Current Evidence on the Antimicrobial Treatment and Chemoprophylaxis of Human Leptospirosis: A Meta-Analysis
Abstract
:1. Introduction
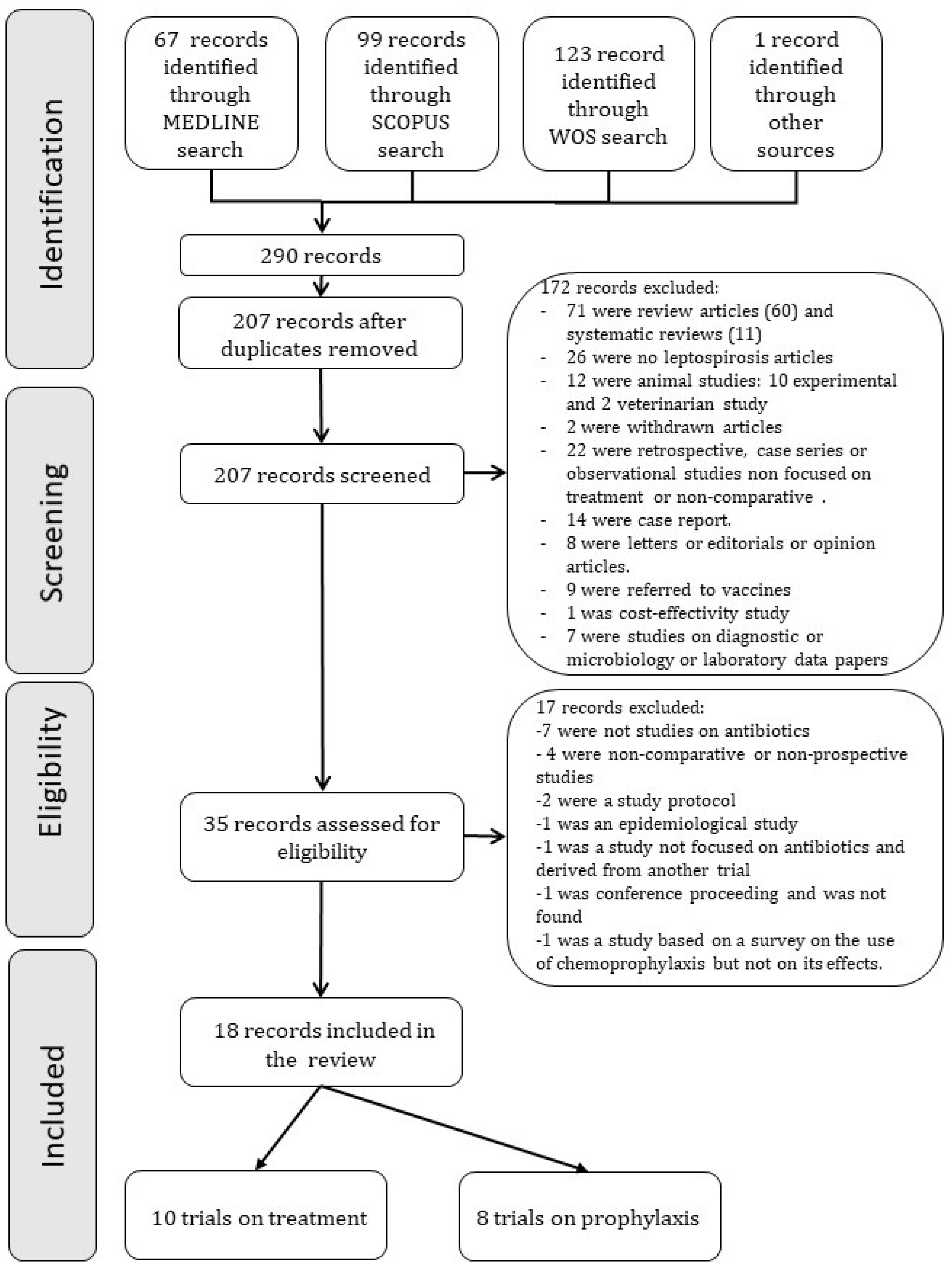
2. Material and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Outcome Measures
- Duration of hospitalization, defervescence time, kidney failure, liver failure, recovery time from kidney or liver failure, and death;
- For prophylaxis trials, the rate of new cases of leptospirosis in each group was collected.
2.4. Data Extraction
- -
- Author and year of publication;
- -
- Type of study;
- -
- Number of patients;
- -
- Diagnostic criteria;
- -
- Treatment regimens with antibiotics, with its duration, route of administration, and dose;
- -
- If the antibiotic treatment was started as prophylaxis or treatment for the infection;
- -
- Duration of hospitalization, time of defervescence, the incidence of renal or liver failure, recovery time from renal or liver failure, and mortality.
