Approaching In Vivo Models of Pneumococcus–Host Interaction: Insights into Surface Proteins, Capsule Production, and Extracellular Vesicles
Abstract
:1. Introduction
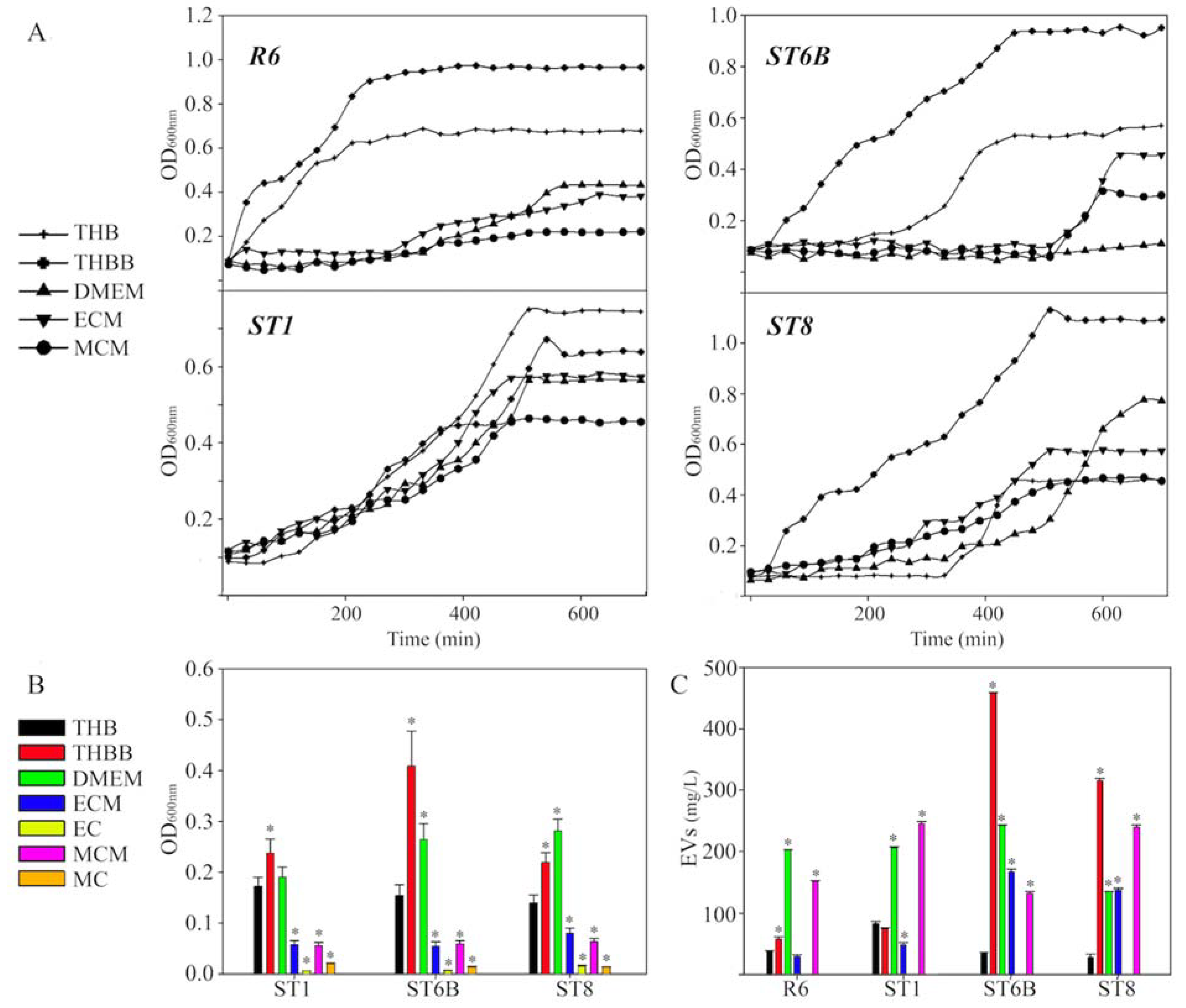
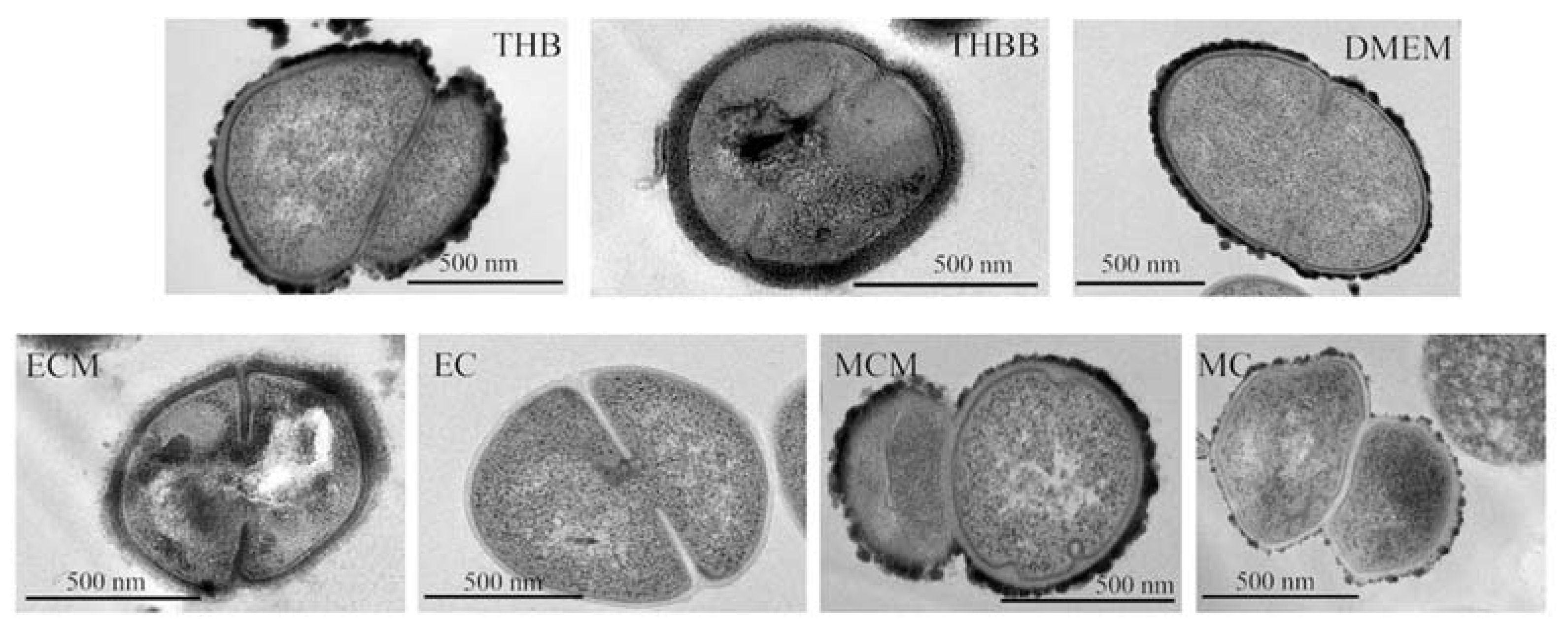
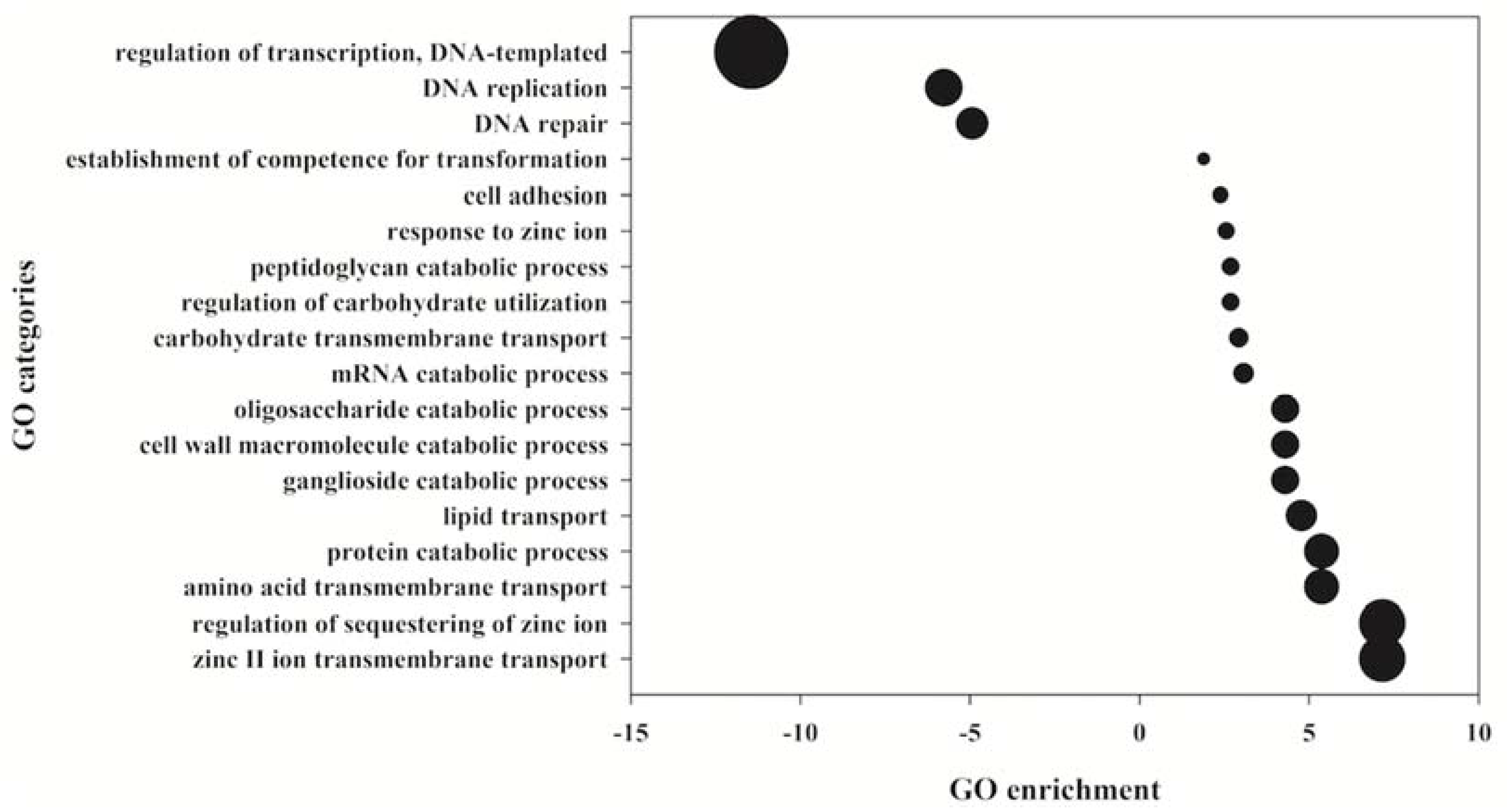
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Bacterial Strains, and Growth
4.2. “Shaving” of Pneumococcal Living Cells
4.3. LC-MS/MS Analysis
4.4. Protein Identification by Database Searching
4.5. Pneumococcal EVs Production and Quantification
4.6. Capsule Quantification
4.7. Electron Microscopy
4.8. Statistics and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blasi, F.; Mantero, M.; Santus, P.; Tarsia, P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012, 18, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Freimanis Hance, L.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010, 7, e1000348. [Google Scholar] [CrossRef] [Green Version]
- Pittet, L.; Posfay-Barbe, K.M. Pneumococcal vaccines for children: A global public health priority. Clin. Microbiol. Infect. 2012, 18, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Lagousi, T.; Basdeki, P.; Routsias, J.; Spoulou, V. Novel Protein-Based Pneumococcal Vaccines: Assessing the Use of Distinct Protein Fragments Instead of Full-Length Proteins as Vaccine Antigens. Vaccines 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Kuster, S.; Rudnick, W.; Shigayeva, A.; Green, K.; Baqi, M.; Gold, W.L.; Lovinsky, R.; Muller, M.P.; Powis, J.; Rau, N.; et al. Previous Antibiotic Exposure and Antimicrobial Resistance in Invasive Pneumococcal Disease: Results From Prospective Surveillance. Clin. Infect. Dis. 2014, 59, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondi, T.; Canessa, C.; Lippi, F.; Iacopelli, J.; Nieddu, F.; Azzari, C. Streptococcus pneumoniae: Elusive mechanisms of the body’s defense systems. J. Prev. Med. Hyg. 2012, 53, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wantuch, P.L.; Avci, F.Y. Current status and future directions of invasive pneumococcal diseases and prophylactic approaches to control them. Hum. Vaccines Immunother. 2018, 14, 2303–2309. [Google Scholar] [CrossRef] [Green Version]
