A Comparison of Pathogenicity and Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with Mycoplasma hyopneumoniae and PCV2
Abstract
:1. Introduction
2. Results
2.1. Clinical Signs
2.2. Growth Performance
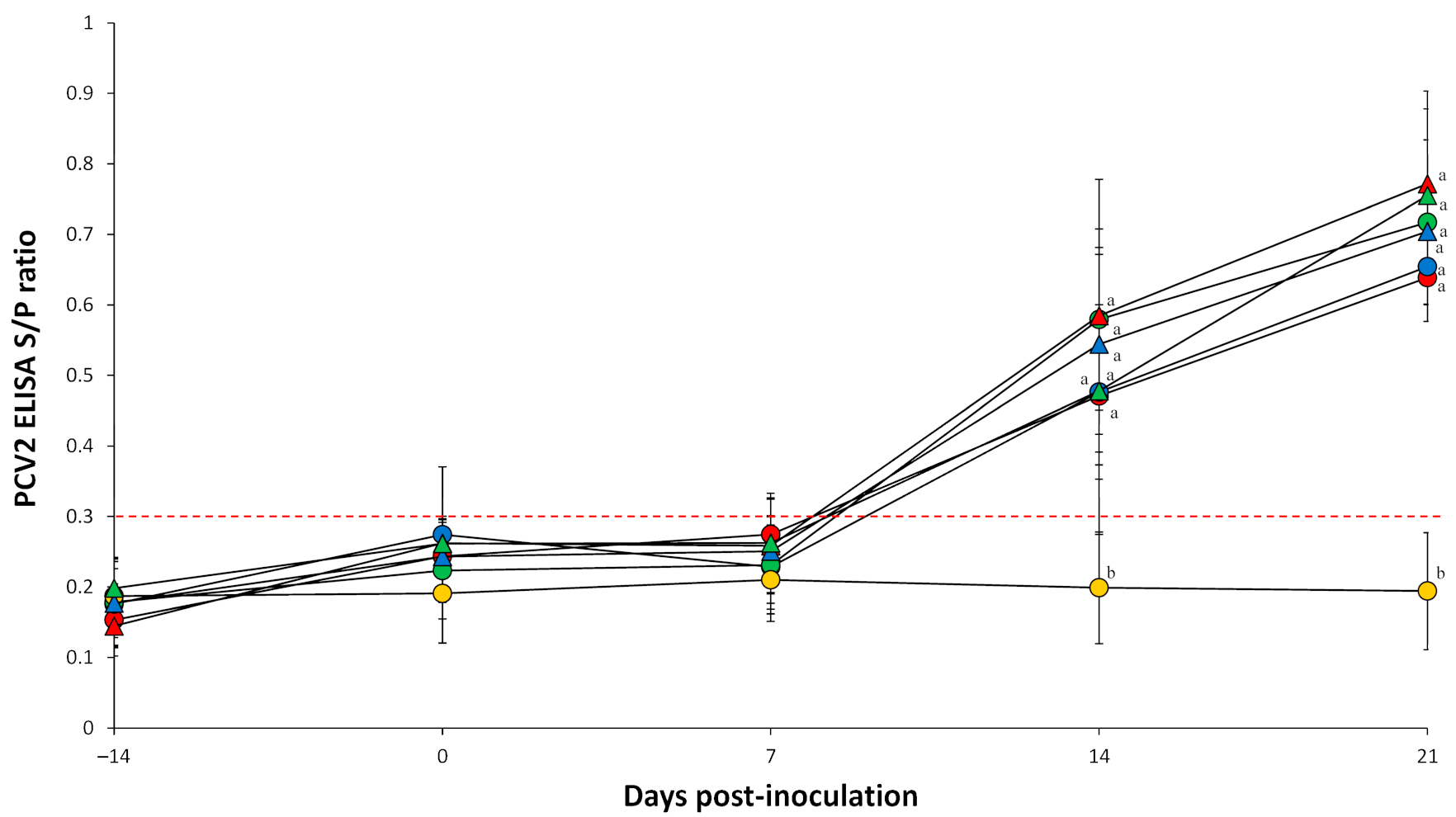
2.3. Enzyme-Linked Immunosorbent Assay
2.4. Quantification of PCV2 DNA in Blood
2.5. Quantification of Mycoplasma hyopneumoniae DNA in Larynx
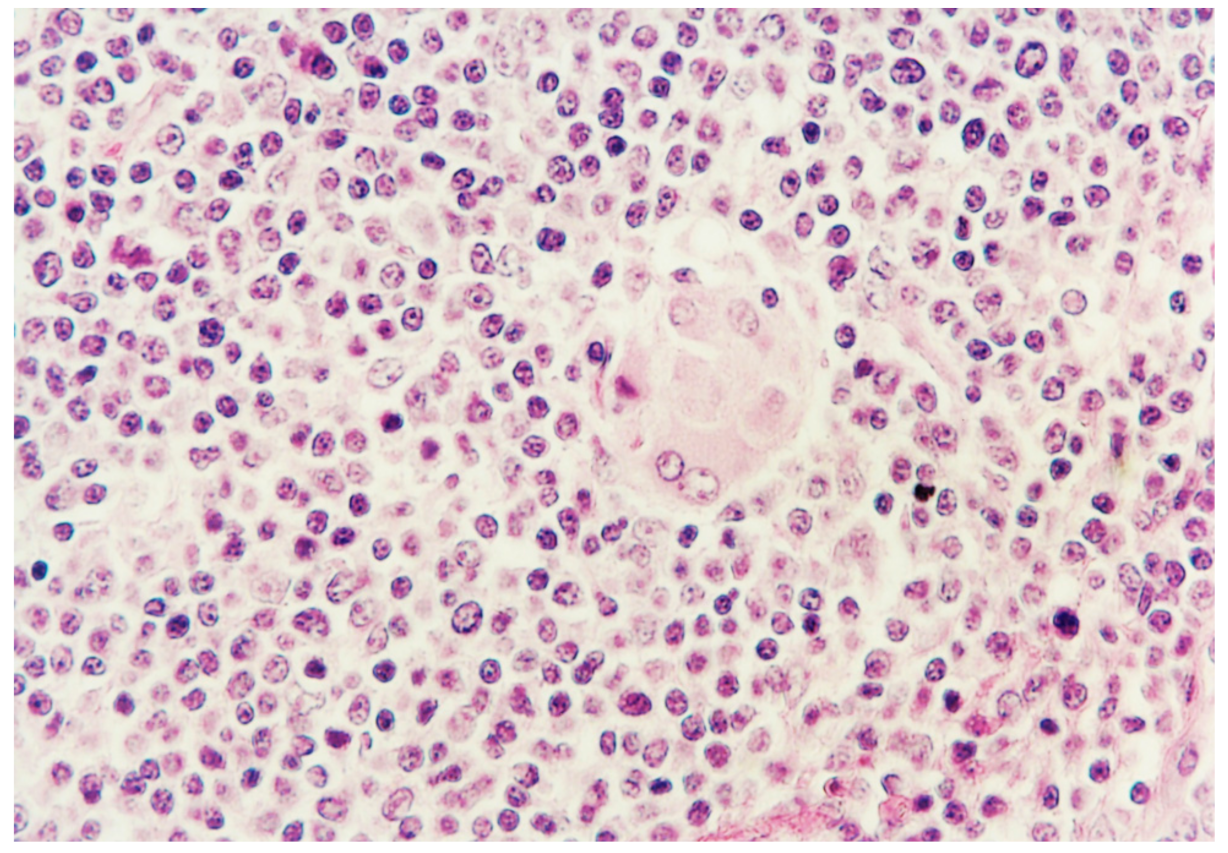
2.6. Histopathology
2.7. Immunohistochemistry
2.8. Correlation between PCV2 Viremia and Lymphoid Lesions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Clinical Observation
4.4. Growth Performance
4.5. Quantification of PCV2 DNA
4.6. Quantification of M. hyopneumoniae DNA
4.7. Serology
4.8. Histopathology
4.9. Immunohistochemistry
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016, 212, 1–6. [Google Scholar] [CrossRef]
- Kim, J.; Chung, H.-K.; Chae, C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 2003, 166, 251–256. [Google Scholar] [CrossRef]
- Chae, C. Postweaning multisystemic wasting syndrome: A review of aetiology, diagnosis and pathology. Vet. J. 2004, 168, 41–49. [Google Scholar] [CrossRef]
- Chae, C. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 2005, 169, 326–336. [Google Scholar] [CrossRef]
- Maes, D.; Segales, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, F.J.; Halbur, P.G.; Opriessnig, T.; Sorden, S.D.; Villar, D.; Janke, B.H.; Yaeger, M.J.; Larson, D.J.; Schwartz, K.J.; Yoon, K.J.; et al. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting. J. Vet. Diagn. Investig. 2002, 14, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Opriessnig, T.; Thacker, E.L.; Yu, S.; Fenaux, M.; Meng, S.J.; Halbur, P.G. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and Porcine Circovirus type 2. Vet. Pathol. 2004, 41, 624–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzo, G.; Segalés, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, B.; Vena, M.M.; Cavalier, A.; Lannic, J.L.; Gouranton, J.; Kobisch, M. Electron microscopic observation of the respiratory tract of SPF piglets inoculated with Mycoplasma hyopneumoniae. Vet. Microbiol. 1992, 30, 329–341. [Google Scholar] [CrossRef]
- Mebus, C.A.; Underdahl, N.R. Scanning electron microscopy of trachea and bronchi from gnotobiotic pigs inoculated with Mycoplasma hyopneumoniae. Am. J. Vet. Res. 1977, 38, 1249–1254. [Google Scholar]