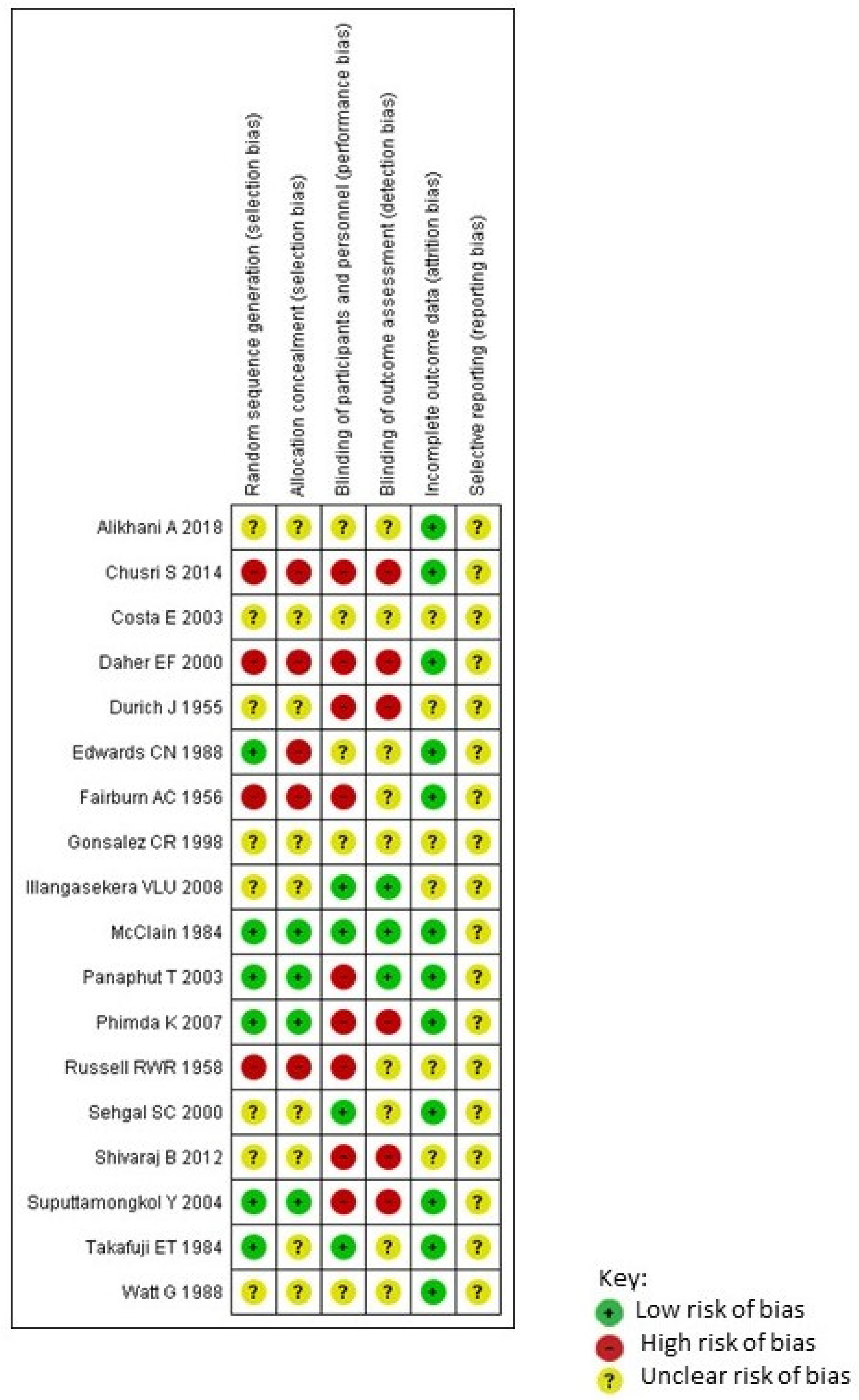
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cagliero, J.; Villanueva, S.Y.A.M.; Matsui, M. Leptospirosis pathophysiology: Into the storm of cytokines. Front. Cell Infect Microbiol. 2018, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef] [Green Version]
- De Brito, T.; Silva, A.M.G.D.; Abreu, P.A.E. Pathology and pathogenesis of human leptospirosis: A commented review. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e23. [Google Scholar] [CrossRef]
- Le Turnier, P.; Epelboin, L. Mise au point sur la leptospirose [Update on leptospirosis]. Rev. Med. Interne. 2019, 40, 306–312. [Google Scholar] [CrossRef]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Soo, Z.M.P.; Khan, N.A.; Siddiqui, R. Leptospirosis: Increasing importance in developing countries. Acta Trop. 2020, 201, 105183. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sean, T.C.; Bhavya, K.S.; Sahithya, C.S.; Chan-Drasekaran, S.; Palanisamy, R.; Robinson, E.R.; Subbiah, S.K.; Mok, P.L. Leptospiral infection, pathogenesis and its diagnosis—A review. Pathogens 2021, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Goarant, C. Leptospirosis: Risk factors and management challenges in developing countries. Res. Rep. Trop. Med. 2016, 7, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [Green Version]
- Griffith, M.E.; Hospenthal, D.R.; Murray, C.K. Antimicrobial therapy of leptospirosis. Curr. Opin. Infect. Dis. 2006, 19, 533–537. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; Version 6. [Google Scholar]
- Fairburn, A.C.; Semple, S.J. Chloramphenicol and penicillin in the treatment of leptospirosis among British troops in Malaya. Lancet 1956, 270, 13–16. [Google Scholar] [CrossRef]
- Russell, R.W. Treatment of leptospirosis with oxytetracycline. Lancet 1958, 2, 1143–1145. [Google Scholar] [CrossRef]
- McClain, J.B.; Ballou, W.R.; Harrison, S.M.; Steinweg, D.L. Doxycycline therapy for leptospirosis. Ann. Intern. Med. 1984, 100, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Padre, L.P.; Tuazon, M.L.; Calubaquib, C.; Santiago, E.; Ranoa, C.P.; Laughlin, L.W. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 1988, 1, 433–435. [Google Scholar] [CrossRef]
- Edwards, C.N.; Nicholson, G.D.; Hassell, T.A.; Everard, C.O.; Callender, J. Penicillin therapy in icteric leptospirosis. Am. J. Trop. Med. Hyg. 1988, 39, 388–390. [Google Scholar] [CrossRef]
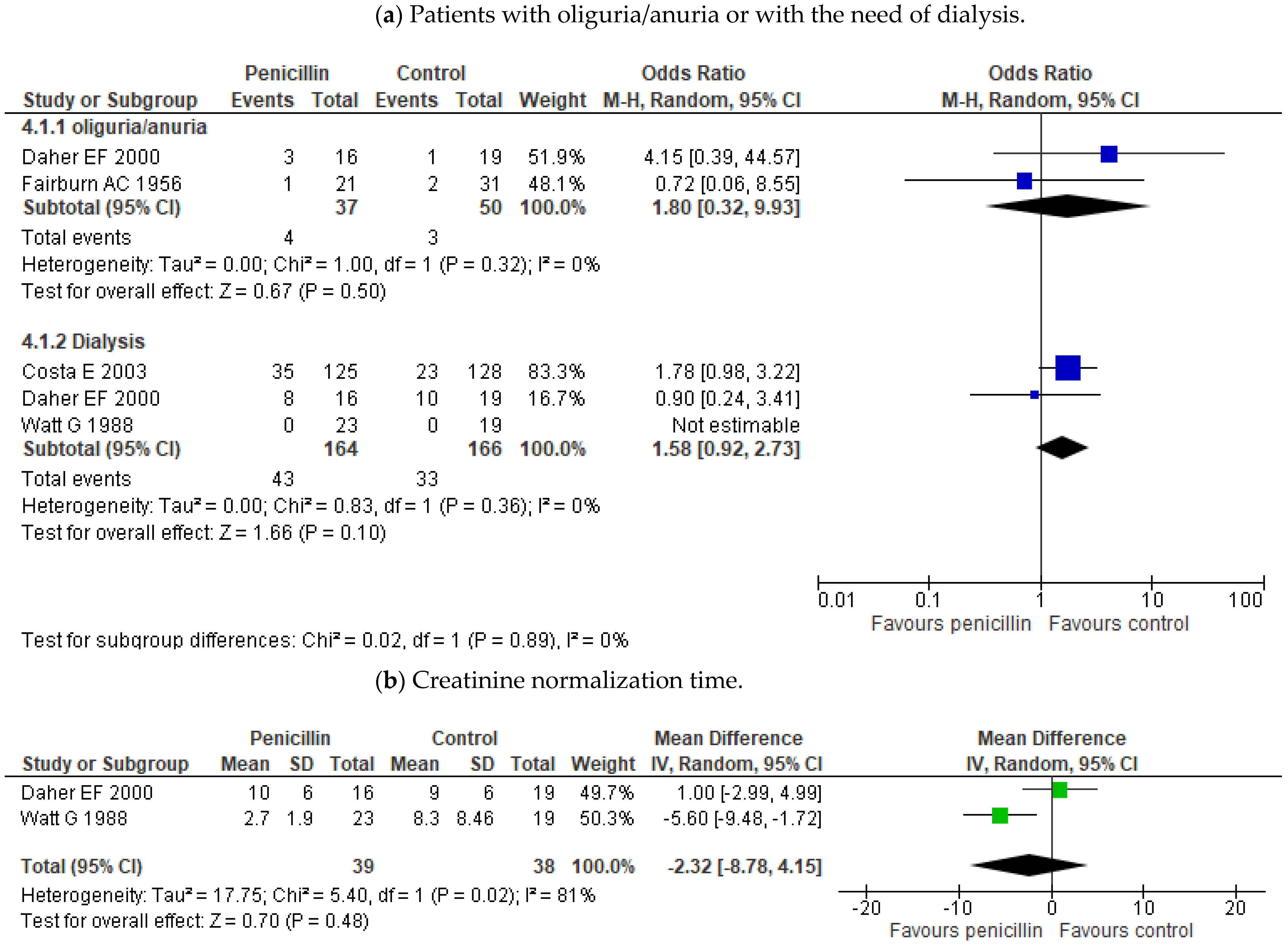