- Porat, N.; Trefler, R.; Godoy, D.; Bilek, N.; Arguedas, A.; Spratt, B.G.; Brilla, E.; Loaiza, C.; Dagan, R. Emergence of Penicillin—Nonsusceptible Streptococcus pneumoniae Clones Expressing Serotypes Not Present in the Antipneumococcal Conjugate Vaccine. J. Infect. Dis. 2004, 190, 2154–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Genet. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Morimura, A.; Hamaguchi, S.; Akeda, Y.; Tomono, K. Mechanisms Underlying Pneumococcal Transmission and Factors Influencing Host-Pneumococcus Interaction: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 639450. [Google Scholar] [CrossRef]
- Navarre, W.W.; Schneewind, O. Surface Proteins of Gram-Positive Bacteria and Mechanisms of Their Targeting to the Cell Wall Envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandi, G. Genomics and Proteomics in Reverse Vaccines. Methods Biochem. Anal. 2005, 49, 379–393. [Google Scholar] [CrossRef]
- Abril, A.O.; Jiménez-Munguía, I.; Gascón, L.G.; Rodríguez-Ortega, M.J. Surfomics: Shaving live organisms for a fast proteomic identification of surface proteins. J. Proteom. 2014, 97, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.O.; Prados-Rosales, R.; McConnell, M.J.; Martín-Peña, R.; González-Reyes, J.A.; Jiménez-Munguía, I.; Gascón, L.G.; Fernández, J.; Luque-Garcia, J.L.; García-Lidón, C.; et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteom. 2014, 106, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Mitsuwan, W.; Jiménez-Munguía, I.; Visutthi, M.; Sianglum, W.; Rodríguez-Ortega, M.J.; Voravuthikunchai, S.P.; Jover, A.; Barcenilla, F.; García, M.; Pujol, M.; et al. Rhodomyrtone decreases Staphylococcus aureus SigB activity during exponentially growing phase and inhibits haemolytic activity within membrane vesicles. Microb. Pathog. 2019, 128, 112–118. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, H. Characterization and function of membrane vesicles in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 1795–1801. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Wolff, S.; Hocke, A.; Rosseau, S.; Müller, E.; Rohde, M. Illustration of Pneumococcal Polysaccharide Capsule during Adherence and Invasion of Epithelial Cells. Infect. Immun. 2005, 73, 4653–4667. [Google Scholar] [CrossRef] [Green Version]