- Le Carrou, J.; Laurentie, M.; Kobisch, M.; Gautier-Bouchardon, A.V. Persistence of Mycoplasma hyopneumoniae in experimentally infected pigs after marbofoxacin treatment and detection of mutations in the parC gene. Antimicrob. Agents Chemother. 2006, 50, 1959–1966. [Google Scholar] [CrossRef] [Green Version]
- Marois, C.; Le Carrou, J.; Kobisch, M.; Gautier-Bouchardon, A.V. Isolation of Mycoplasma hyopneumoniae from different sampling sites in experimentally infected and contact SPF piglets. Vet. Microbiol. 2007, 120, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Rodriguez, F.; Quesada, O.; Poveda, J.B.; Fernandez, A.; Lorenzo, H. Immunohistochemical detection of interleukin-12 and interferon-γ in pigs experimentally infected with Mycoplasma hyopneumoniae. J. Comp. Pathol. 2007, 136, 79–82. [Google Scholar] [CrossRef]
- Meerts, P.; Misinzo, G.; Nauwynck, H.J. Enhancement of porcine circovirus type 2 replication in porcine cell lines by IFN-γ before and after treatment and by IFN-α after treatment. J. Interferon Cytokine Res. 2005, 25, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, M.; Opriessnig, T.; Halbur, P.G.; Elvinger, F.; Meng1, X.J. Two Amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J. Virol. 2004, 78, 13440–13446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Fu, Y.; Wang, Y.; Lu, Y.; Wei, Y.; Tang, Q.; Fan, P.; Liu, J.; Zhang, L.; Zhang, F.; et al. A porcine circovirus type 2 (PCV2) mutant with 234 amino acids in capsid protein showed more virulence in vivo, compared with classical PCV2a/b strain. PLoS ONE 2012, 7, e41463. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Chae, C. The prevalence of porcine circovirus type 2e (PCV2e) in Korean slaughter pig lymph nodes when compared with other PCV2 genotypes. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yin, S.; Shang, Y.; Liu, B.; Yuan, L.; Zafae Khan, M.U.; Liu, X.; Xai, J. Phylogenetic and genetic variation analyses of porcine circovirus type 2 isolated from China. Transbound. Emerg. Dis. 2018, 65, e383–e392. [Google Scholar] [CrossRef]
- Dinh, P.X.; Nguyen, M.N.; Nguyen, H.T.; Tran, V.H.; Tran, Q.D.; Dang, K.H.; Le, H.T.; Nguyen, N.T.T.; Nguyen, T.T.; Do, D.T. Porcine circovirus genotypes and their copathogens in pigs with respiratory disease in southern provinces of Vietnam. Arch. Virol. 2021, 166, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Thangthamniyom, N.; Sangthong, P.; Poolperm, P.; Thanantong, N.; Boonsoongnern, A.; Hansoongnern, P.; Semkum, P.; Petcharat, N.; Lekcharoensuk, P. Genetic diversity of porcine circovirus type 2 (PCV2) in Thailand during 2009–2015. Vet. Microbiol. 2017, 208, 239–246. [Google Scholar] [CrossRef]