- Daher, E.F.; Nogueira, C.B. Evaluation of penicillin therapy in patients with leptospirosis and acute renal failure. Rev. Inst. Med. Trop. Sao Paulo 2000, 42, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Lopes, A.A.; Sacramento, E.; Costa, Y.A.; Matos, E.D.; Lopes, M.B.; Bina, J.C. Penicillin at the late stage of leptospirosis: A randomized controlled trial. Rev. Inst. Med. Trop. Sao Paulo 2003, 45, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Panaphut, T.; Domrongkitchaiporn, S.; Vibhagool, A.; Thinkamrop, B.; Susaengrat, W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin. Infect. Dis. 2003, 36, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Suputtamongkol, Y.; Niwattayakul, K.; Suttinont, C.; Losuwanaluk, K.; Limpaiboon, R.; Chierakul, W.; Wuthiekanun, V.; Triengrim, S.; Chenchittikul, M.; White, N.J. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin. Infect. Dis. 2004, 39, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Phimda, K.; Hoontrakul, S.; Suttinont, C.; Chareonwat, S.; Losuwanaluk, K.; Chueasuwanchai, S.; Chierakul, W.; Suwancharoen, D.; Silpasakorn, S.; Saisongkorh, W.; et al. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob. Agents Chemother. 2007, 51, 3259–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durich, J.; Pumarola, A. La endemia de leptospirosis en la provincia de Valencia. ensayo de métodos profilácticos (1954) [Endemic aspects of leptospirosis in the province of Valencia; trial of preventive methods (1954)]. Med. Esp. 1955, 34, 83–90. [Google Scholar] [PubMed]
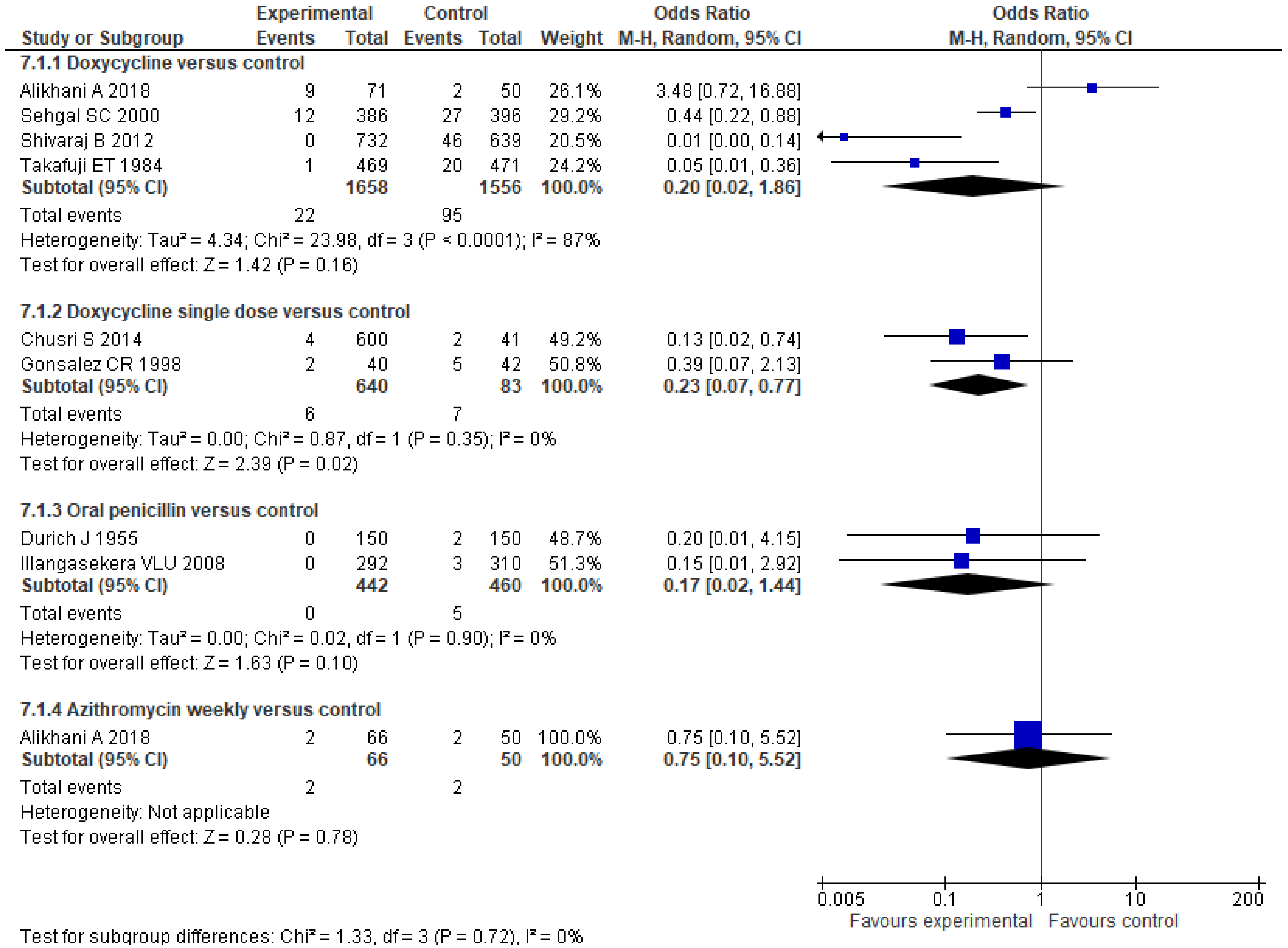
- Takafuji, E.T.; Kirkpatrick, J.W.; Miller, R.N.; Karwacki, J.J.; Kelley, P.W.; Gray, M.R.; McNeill, K.M.; Timboe, H.L.; Kane, R.E.; Sanchez, J.L. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N. Engl. J. Med. 1984, 310, 497–500. [Google Scholar] [CrossRef] [Green Version]
- Gonsalez, C.R.; Casseb, J.; Monteiro, F.G.; Paula-Neto, J.B.; Fernandez, R.B.; Silva, M.V.; Camargo, E.D.; Mairinque, J.M.; Tavares, L.C. Use of doxycycline for leptospirosis after high-risk exposure in São Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 1998, 40, 59–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, S.C.; Sugunan, A.P.; Murhekar, M.V.; Sharma, S.; Vijayachari, P. Randomized controlled trial of doxycycline prophylaxis against leptospirosis in an endemic area. Int. J. Antimicrob. Agents 2000, 13, 249–255. [Google Scholar] [CrossRef]
- Illangasekera, V.L.; Kularatne, S.A.; Kumarasiri, P.V.; Pussepitiya, D.; Premaratne, M.D. Is oral penicillin an effective chemoprophylaxis against leptospirosis? A placebo controlled field study in the Kandy District, Sri Lanka. Southeast Asian J. Trop. Med. Public Health 2008, 39, 882–884. [Google Scholar]
- Shivaraj, B.; Ts, R.; Anithraj, B.Y.; Bayari, R. A study on prophylactic doxycycline to reduce the incidence of leptospirosis among paddy field farmers in a coastal district of India. Int. J. Infect. Dis. 2012, 16, E462. [Google Scholar] [CrossRef] [Green Version]
- Chusri, S.; McNeil, E.B.; Hortiwakul, T.; Charernmak, B.; Sritrairatchai, S.; Santimaleeworagun, W.; Pattharachayakul, S.; Suksanan, P.; Thaisomboonsuk, B.; Jarman, R.G. Single dosage of doxycycline for prophylaxis against leptospiral infection and leptospirosis during urban flooding in southern Thailand: A non-randomized controlled trial. J. Infect. Chemother. 2014, 20, 709–715. [Google Scholar] [CrossRef]
