Loop-Mediated Isothermal Amplification (LAMP) Assay for Detecting Burkholderia cepacia Complex in Non-Sterile Pharmaceutical Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. LAMP Primer Design
2.2. Bacterial Strains and DNA Preparation
2.3. LAMP and qPCR Assay
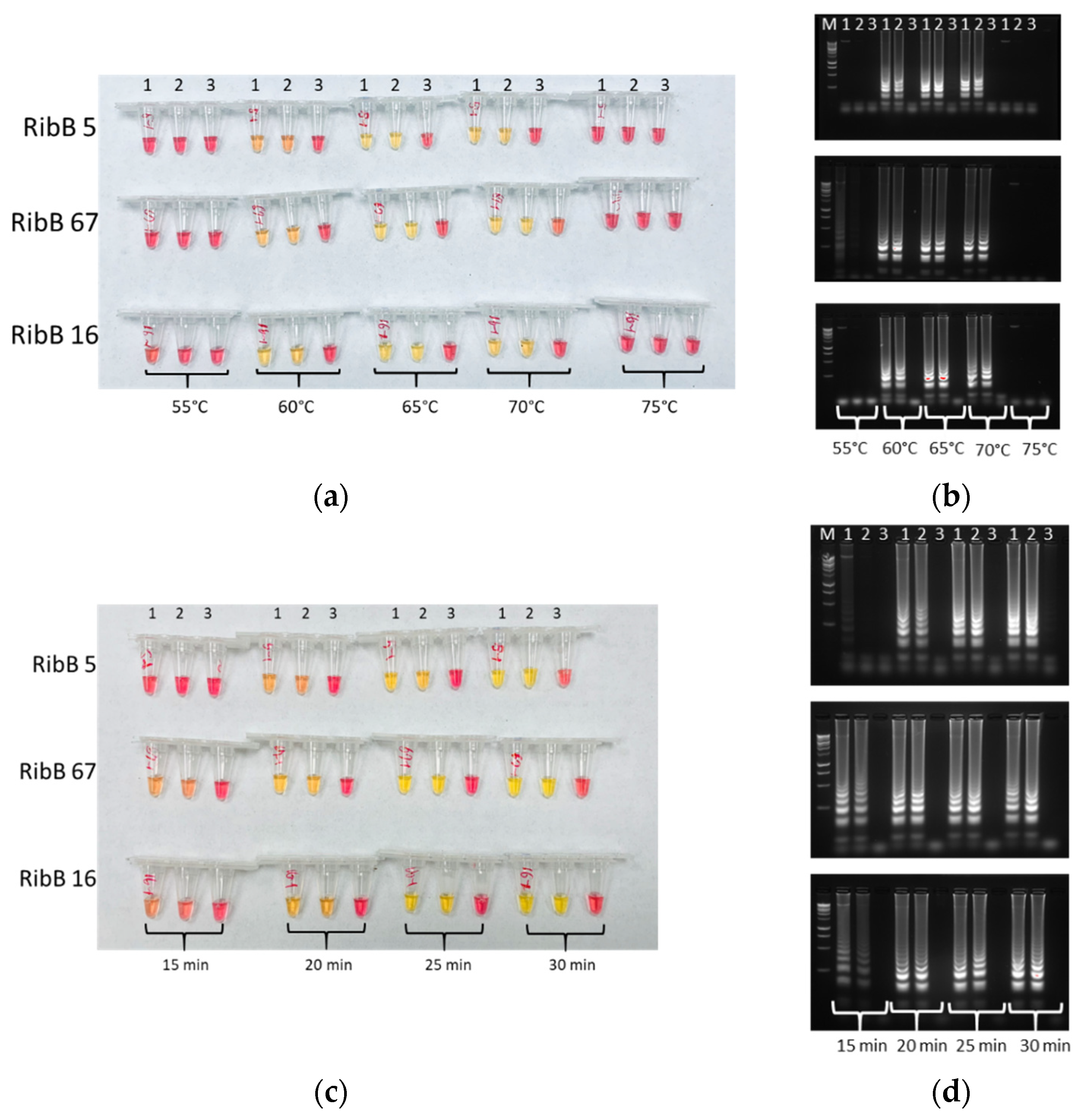
2.4. Optimization of LAMP
2.5. True-Positive Rate (Sensitivity) and True-Negative rate (Specificity) in Analysis Using LAMP
2.6. Limit of Detection (LOD) and Limit of Quantification (LOQ) of LAMP
2.7. Evaluation of LAMP in Autoclaved Nuclease-Free Water and Antiseptic Solutions
2.7.1. Autoclaved Nuclease-Free Water
2.7.2. Chlorhexidine Gluconate (CHX) and Benzalkonium Chloride (BZK)
3. Results
3.1. Primers Designed for LAMP
3.2. Optimal Conditions of LAMP
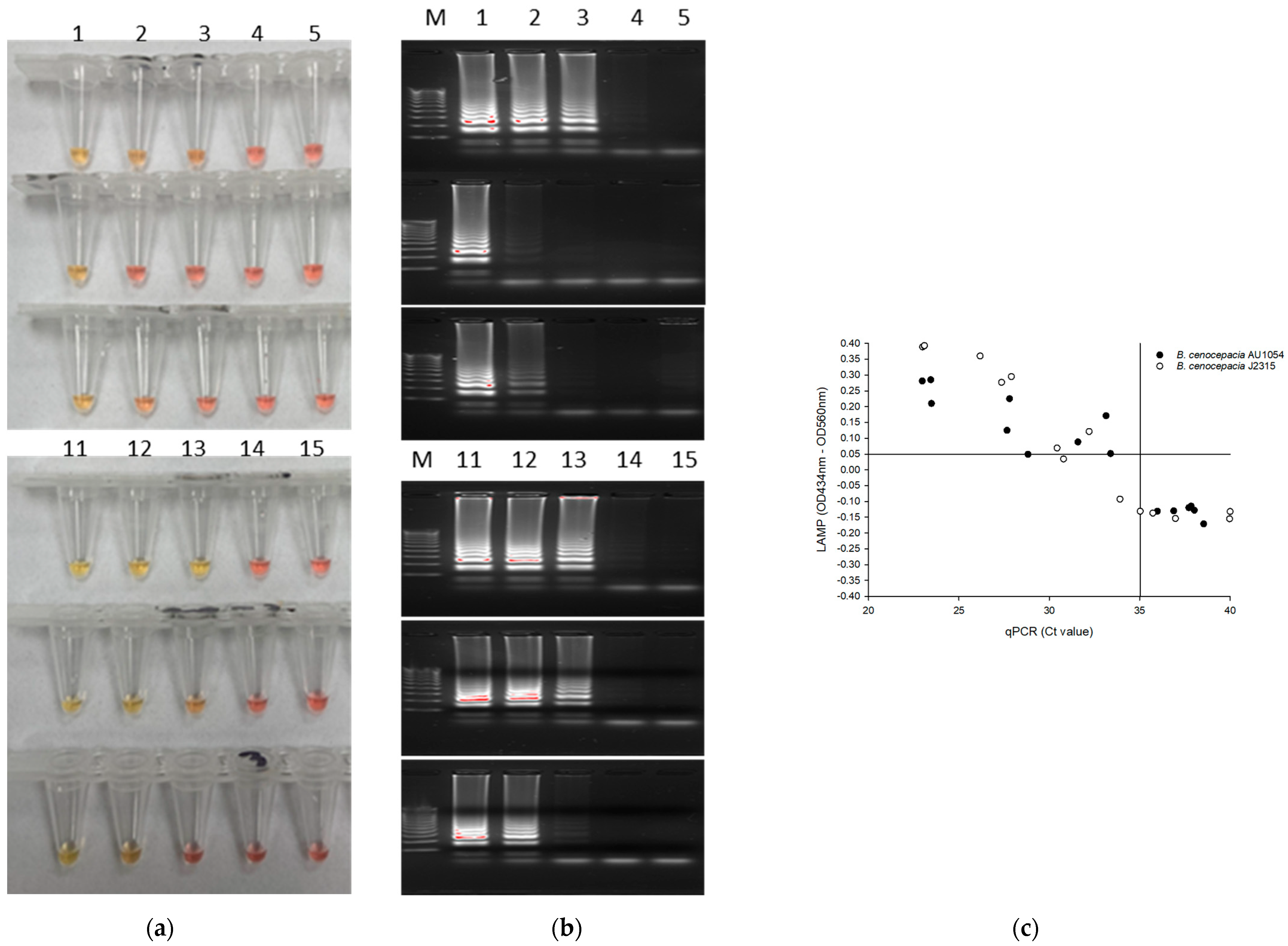
3.3. Specificity Analysis of LAMP
3.4. Limit of Quantification (LOQ) and Limit of Detection (LOD) of the LAMP Assay
3.5. Testing BCC Detection from Autoclaved Nuclease-Free Water, and Antiseptic Solutions with the LAMP Assay
3.5.1. Nuclease-Free Water
3.5.2. CHX
3.5.3. BZK
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Depoorter, E.; De Canck, E.; Peeters, C.; Wieme, A.D.; Cnockaert, M.; Zlosnik, J.E.A.; LiPuma, J.J.; Coenye, T.; Vandamme, P. Burkholderia cepacia complex taxon K: Where to split? Front. Microbiol. 2020, 11, 1594. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sa-Correia, I. Burkholderia cepacia complex bacteria: A feared contamination risk in water-based pharmaceutical products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef] [PubMed]
- LiPuma, J.J.; Dulaney, B.J.; McMenamin, J.D.; Whitby, P.W.; Stull, T.L.; Coenye, T.; Vandamme, P. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 1999, 37, 3167–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, L. Microbial diversity in pharmaceutical product recalls and environments. PDA J. Pharm. Sci. Technol. 2007, 61, 383–399. [Google Scholar]
- Sutton, S.; Jimenez, L. A review of reported recalls involving microbiological control 2004–2011 with emphasis on FDA considerations of “objectionable organisms”. Am. Pharm. Review. 2012, 15, 42. [Google Scholar]
- Jimenez, L. Analysis of FDA enforcement reports (2012–2019) to determine the microbial diversity in contaminated non-sterile and sterile Drugs. Am. Pharm. Review. 2019, 1–21. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/518912-Analysis-of-FDA-Enforcement-Reports-2012–2019-to-Determine-the-Microbial-Diversity-in-Contaminated-Non-Sterile-and-Sterile-Drugs/ (accessed on 17 July 2021).