- Tsai, G.-T.; Lin, Y.-C.; Lin, W.-H.; Lin, J.-H.; Chiou, M.-T.; Liu, H.-F.; Lin, C.-N. Phylogeographic and genetic characterization of porcine circovirus type 2 in Taiwan from 2001–2017. Sci. Rep. 2019, 9, 10782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.; Kang, I.; Oh, T.; Yang, S.; Park, K.H.; Min, K.-D.; Ham, H.J.; Chae, C. Comparative study of the virulence of 3 major Korean porcine circovirus type 2 genotypes (a, b, and d). Can. J. Vet. Res. 2020, 44, 235–240. [Google Scholar]
- Opriessnig, T.; Xiao, C.-T.; Gerber, P.F.; Halbur, P.G.; Matzinger, S.R.; Meng, X.-J. Mutant USA strain of porcine circovirus type 2 (mPCV2) exhibits similar virulence to the classical PCV2a and PCV2b strains in caesarean-derived, colostrum-deprived pigs. J. Gen. Virol. 2014, 95, 2495–2503. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Park, C.; Choi, K.; Chae, C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet. Microbiol. 2015, 177, 43–52. [Google Scholar] [CrossRef]
- Gagnon, C.A.; Del Castillo, J.R.E.; Music, N.; Fontaine, G.; Harel, J.; Tremblay, D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J. Vet. Diagn. Investig. 2008, 20, 545–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubosson, C.R.; Conzelmann, C.; Miserez, R.; Boerlin, P.; Frey, J.; Zimmermann, W.; Häni, H.; Kuhnert, P. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet. Microbiol. 2004, 102, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wasilk, A.; Callahan, J.D.; Christopher-Hennings, J.; Gay, T.A.; Fang, Y.; Dammen, M.; Reos, M.E.; Torremorell, M.; Polson, D.; Mellencamp, M.; et al. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J. Clin. Microbiol. 2004, 42, 4453–4461. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Choi, C.; Chae, C. Chronologic localization of Mycoplasma hyopneumoniae in experimentally infected pigs. Vet. Pathol. 2002, 39, 584–587. [Google Scholar] [CrossRef] [Green Version]
- Beaver, B.V.; Reed, W.; Leary, S.; McKiernan, B.; Bain, F.; Schultz, R.; Bennet, B.T.; Pascoe, P.; Shull, E.; Cork, L.C.; et al. 2000 Report of the AVMA panel on euthanasia. J. Am. Vet. Med. Assoc. 2001, 218, 669–696. [Google Scholar]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.-J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chae, C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J. Comp. Pathol. 2004, 131, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.H.; Han, K.; Seo, H.W.; Oh, Y.; Park, C.; Kang, I.; Chae, C. Comparative efficacy of commercial Mycoplasma hyopneumoniae and porcine circovirus 2 (PCV2) vaccines in pigs experimentally infected with M. hyopneumoniae and PCV2. Vaccine 2011, 29, 3206–3212. [Google Scholar] [CrossRef] [PubMed]