- Alikhani, A.; Salehifar, E.; Zameni, F.; Rafiei, A.; Yazdani-Charati, J.; Delavaryan, L.; Akbari, A.; Babamahmoudi, F. Comparison of azithromycin vs. doxycycline prophylaxis in leptospirosis, a randomized double blind placebo-controlled trial. J. Infect. Dev. Ctries. 2018, 12, 991–995. [Google Scholar] [CrossRef]
- Charan, J.; Saxena, D.; Mulla, S.; Yadav, P. Antibiotics for the treatment of leptospirosis: Systematic review and meta-analysis of controlled trials. Int. J. Prev. Med. 2013, 4, 501–510. [Google Scholar]
- Brett-Major, D.M.; Coldren, R. Antibiotics for leptospirosis. Cochrane Database Syst. Rev. 2012, CD008264. [Google Scholar] [CrossRef] [PubMed]
- Niwattayakul, K.; Kaewtasi, S.; Chueasuwanchai, S.; Hoontrakul, S.; Chareonwat, S.; Suttinont, C.; Phimda, K.; Chierakul, W.; Silpasakorn, S.; Suputtamongkol, Y. An open randomized controlled trial of desmopressin and pulse dexamethasone as adjunct therapy in patients with pulmonary involvement associated with severe leptospirosis. Clin. Microbiol. Infect. 2010, 16, 1207–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, C.; Lakshitha de Silva, N.; Goonaratne, R.; Samarasekara, K.; Wijesinghe, I.; Parththipan, B.; Rajapakse, S. High dose corticosteroids in severe leptospirosis: A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.V.; Vasava, A.H.; Patel, T.C.; Bhatia, L.C. Cyclophosphamide in pulmonary alveolar hemorrhage due to leptospirosis. Indian J. Crit. Care Med. 2009, 13, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, A.M. Use of ciprofloxacin for treating leptospirosis-need for clinical trials. J. Med. Microbiol. 2005, 54, 907. [Google Scholar] [CrossRef]


| Author (Year) and Reference | Treatment/Prophylaxis | Type of Study | Patients | Treatment Groups | n | Outcomes |
|---|---|---|---|---|---|---|
| Fairburn AC (1956) [13] | Treatment | Controlled trial | Soldiers admitted to one of two military hospitals in Malaya with agglutination test positive. | Penicillin 600,000 UI/6h versus chloramphenicol 0.5 g/6 h versus no antibiotics (for at least five days) | 21 vs. 31 vs. 31 | Duration of fever Duration of symptoms “After-peaks” of pyrexia Complications: Jaundice, oliguria, anuria, and uremia |
| Ross Russell RW (1958) [14] | Treatment | Placebo-controlled trial | 52 consecutive patients admitted to the military hospital with leptospirosis confirmed by blood-culture or serological tests. | Oxytetracycline 1.5 g followed by 0.5 g/6 h for at least 5 days versus placebo | 27 vs. 25 | Duration of pyrexia; Duration of symptoms; Incidence of jaundice; Urea levels. |