- Paton, J.C.; Trappetti, C. Streptococcus pneumoniae Capsular Polysaccharide. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Codemo, M.; Muschiol, S.; Iovino, F.; Nannapaneni, P.; Plant, L.; Wai, S.N.; Henriques-Normark, B. Immunomodulatory Effects of Pneumococcal Extracellular Vesicles on Cellular and Humoral Host Defenses. mBio 2018, 9, e00559-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaya-Abril, A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Obando, I.; Rodríguez-Ortega, M.J. Identification of Potential New Protein Vaccine Candidates through Pan-Surfomic Analysis of Pneumococcal Clinical Isolates from Adults. PLoS ONE 2013, 8, e70365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abril, A.O.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Obando, I.; Rodríguez-Ortega, M.J. A Pneumococcal Protein Array as a Platform to Discover Serodiagnostic Antigens Against Infection. Mol. Cell. Proteom. 2015, 14, 2591–2608. [Google Scholar] [CrossRef] [Green Version]
- Ardanuy, C.; Marimón, J.M.; Calatayud, L.; Giménez, M.; Alonso, M.; Grau, I.; Pallarés, R.; Pérez-Trallero, E.; Liñares, J. Epidemiology of Invasive Pneumococcal Disease in Older People in Spain (2007–2009): Implications for Future Vaccination Strategies. PLoS ONE 2012, 7, e43619. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Yi, R.; Jiang, Y.; Xu, S.; Qin, P.; Liang, Z.; Chen, J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae causing invasive diseases in China: A meta-analysis. BMC Pediatr. 2019, 19, 424. [Google Scholar] [CrossRef] [Green Version]
- Massora, S.; Lessa, F.C.; Moiane, B.; Pimenta, F.C.; Mucavele, H.; Chaúque, A.; Cossa, A.; Verani, J.R.; Tembe, N.; Carvalho, M.D.G.; et al. Invasive disease potential of Streptococcus pneumoniae serotypes before and after 10-valent pneumococcal conjugate vaccine introduction in a rural area, southern Mozambique. Vaccine 2019, 37, 7470–7477. [Google Scholar] [CrossRef]