| Body Weight (kg) | ADWG (Gram/Pig/Day) | ||
|---|---|---|---|
| Groups | 28 Days Old (−14 dpi) | 63 Days Old (21 dpi) | between 28 and 63 Days Old |
| M. hyopneumoniae/PCV2a | 6.47 ± 0.40 | 14.92 ± 0.61 a | 241.43 ± 21.44 a |
| M. hyopneumoniae/PCV2b | 6.50 ± 0.24 | 15.02 ± 0.63 a | 243.33 ± 25.39 a |
| M. hyopneumoniae/PCV2d | 6.42 ± 0.35 | 14.80 ± 1.07 a | 239.52 ± 36.96 a |
| PCV2a | 6.47 ± 0.34 | 17.00 ± 0.93 b | 300.95 ± 27.62 b |
| PCV2b | 6.28 ± 0.38 | 16.97 ± 0.97 b | 305.24 ± 25.26 b |
| PCV2d | 6.38 ± 0.39 | 16.93 ± 1.01 b | 301.43 ± 21.51 b |
| Negative control | 6.53 ± 0.32 | 18.02 ± 0.75 b | 328.10 ± 18.73 b |
| Groups | Microscopic Lymphoid Lesion Scores (Ranged from 0 to 5) | PCV2-Antigen Positive Cells Within Lymphoid Lesion |
|---|---|---|
| M. hyopneumoniae/PCV2a | 3.10 ± 0.28 a | 34.61 ± 6.78 a |
| M. hyopneumoniae/PCV2b | 3.13 ± 0.45 a | 34.83 ± 6.46 a |
| M. hyopneumoniae/PCV2d | 3.87 ± 0.50 b | 45.39 ± 4.88 b |
| PCV2a | 1.80 ± 0.25 c | 22.17 ± 4.57 c |
| PCV2b | 1.73 ± 0.50 c | 21.28 ± 6.33 c |
| PCV2d | 1.77 ± 0.34 c | 22.72 ± 7.17 c |
| Negative control | 0 ± 0 | 0 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, T.; Suh, J.; Park, K.H.; Yang, S.; Cho, H.; Chae, C. A Comparison of Pathogenicity and Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with Mycoplasma hyopneumoniae and PCV2. Pathogens 2021, 10, 979. https://doi.org/10.3390/pathogens10080979
Oh T, Suh J, Park KH, Yang S, Cho H, Chae C. A Comparison of Pathogenicity and Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with Mycoplasma hyopneumoniae and PCV2. Pathogens. 2021; 10(8):979. https://doi.org/10.3390/pathogens10080979
Chicago/Turabian StyleOh, Taehwan, Jeongmin Suh, Kee Hwan Park, Siyeon Yang, Hyejean Cho, and Chanhee Chae. 2021. "A Comparison of Pathogenicity and Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with Mycoplasma hyopneumoniae and PCV2" Pathogens 10, no. 8: 979. https://doi.org/10.3390/pathogens10080979
APA StyleOh, T., Suh, J., Park, K. H., Yang, S., Cho, H., & Chae, C. (2021). A Comparison of Pathogenicity and Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with Mycoplasma hyopneumoniae and PCV2. Pathogens, 10(8), 979. https://doi.org/10.3390/pathogens10080979