| McClain (1984) [15] | Treatment | Randomized, double-blind controlled trial | Any febrile patient returning from jungle training school without another cause of fever identified and isolation of leptospira in blood or urine, or a fourfold rise in serum antibody titers. | Doxycycline 100 mg/12 h for 7 days versus placebo | 14 vs. 15 | Duration of fever; Duration of signs and symptoms; Negativization of Leptospira in cultures and MAT. |
| Watt G (1988) [16] | Treatment | Double-blind placebo-controlled trial | Patients (>16 years) with a high likelihood of leptospirosis and also have leptospirosis confirmed with a fourfold or greater rise in antibody titer by microagglutination test or isolation in blood or urine. | Penicillin G 1.5 MU/6h for 7 days versus placebo | 23 vs. 19 | Duration of fever; Duration of symptoms. Duration of hospital stay; Number of patients from whom leptospires were isolated after treatment; Raised serum creatinine levels, hepatic tenderness, and hematological and biochemical variables. |
| Edwards CN (1988) [17] | Treatment | Randomized controlled trial | Patients with a history and physical findings compatible with symptoms of leptospirosis and have a fourfold rise in titer or an initial titer ≥600 in the MAT; a titer 1:80 IgM on an ELISA; a 4+ Patoc I antigen titer; positive leptospira cultures. | Penicillin 2 MU/6 h iv 5 days vs. placebo | 38 vs. 41 | Time to defervescence; Time to return of biochemical normality; Absence or positive urine culture; Complications; Mortality; Iritis. |
| Daher EF (2000) [18] | Treatment | Probably non randomized † | Patients with leptospirosis and acute renal failure (plasma creatinine > 1.5 mg/dL) and jaundice on admission. Diagnosis of leptospirosis clinical ald IgM ≥ 1:400 on the 15th day after the onset of symptoms. | Penicillin 6 MU/day iv for 8 days vs. no antibiotics | 16 vs. 19 | Days of hospitalization; Duration of fever Time to normalization of creatinine, bilirubin (or one-third of maximum value), and platelet count; Dialytic treatment; Values of serum and urine biochemical parameters; Hospital mortality; Presence of oliguria; Positive fluid balance (%); Episodes of bleeding; X-ray evidence of pneumonitis. |
| Costa E (2003) [19] | Treatment | Randomized controlled trial | Patients with> 4 days with symptoms; ≥26 points in the WHO probability score for leptospirosis; Macroscopic slide test, microagglutination test, and blood culture for microbiologic confirmation in Salvador. | Penicillin 6 mU/day (1 mU/4 h) for seven days versus control (no antibiotic) | 125 vs. 128 | In-Hospital death; Length of hospital stay. |
| Panaphut T (2003) [20] | Treatment | Randomized trial. Open-label | Patients (>16 years) with severe leptospirosis, defined by the presence of jaundice, a serum creatine level of >180 µmol/L, or a mean arterial pressure <70 mmHg. Serology diagnostic. WHO criteria. | Ceftriaxone 1 g/day for 7 days versus penicillin G 1.5 MU/6 h for 7 days *. | 87 vs. 86 | Time to fever resolution Hospital mortality Time to resolution of organ dysfunction (renal failure, hepatic dysfunction, and thrombocytopenia) |