- Vanderkooi, O.G.; Church, D.L.; MacDonald, J.; Zucol, F.; Kellner, J. Community-Based Outbreaks in Vulnerable Populations of Invasive Infections Caused by Streptococcus pneumoniae Serotypes 5 and 8 in Calgary, Canada. PLoS ONE 2011, 6, e28547. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Feikin, D.R.; Klugman, K.P. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 2005, 5, 83–93. [Google Scholar] [CrossRef]
- Hyams, C.; Camberlein, E.; Cohen, J.M.; Bax, K.; Brown, J.S. The Streptococcuspneumoniae Capsule Inhibits Complement Activity and Neutrophil Phagocytosis by Multiple Mechanisms. Infect. Immun. 2010, 78, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilley, R.P.; Orihuela, C.J. Pneumococci in biofilms are non-invasive: Implications on nasopharyngeal colonization. Front. Cell. Infect. Microbiol. 2014, 4, 163. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, M.J.; Norais, N.; Bensi, G.; Liberatori, S.; Capo, S.; Mora, M.; Scarselli, M.; Doro, F.; Ferrari, G.; Garaguso, I.; et al. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat. Biotechnol. 2006, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Abril, A.; Gascón, L.G.; Jiménez-Munguía, I.; Obando, I.; Rodríguez-Ortega, M.J. Another turn of the screw in shaving Gram-positive bacteria: Optimization of proteomics surface protein identification in Streptococcus pneumoniae. J. Proteom. 2012, 75, 3733–3746. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, M.J.; Luque, I.; Tarradas, C.; Bárcena, J.A. Overcoming function annotation errors in the Gram-positive pathogen Streptococcus suis by a proteomics-driven approach. BMC Genom. 2008, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Garibaldi, M.; Rodríguez-Ortega, M.J.; Mandanici, F.; Cardaci, A.; Midiri, A.; Papasergi, S.; Gambadoro, O.; Cavallari, V.; Teti, G.; Beninati, C. Immunoprotective activities of a Streptococcus suis pilus subunit in murine models of infection. Vaccine 2010, 28, 3609–3616. [Google Scholar] [CrossRef]