- USP. <60> Microbiological Examination of Nonsterile Products-Tests for Burkholderia Cepacia Complex. 2018. Available online: http://www.usppf.com/pf/pub/data/v445/CHA_IPR_445_c60.xml (accessed on 17 July 2021).
- FDA. Pharmaceutical Microbiology Manual. 2020. Available online: https://www.fda.gov/media/88801/download. (accessed on 17 July 2021).
- Ahn, Y.; Kim, J.M.; Ahn, H.; Lee, Y.J.; LiPuma, J.J.; Hussong, D.; Cerniglia, C.E. Evaluation of liquid and solid culture media for the recovery and enrichment of Burkholderia cenocepacia from distilled water. J. Ind. Microbiol. Biotechnol. 2014, 41, 1109–1118. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, U.J.; Lee, Y.J.; LiPuma, J.J.; Hussong, D.; Marasa, B.; Cerniglia, C.E. Oligotrophic media compared with a tryptic soy agar or broth for the recovery of Burkholderia cepacia complex from different storage temperatures and culture conditions. J. Microbiol. Biotechnol. 2019, 29, 1495–1505. [Google Scholar] [CrossRef]
- Ahn, Y.; Gibson, B.; Williams, A.; Alusta, P.; Buzatu, D.A.; Lee, Y.J.; LiPuma, J.J.; Hussong, D.; Marasa, B.; Cerniglia, C.E. A comparison of culture-based, real-time PCR, droplet digital PCR and flow cytometric methods for the detection of Burkholderia cepacia complex in nuclease-free water and antiseptics. J. Ind. Microbiol. Biotechnol. 2020, 47, 475–484. [Google Scholar] [CrossRef]
- Attia, M.A.; Ali, A.E.; Essam, T.M.; Amin, M.A. Direct detection of Burkholderia cepacia in susceptible pharmaceutical products using semi-nested PCR. PDA J. Pharm. Sci. Technol. 2016, 70, 99–108. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Pitondo-Silva, A.; Braz, V.S.; Gallo, I.F.L.; Stehling, E.G. Evaluation of different molecular and phenotypic methods for identification of environmental Burkholderia cepacia complex. World J. Microbiol. Biotechnol. 2019, 35, 39. [Google Scholar] [CrossRef]
- Jimenez, L.; Jashari, T.; Vasquez, J.; Zapata, S.; Bochis, J.; Kulko, M.; Ellman, V.; Gardner, M.; Choe, T. Real-time PCR detection of Burkholderia cepacia in pharmaceutical products contaminated with low levels of bacterial contamination. PDA J. Pharm. Sci. Technol. 2018, 72, 73–80. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Bischof, J.; Byrne, S.K.; Radomski, C.; Davies, J.E.; Av-Gay, Y.; Vandamme, P. DNA-Based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 2000, 38, 3165–3173. [Google Scholar] [CrossRef] [Green Version]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic. Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao Thi, V.L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Tanner, N.A.; Zhang, Y.; Evans, T.C., Jr. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques 2015, 58, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Augustine, R.; Hasan, A.; Das, S.; Ahmed, R.; Mori, Y.; Notomi, T.; Kevadiya, B.D.; Thakor, A.S. Loop-mediated isothermal amplification (LAMP): A rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology 2020, 9, 182. [Google Scholar] [CrossRef]
- Becherer, L.; Bakheit, M.; Frischmann, S.; Stinco, S.; Borst, N.; Zengerle, R.; von Stetten, F. Simplified real-time multiplex detection of loop-mediated isothermal amplification using novel mediator displacement probes with universal reporters. Anal. Chem. 2018, 90, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Meumann, E.; Thanwisai, A.; Limmathurotsakul, D.; Wuthiekanun, V.; Wannapasni, S.; Tumapa, S.; Day, N.P.; Peacock, S.J. Loop-mediated isothermal amplification method targeting the TTS1 gene cluster for detection of Burkholderia pseudomallei and diagnosis of melioidosis. J. Clin. Microbiol. 2008, 46, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzai, S.; Safi, S.; Mossavari, N.; Afshar, D.; Bolourchian, M. Development of a loop-mediated isothermal amplification assay for rapid detection of Burkholderia mallei. Cell Mol. Biol. 2016, 62, 32–36. [Google Scholar]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): Expansion of its practical application as a tool to achieve universal health coverage. J. Infect. Chemother. 2020, 26, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Pal, V.; Tripathi, N.K.; Goel, A.K. A real-time loop mediated isothermal amplification assay for molecular detection of Burkholderia mallei, the aetiological agent of a zoonotic and re-emerging disease glanders. Acta. Trop. 2019, 194, 189–194. [Google Scholar] [CrossRef]
- FDA. Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests-Guidance for Industry and FDA Staff; Center for Devices and Radiological Health: Silver Spring, MD, USA, 2007. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-guidance-reporting-results-studies-evaluating-diagnostic-tests-guidance-industry-and-fda (accessed on 17 July 2021).