| Suputtamongkol Y (2004) [21] | Treatment | Randomized, open trial | Patients with suspected severe leptospirosis in some hospitals in Thailand, whom had isolated leptospires from blood or a 4′fold or greater increase in the antibody titer or a single or stable antibody titer of ≥1:400. | Penicillin G 1.5 MU/6 h versus Cefotaxime 1 g/6 h versus Doxycycline 200 mg first dose followed by 100 mg/12 h iv. 7 days **. | 181 vs. 172 vs. 187 (87 vs. 81 vs. 88 with confirmed leptospirosis) | Mortality; Time to defervescence; Duration of hospitalization. |
| Phimda K (2007) [22] | Treatment | Randomized trial | Patients with suspected Leptospirosis and scrub typhus in Thailand Leptospirosis: isolation from blood or positive serologic test (fourfold or greater rise in antibody titer or at least 1:400 on a single specimen). | Doxycycline 200 mg the first dose followed by 100 mg/12 horas seven days versus Azithromycin 1 g initially followed by 500 mg once daily for 2 days. | 34:35 (only leptospirosis) | Time of fever clearance; % of afebrile patients at 48 h post-treatment. |
| Durich J (1956) [23] | Chemoprophylaxis | Probably non randomized † | Rice reapers in the province of Valencia (Spain) | Penicillin procain 100,000 U/12 h vo (for 100 reapers) or dipenicillin N,N’dibenciletilendiamine 100000U/12 h (for 50 reapers) versus control. | 150 vs. 150 | New cases of leptospirosis. |
| Takafuji ET (1984) [24] | Chemoprophylaxis | Double-blind, placebo-controlled trial | Healthy volunteers at Jungle Operations Training Center (Panama). | Doxycycline 200 mg weekly vs. placebo. | 469:471 | New cases of leptospirosis. |
| Gonsalez CR (1998) [25] | Chemoprophylaxis | Double-blind, randomized, placebo-controlled trial | Residents of a small community in Sao Paulo region, an area at high risk for flooding. | Doxycycline 200 mg single dose vs. placebo. | 40 vs. 42 | New confirmed cases (symptomatic). Seropositivity |
| Sehgal SC (2000) [26] | Chemoprophylaxis | Randomized controlled-trial | Healthy persons (>10 years old) residing in Diglipur (North Andaman), India. | Doxycycline 200/week versus placebo (12 weeks). | 386 vs. 396 | New cases of leptospirosis. Seropositivity |
| Illangasekera VLU (2008) [27] | Chemoprophylaxis | Randomized, double-blind Placebo-controlled trial | Healthy persons (male farmers) between 20–80 years of age from Central Province, Sri Lanka. | Oral Penicillin 500 mg/bid versus placebo during 1 month. | 292 vs. 310 | New cases of leptospirosis. |
| Shivaraj B (2012) [28] | Chemoprophylaxis | Randomized controlled trial | Paddy field farmers in Karnataka state, India. | Doxycycline 200 mg/week for 5 weeks and Information Education Communication activity versus none treatment. | 732 vs. 639 | New cases of leptospirosis. |