- Mandanici, F.; Gascón, L.G.; Garibaldi, M.; Olaya-Abril, A.; Luque, I.; Tarradas, C.; Mancuso, G.; Papasergi, S.; Bárcena, J.A.; Teti, G.; et al. A surface protein of Streptococcus suis serotype 2 identified by proteomics protects mice against infection. J. Proteom. 2010, 73, 2365–2369. [Google Scholar] [CrossRef]
- Gascón, L.G.; Luque, I.; Abril, A.O.; Jiménez-Munguía, I.; Orbegozo-Medina, R.A.; Peralbo, E.; Tarradas, C.; Rodríguez-Ortega, M.J. Exploring the pan-surfome of Streptococcus suis: Looking for common protein antigens. J. Proteom. 2012, 75, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, E.P.; Rodríguez-Franco, A.; Rodríguez-Ortega, M.J. Proteomic and Bioinformatic Analysis of Streptococcus suis Human Isolates: Combined Prediction of Potential Vaccine Candidates. Vaccines 2020, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Solis, N.; Larsen, M.R.; Cordwell, S.J. Improved accuracy of cell surface shaving proteomics in Staphylococcus aureus using a false-positive control. Proteomics 2010, 10, 2037–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis, N.; Cain, J.A.; Cordwell, S.J. Comparative analysis of Staphylococcus epidermidis strains utilizing quantitative and cell surface shaving proteomics. J. Proteom. 2016, 130, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Hebraud, M.; Chafsey, I.; Chambon, C.; Viala, D.; Torres, C.; Poeta, P.; Igrejas, G. Surfaceome and exoproteome of a clinical sequence type 398 methicillin resistant Staphylococcus aureus strain. Biochem. Biophys. Rep. 2015, 3, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Galán-Relaño, Á.; Gómez-Gascón, L.; Rodríguez-Franco, A.; Luque, I.; Huerta, B.; Tarradas, C.; Rodríguez-Ortega, M.J. Search of Potential Vaccine Candidates against Trueperella pyogenes Infections through Proteomic and Bioinformatic Analysis. Vaccines 2020, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Giefing, C.; Meinke, A.L.; Hanner, M.; Henics, T.; Minh, D.B.; Gelbmann, D.; Lundberg, U.; Senn, B.M.; Schunn, M.; Habel, A.; et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 2008, 205, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.F.; Marquart, M.E.; McDaniel, L.S. Localization of PcsB of Streptococcus pneumoniae and Its Differential Expression in Response to Stress. J. Bacteriol. 