, 28, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.E. Time to focus on improving the contraceptive method mix in high HIV prevalence settings and let go of unanswerable questions. Contraception 2014, 90, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Soros, V.B.; Greene, W.C. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J. Virol. 2004, 78, 1375–1383. [Google Scholar] [CrossRef]
- Chikhlikar, P.; de Barros Arruda, L.; Maciel, M.; Silvera, P.; Lewis, M.G.; August, J.T.; Marques, E.T. DNA encoding an HIV-1 Gag/human lysosome-associated membrane protein-1 chimera elicits a broad cellular and humoral immune response in Rhesus macaques. PLoS ONE 2006, 1, e135. [Google Scholar] [CrossRef]
- Starodubova, E.S.; Isaguliants, M.G.; Kuzmenko, Y.V.; Latanova, A.A.; Krotova, O.A.; Karpov, V.L. Fusion to the Lysosome Targeting Signal of the Invariant Chain Alters the Processing and Enhances the Immunogenicity of HIV-1 Reverse Transcriptase. Acta Nat. 2014, 6, 61–68. [Google Scholar] [CrossRef]
- Kubo, Y.; Izumida, M.; Yashima, Y.; Yoshii-Kamiyama, H.; Tanaka, Y.; Yasui, K.; Hayashi, H.; Matsuyama, T. Gamma-interferon-inducible, lysosome/endosome-localized thiolreductase, GILT, has anti-retroviral activity and its expression is counteracted by HIV-1. Oncotarget 2016, 7, 71255–71273. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, R.; Zhou, T.; Su, P.; Zhang, X.; Qiu, X.; Li, H.; Li, S.; Yu, C.; Wang, B.; et al. HIV-1 Nef Antagonizes SERINC5 Restriction by Downregulation of SERINC5 via the Endosome/Lysosome System. J. Virol. 2018, 92, 11. [Google Scholar] [CrossRef]
- Marques, E.T., Jr.; Chikhlikar, P.; de Arruda, L.B.; Leao, I.C.; Lu, Y.; Wong, J.; Chen, J.S.; Byrne, B.; August, J.T. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J. Biol. Chem. 2003, 278, 37926–37936. [Google Scholar] [CrossRef]
- Fields, J.; Dumaop, W.; Eleuteri, S.; Campos, S.; Serger, E.; Trejo, M.; Kosberg, K.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: Implications for HIV-associated neurocognitive disorders. J. Neurosci. 2015, 35, 1921–1938. [Google Scholar] [CrossRef]
- Santerre, M.; Arjona, S.P.; Allen, C.N.; Callen, S.; Buch, S.; Sawaya, B.E. HIV-1 Vpr protein impairs lysosome clearance causing SNCA/alpha-synuclein accumulation in neurons. Autophagy 2021, 17, 1768–1782. [Google Scholar] [CrossRef]
- Moorjani, H.; Craddock, B.P.; Morrison, S.A.; Steigbigel, R.T. Impairment of phagosome-lysosome fusion in HIV-1-infected macrophages. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 13, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Fredericksen, B.L.; Wei, B.L.; Yao, J.; Luo, T.; Garcia, J.V. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 2002, 76, 11440–11446. [Google Scholar] [CrossRef]
- Wei, B.L.; Denton, P.W.; O′Neill, E.; Luo, T.; Foster, J.L.; Garcia, J.V. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J. Virol. 2005, 79, 5705–5712. [Google Scholar] [CrossRef]
- Rowell, J.F.; Ruff, A.L.; Guarnieri, F.G.; Staveley-O’ Carroll, K.; Lin, X.; Tang, J.; August, J.T.; Siliciano, R.F. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J. Immunol. 1995, 155, 1818–1828. [Google Scholar] [PubMed]
- Africander, D.; Louw, R.; Verhoog, N.; Noeth, D.; Hapgood, J.P. Differential regulation of endogenous pro-inflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception 2011, 84, 423–435. [Google Scholar] [CrossRef]
- Ratner, L.; Haseltine, W.; Patarca, R.; Livak, K.J.; Starcich, B.; Josephs, S.F.; Doran, E.R.; Rafalski, J.A.; Whitehorn, E.A.; Baumeister, K.; et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 1985, 313, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Sarngadharan, M.G.; Read, E.; Gallo, R.C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 1984, 224, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Read-Connole, E.; Gallo, R.C. T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet 1984, 2, 1472–1473. [Google Scholar] [CrossRef]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choe, J.S.; Seo, H.R.; Park, J.S. Effect of Poly (ethylene glycol) Grafting on Polyethylenimine as a Gene Transfer Vector in vitro. Bull. Korean Chem. Soc. 2001, 22, 46–52. [Google Scholar]
- Fichorova, R.N.; Rheinwald, J.G.; Anderson, D.J. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 1997, 57, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Wehrly, K.; Chesebro, B. P24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 1997, 12, 288–293. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Q.; Yu, X.; Kong, W.; Hildreth, J.E.; Liu, B. N-terminal hemagglutinin tag renders lysine-deficient APOBEC3G resistant to HIV-1 Vif-induced degradation by reduced polyubiquitination. J. Virol. 2011, 85, 4510–4519. [Google Scholar] [CrossRef][Green Version]
- Drosten, C.; Muller-Kunert, E.; Dietrich, M.; Gerdes, J.; Schmitz, H. Topographic and quantitative display of integrated human immunodeficiency virus-1 provirus DNA in human lymph nodes by real-time polymerase chain reaction. J. Mol. Diagn. 2005, 7, 219–225. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).