- Price, E.P.; Dale, J.L.; Cook, J.M.; Sarovich, D.S.; Seymour, M.L.; Ginther, J.L.; Kaufman, E.L.; Beckstrom-Sternberg, S.M.; Mayo, M.; Kaestli, M.; et al. Development and validation of Burkholderia pseudomallei-specific real-time PCR assays for clinical, environmental or forensic detection applications. PLoS ONE 2012, 7, e37723. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.; Jones, K.N.; Whaley, E.M.; Koy, T.H.; Revell, P.A.; Taylor, R.S.; Bernhardt, M.B.; Wagner, J.L.; Dunn, J.J.; LiPuma, J.J.; et al. An outbreak of Burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control. Hosp. Epidemiol. 2017, 38, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Vanlaere, E.; Baldwin, A.; Gevers, D.; Henry, D.; De Brandt, E.; LiPuma, J.J.; Mahenthiralingam, E.; Speert, D.P.; Dowson, C.; Vandamme, P. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int. J. Syst Evol. Microbiol. 2009, 59, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.; Saxena, A.; Singh, S.; Goel, A.K.; Kumar, J.S.; Parida, M.M.; Rai, G.P. Development of a real-time loop-mediated isothermal amplification assay for detection of Burkholderia mallei. Transbound. Emerg. Dis. 2018, 65, e32–e39. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-mediated isothermal amplification (LAMP)—review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef] [Green Version]
- Gadkar, V.J.; Goldfarb, D.M.; Gantt, S.; Tilley, P.A.G. Real-time detection and monitoring of loop mediated amplification (LAMP) reaction using self-quenching and de-quenching fluorogenic probes. Sci. Rep. 2018, 8, 5548. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Li, X.; Liu, W.; Lu, C.; Lu, X.; Ma, L.; Li, Y.Y.; Wei, C. GLAPD: Whole genome based LAMP primer design for a set of target genomes. Front. Microbiol. 2019, 10, 2860. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.; Babu, D.; Reiz, B.; Whittal, R.; Suh, L.Y.K.; Siraki, A.G. Caution for the routine use of phenol red - It is more than just a pH indicator. Chem. Biol. Interact. 2019, 310, 108739. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Huang, C.F.; Shen, L.J.; Wang, H.J.; Lin, C.Y.; Wu, F.L. Formulation and stability of an extemporaneous 0.02% chlorhexidine digluconate ophthalmic solution. J. Formos. Med. Assoc. 2015, 114, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. The Use of Loop-Mediated Isothermal Amplification (TB-LAMP) for the Diagnosis of Pulmonary Tuberculosis: Policy Guidance. 2016. Available online: https://apps.who.int/iris/handle/10665/249154 (accessed on 17 July 2021).