2007, 189, 4544–4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Munguía, I.; Calderón-Santiago, M.; Franco, A.R.; Priego-Capote, F.; Rodríguez-Ortega, M.J. Multi-omic profiling to assess the effect of iron starvation inStreptococcus pneumoniaeTIGR4. PeerJ 2018, 6, e4966. [Google Scholar] [CrossRef] [Green Version]
- Bensing, B.A.; Rubens, C.E.; Sullam, P.M. Genetic Loci of Streptococcus mitis That Mediate Binding to Human Platelets. Infect. Immun. 2001, 69, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siboo, I.R.; Bensing, B.A.; Sullam, P.M. Genomic Organization and Molecular Characterization of SM1, a Temperate Bacteriophage of Streptococcus mitis. J. Bacteriol. 2003, 185, 6968–6975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-C.; Lin, T.-L.; Lin, C.-M.; Wang, J.-T. Identification of PblB mediating galactose-specific adhesion in a successful Streptococcus pneumoniae clone. Sci. Rep. 2015, 5, 12265. [Google Scholar] [CrossRef] [Green Version]
- Tunjungputri, R.N.; Mobegi, F.M.; Cremers, A.; Jongh, C.E.v.d.G.-d.; Ferwerda, G.; Meis, J.F.; Roeleveld, N.; Bentley, S.D.; Pastura, A.S.; van Hijum, S.; et al. Phage-Derived Protein Induces Increased Platelet Activation and Is Associated with Mortality in Patients with Invasive Pneumococcal Disease. mBio 2017, 8, e01984-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Càmara, J.; Cubero, M.; Martín-Galiano, A.J.; García, E.; Grau, I.; Nielsen, J.B.; Worning, P.; Tubau, F.; Pallares, R.; Domínguez, M.A.; et al. Evolution of the β-lactam-resistant Streptococcus pneumoniae PMEN3 clone over a 30 year period in Barcelona, Spain. J. Antimicrob. Chemother. 2018, 73, 2941–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Ortega, M.J. “Shaving” Live Bacterial Cells with Proteases for Proteomic Analysis of Surface Proteins. Methods Mol. Biol. 2018, 1722, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Boekhorst, J.; Francke, C.; Siezen, R.J. LocateP: Genome-scale subcellular-location predictor for bacterial proteins. BMC Bioinform. 