| Chusri S (2014) [29] | Chemoprophylaxis | Non-randomized trial | Residents in Hat Yai City (Thailand), aged 18 years or above and exposed to flood water. | Doxycycline 200 mg single dose versus no treatment. | 600 vs. 41 | Seroconversion New cases of symptomatic leptospirosis. |
| Alikhani A (2018) [30] | Chemoprophylaxis | Randomized, double-blind, placebo-controlled trial | Paddy field workers (from 18 to 65 years); Three endemic cities for leptospirosis in Mazandaran in the north of Iran. | Azithromycin 500 mg/week versus doxycycline 200 mg/week versus placebo during 1 month. | 66 vs. 71 vs. 50 | Cases of leptospirosis (symptomatic); Seropositivity for leptospirosis. |
| Author (Year) and Reference | Treatment Groups | Mortality | Duration of Fever | Hospital Stay | Oliguria/Anuria | Dialysis | Creatinine Normalization Time | Jaundice | Liver Function Tests Normalization Time |
|---|---|---|---|---|---|---|---|---|---|
| Fairburn AC (1956) [13] | Penicillin 600,000 U/6 h | NR | 7.6 | NR | 1 (4.8%) | NR | NR | 0 (0%) | NR |
| Chloramphenicol 0.5g/6h | NR | 8.8 | NR | 1 (3.2%) | NR | NR | 1 (3.2%) | NR | |
| control | NR | 9 | NR | 2 (6.5%) | NR | NR | 2 (6.5%) | NR | |
| Ross Russell (1958) [14] | Oxytetracycline 1.5g followed by 0.5g/6h for at least 5 days | NR | 6.4 | NR | NR | NR | NR | 6 (22.2%) | NR |
| placebo | NR | 9.4 | NR | NR | NR | NR | 5 (20%) | NR | |
| McClain (1984) [15] | Doxiciclina 100 mg cada 12 horas durante 7 días | NR | 3.7 ± 0.3 | NR | NR | NR | NR | NR | NR |
| placebo | NR | 5.4 ± 0.3 | NR | NR | NR | NR | NR | NR | |
| Watt G (1988) [16] | Penicillin G 1.5 MU/6h for 7 days | 0/23 | 4.7 ± 4.19 | NR | NR | 0 (0%) | 2.7±1.9 | NR | NR |
| placebo | 0/19 | 11.6 ± 8.34 | NR | NR | 0 (0%) | 8.3±8.46 | NR | NR | |
| Edwards CN (1988) [17] | Penicillin 2 MU/6h iv 5 days | 1/38 | 6.9 ± 3.8 | NR | NR | NR | NR | NR | 5.7 ± 3 |
| placebo | 3/41 | 6.6 ± 3.2 | NR | NR | NR | NR | NR | 5.65 ± 3.7 | |
| Daher EF (2000) [18] | Penicillin 6 MU/day iv for 8 days | 1/16 | 3 ± 4 | 12 ± 6 | 3 (19%) | 8 (50%) | 10±6 | NR | 8 ± 3 |
| no antibiotics | 0/19 | 2 ± 3 | 11 ± 5 | 1 (5%) | 10 (52%) | 9±6 | NR | 8 ± 3 | |
| Costa E (2003) [19] | Penicillin 6 mU/day (1 mU/4h) for seven days | 15/125 | NR | 8.9 ± 3.9 | NR | 35 (28%) | NR | NR | NR |
| control | 8/128 | NR | 8.8 ± 3.6 | NR | 23 (18%) | NR | NR | NR | |
| Panaphut T (2003) [20] | penicillin G 1.5 MU/6h for 7 days | 5/86 | 3 * | NR | NR | NR | NR | NR | NR |
| Ceftriaxone 1 g/day for 7 days | 5/87 | 3 * | NR | NR | NR | NR | NR | NR | |
| Suputtamongkol Y (2004) [21] | Penicillin G 1.5 MU/6h 7 days | 2/87 | 72 (12–240) | 6 (2–21) | NR | NR | NR | NR | NR |
| Cefotaxime 1 g/6 h 7 days | 0/88 | 60 (8–192) | 5.5 (3–37) | NR | NR | NR | NR | NR | |
| Doxycycline 200 mg first dose followed by 100 mg/12 h iv. 7 days. | 2/81 | 72 (12–264) | 5 (2–28) | NR | NR | NR | NR | NR | |