- Nguyen, A.V.; Orlofsky, A.; Pubill, K.; Tawde, M.; Li, G.; Mata, D.; Bermudes, O.; Fernandez, M.; Santana, J.; Kim, W.; et al. Loop-mediated isothermal amplification (LAMP) as a rapid, affordable and effective tool to involve students in undergraduate research. Front. Microbiol. 2020, 11, 603381. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequences (5′-3′) | Length (bp) | ||
|---|---|---|---|---|
| qPCR | LAMP | |||
| RibB5 | RibB5-F | RibB5 F3 | GGCCGGATGGTGATCCT | 17 |
| RibB5-R | RibB5 B3 | GTCATCAGCGGCAGGTG | 17 | |
| RibB5 FIP | TCCGGTGTGACGAATTCGGC- CGAAGAAGACCGCGAAAACG | 40 | ||
| RibB5 BIP | AACTTCATGGCCAAGTACGGCC-AAGCTGCTTGCAGCGTT | 39 | ||
| RibB5 LF | GATCACGAGGTCGCCCT | 17 | ||
| RibB5 LB | CGGCCTGGTTTGTCTGA | 17 | ||
| RibB67 | RibB67-F | RibB67 F3 | GCGAYACGAAGGAACAYCTG | 20 |
| RibB67-R | RibB67 B3 | CGTAGCCGGACATGCTG | 17 | |
| RibB67 FIP | TGAAATCGACCGGCCGGC-TCTTCAAGGCGTTCGACGAG | 38 | ||
| RibB67 BIP | AGATCCTGCGCGATGTCGG-CCAGCTTGCGCGGATTC | 36 | ||
| RibB67 LF | TGAGCGCAGCGGCCTTTTCTT | 21 | ||
| RibB67 LB | TCGGCAAGATGCAGGTGC | 18 | ||
| RibB16 | RibB16-F | RibB16 F3 | ARGGCGACCTCGTGATC | 17 |
| RibB16-R | RibB16 B3 | CGAGATGCCGGTCGTCAC | 18 | |
| RibB16 FIP | TCAGACAAAYCAGGCCGCG-CGTCACACCGGAAGCGAT | 37 | ||
| RibB16 BIP | ACGCTGCAAGCAGCTTCACC-CGATGCTGACCGTGAACG | 38 | ||
| RibB16 LF | CCGTACTTGGCCATGAAGTT | 20 | ||
| RibB16 LB | GCTGATGACCTACCGCAAC | 19 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daddy Gaoh, S.; Kweon, O.; Lee, Y.-J.; LiPuma, J.J.; Hussong, D.; Marasa, B.; Ahn, Y. Loop-Mediated Isothermal Amplification (LAMP) Assay for Detecting Burkholderia cepacia Complex in Non-Sterile Pharmaceutical Products. Pathogens 2021, 10, 1071. https://doi.org/10.3390/pathogens10091071
Daddy Gaoh S, Kweon O, Lee Y-J, LiPuma JJ, Hussong D, Marasa B, Ahn Y. Loop-Mediated Isothermal Amplification (LAMP) Assay for Detecting Burkholderia cepacia Complex in Non-Sterile Pharmaceutical Products. Pathogens. 2021; 10(9):1071. https://doi.org/10.3390/pathogens10091071
Chicago/Turabian StyleDaddy Gaoh, Soumana, Ohgew Kweon, Yong-Jin Lee, John J. LiPuma, David Hussong, Bernard Marasa, and Youngbeom Ahn. 2021. "Loop-Mediated Isothermal Amplification (LAMP) Assay for Detecting Burkholderia cepacia Complex in Non-Sterile Pharmaceutical Products" Pathogens 10, no. 9: 1071. https://doi.org/10.3390/pathogens10091071
APA StyleDaddy Gaoh, S., Kweon, O., Lee, Y.-J., LiPuma, J. J., Hussong, D., Marasa, B., & Ahn, Y. (2021). Loop-Mediated Isothermal Amplification (LAMP) Assay for Detecting Burkholderia cepacia Complex in Non-Sterile Pharmaceutical Products. Pathogens, 10(9), 1071. https://doi.org/10.3390/pathogens10091071