2008, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Mitsuwan, W.; Olaya-Abril, A.; Calderón-Santiago, M.; Munguía, I.J.; González-Reyes, J.A.; Priego-Capote, F.; Voravuthikunchai, S.P.; Rodríguez-Ortega, M.J. Integrated proteomic and metabolomic analysis reveals that rhodomyrtone reduces the capsule in Streptococcus pneumoniae. Sci. Rep. 2017, 7, 2715. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Rohde, M. Electron Microscopy to Study the Fine Structure of the Pneumococcal Cell. Methods Mol. Biol. 2019, 1968, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [Green Version]
- Fruzangohar, M.; Ebrahimie, E.; Ogunniyi, A.D.; Mahdi, L.; Paton, J.C.; Adelson, D.L. Comparative GO: A Web Application for Comparative Gene Ontology and Gene Ontology-Based Gene Selection in Bacteria. PLoS ONE 2013, 8, e58759. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Campbell, D.S.; Llinares, M.B.; Okuda, S.; Kawano, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2018, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaya-Abril, A.; González-Reyes, J.A.; Rodríguez-Ortega, M.J. Approaching In Vivo Models of Pneumococcus–Host Interaction: Insights into Surface Proteins, Capsule Production, and Extracellular Vesicles. Pathogens 2021, 10, 1098. https://doi.org/10.3390/pathogens10091098
Olaya-Abril A, González-Reyes JA, Rodríguez-Ortega MJ. Approaching In Vivo Models of Pneumococcus–Host Interaction: Insights into Surface Proteins, Capsule Production, and Extracellular Vesicles. Pathogens. 2021; 10(9):1098. https://doi.org/10.3390/pathogens10091098
Chicago/Turabian StyleOlaya-Abril, Alfonso, José A. González-Reyes, and Manuel J. Rodríguez-Ortega. 2021. "Approaching In Vivo Models of Pneumococcus–Host Interaction: Insights into Surface Proteins, Capsule Production, and Extracellular Vesicles" Pathogens 10, no. 9: 1098. https://doi.org/10.3390/pathogens10091098
APA StyleOlaya-Abril, A., González-Reyes, J. A., & Rodríguez-Ortega, M. J. (2021). Approaching In Vivo Models of Pneumococcus–Host Interaction: Insights into Surface Proteins, Capsule Production, and Extracellular Vesicles. Pathogens, 10(9), 1098. https://doi.org/10.3390/pathogens10091098