| Phimda K (2007) [22] | Doxycycline 200 mg the first dose followed by 100 mg/12 horas seven days | NR | 45 h (8–118 h) | NR | NR | NR | NR | NR | NR |
| Azithromycin 1g initially followed by 500 mg once daily for 2 days. | NR | 40 h (8–136 h) | NR | NR | NR | NR | NR | NR |
| Author (Year) | Treatment Groups | New Symptomatic Cases | Seroconversion |
|---|---|---|---|
| Durich J (1956) [23] | Penicillin procain 100,000 U/12 h vo or dipenicillin N,N’dibenciletilendiamine | 0/150 (0%) | NR |
| Control | 2/150 (1.3%) | NR | |
| Takafuji ET (1984) [24] | Doxycycline 200 mg semanales (3 weeks aprox) | 1/469 (0.2%) | NR |
| placebo | 20/471 (4.2%) | NR | |
| Gonsalez CR (1998) [25] | Doxycycline 200 mg single dose | 2/40 (5%) | IgM: 13/40 (32.5%) |
| placebo | 5/42 (11.9%) | IgM: 11/42 (26.2%) | |
| Sehgal SC (2000) [26] | Doxycycline 200/week (12 weeks) | 12/386 (3.1%) | 112/386 (29%) |
| placebo | 27/396 (6.82%) | 101/396 (25.5%) | |
| Illangasekera VLU (2008) [27] | Oral Penicillin 500 mg/bid for one month | 0/292 (0%) | NR |
| placebo | 3/310 (1%) | NR | |
| Shivaraj B (2012) [28] | Doxycycline 200 mg/week for 5 weeks and IEC | 0/732 (0%) | NR |
| No treatment | 46 */639 (7.29%) | NR | |
| Chusri S (2014) [29] | Doxycycline 200 mg single dose | 4/600 (0.7%) | 17/600 (2.8%) |
| No treatment | 2/41 (4.9%) | 5/41 (12.2%) | |
| Alikhani A (2018) [30] | Azithromycin 500 mg/week | 2/66 (3%) | IgM: 2/66(3%) IgG: 5/66 (7.6%) |
| doxycycline 200 mg/week | 9/71 (12.6%) | IgM: 9/71 (12.6%) IgG: 8/71 (11.3%) | |
| placebo | 2/50 (4%) | IgM: 2/50 (4%) IgG: 12/50 (24%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán Pérez, M.; Blanch Sancho, J.J.; Segura Luque, J.C.; Mateos Rodriguez, F.; Martínez Alfaro, E.; Solís García del Pozo, J. Current Evidence on the Antimicrobial Treatment and Chemoprophylaxis of Human Leptospirosis: A Meta-Analysis. Pathogens 2021, 10, 1125. https://doi.org/10.3390/pathogens10091125
Guzmán Pérez M, Blanch Sancho JJ, Segura Luque JC, Mateos Rodriguez F, Martínez Alfaro E, Solís García del Pozo J. Current Evidence on the Antimicrobial Treatment and Chemoprophylaxis of Human Leptospirosis: A Meta-Analysis. Pathogens. 2021; 10(9):1125. https://doi.org/10.3390/pathogens10091125
Chicago/Turabian StyleGuzmán Pérez, Marta, José Javier Blanch Sancho, Juan Carlos Segura Luque, Fernando Mateos Rodriguez, Elisa Martínez Alfaro, and Julián Solís García del Pozo. 2021. "Current Evidence on the Antimicrobial Treatment and Chemoprophylaxis of Human Leptospirosis: A Meta-Analysis" Pathogens 10, no. 9: 1125. https://doi.org/10.3390/pathogens10091125
APA StyleGuzmán Pérez, M., Blanch Sancho, J. J., Segura Luque, J. C., Mateos Rodriguez, F., Martínez Alfaro, E., & Solís García del Pozo, J. (2021). Current Evidence on the Antimicrobial Treatment and Chemoprophylaxis of Human Leptospirosis: A Meta-Analysis. Pathogens, 10(9), 1125. https://doi.org/10.3390/pathogens10091125







