Implementation of Adenovirus-Mediated Pulmonary Expression of Human ACE2 in HLA Transgenic Mice Enables Establishment of a COVID-19 Murine Model for Assessment of Immune Responses to SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Results
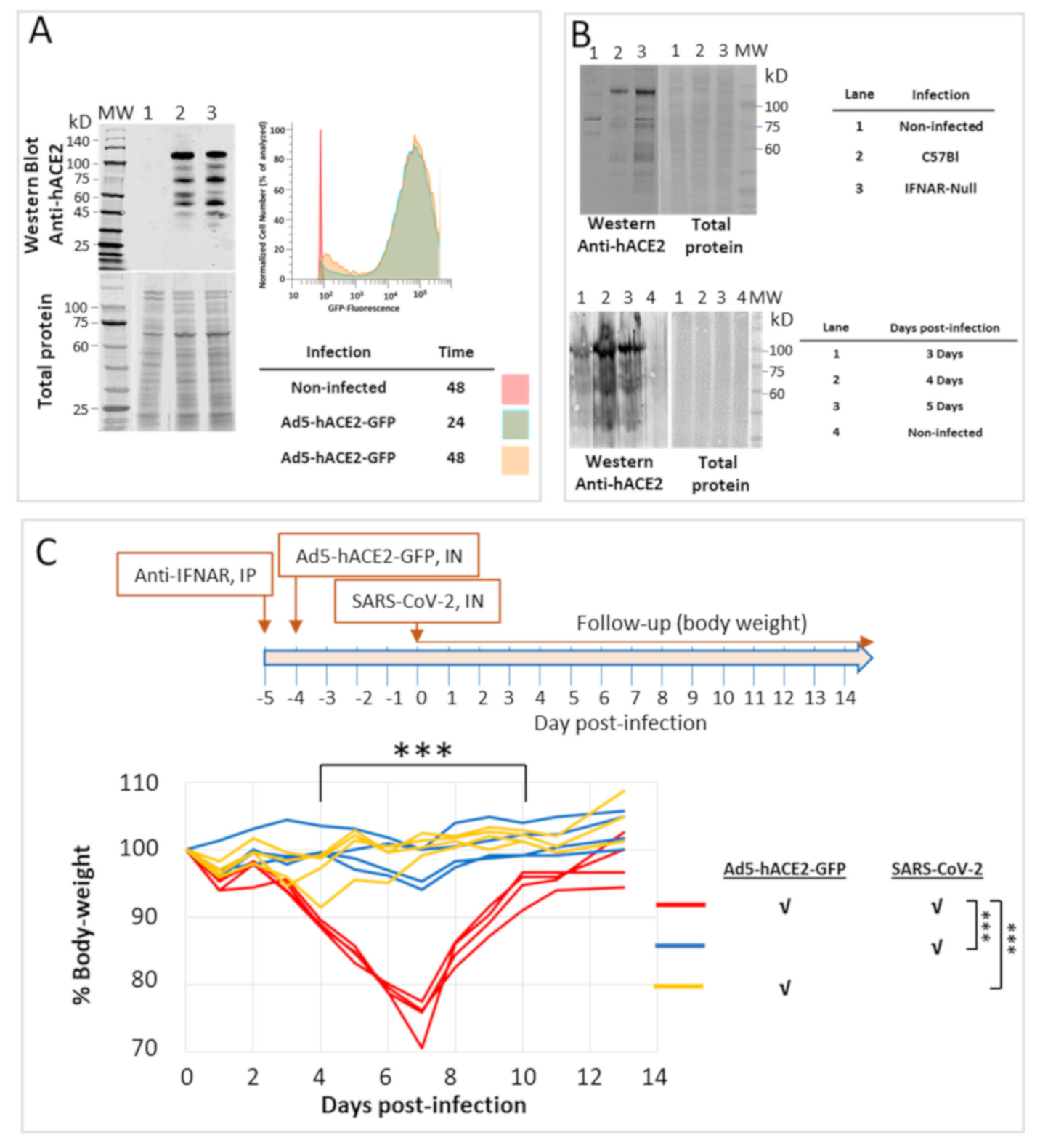
2.1. Transduction of HEK-293 Cells and Intranasal Infection of C57BL/6 Mice with an Adenovirus-hACE2 Vector Result in Expression of hACE2
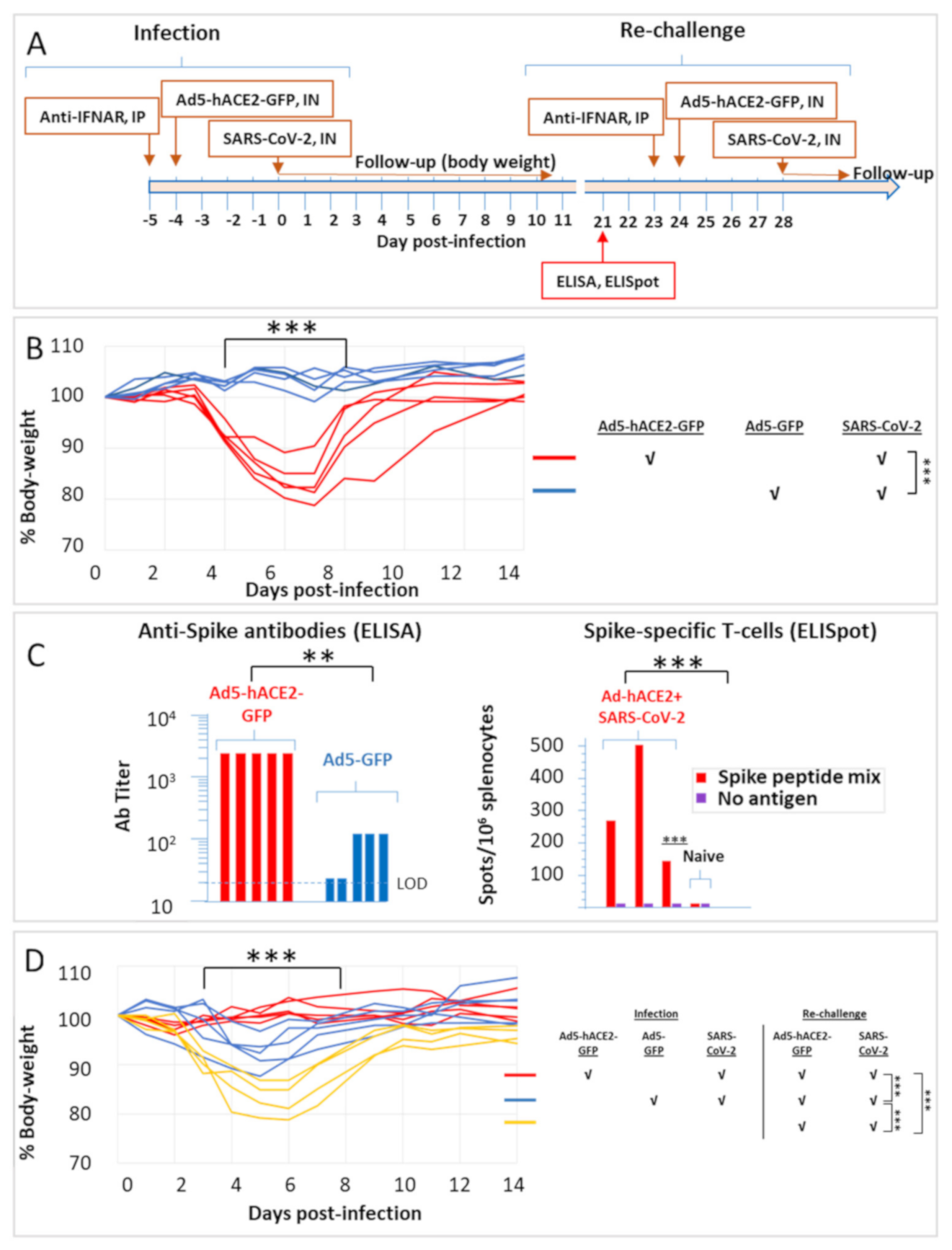
2.2. SARS-CoV-2 Infection of Ad5-hACE2-Transduced HHD and HLA-A2.1 Humanized Transgenic Mice
3. Materials and Methods
3.1. Cells and Virus Strains
3.2. Transduction and Harvest of of Cells for Analysis
3.3. Experimental Animals
3.4. Infection of Animals
3.5. Antibodies
3.6. Collection of Lungs for Evaluation of ACE2 Expression by SDS-PAGE and Western Blot Analysis
3.7. ELISA and ELISPOT
3.8. Flow Cytometry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X. Domains and functions of spike protein in Sars-Cov-2 in the context of vaccine design. Viruses 2021, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, L. ACE2 partially dictates the host range and tropism of SARS-CoV-2. Comput. Struct. Biotechnol. J. 2020, 18, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Noy-Porat, T.; Makdasi, E.; Alcalay, R.; Mechaly, A.; Levy, Y.; Bercovich-Kinori, A.; Zauberman, A.; Tamir, H.; Yahalom-Ronen, Y.; Israeli, M.; et al. A panel of human neutralizing mAbs targeting SARS-CoV-2 spike at multiple epitopes. Nat. Commun. 2020, 11, 4303. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.; Noy-Porat, T.; Mechaly, A.; Makdasi, E.; Levy, Y.; Alcalay, R.; Falach, R.; Aftalion, M.; Epstein, E.; Gur, D.; et al. Post-exposure protection of SARS-CoV-2 lethal infected K18-hACE2 transgenic mice by neutralizing human monoclonal antibody. Nat. Commun. 2021, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Case, J.B.; Winkler, E.S.; Thackray, L.B.; Kafai, N.M.; Bailey, A.L.; McCune, B.T.; Fox, J.M.; Chen, R.E.; Alsoussi, W.B.; et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 2020, 182, 744–753.e4. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhuang, Z.; Zheng, J.; Li, K.; Wong, R.L.; Liu, D.; Huang, J.; He, J.; Zhu, A.; Zhao, J.; et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 2020, 182, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Matsui, M. Identification of HLA-A*02:01-restricted candidate epitopes derived from the non-structural polyprotein 1a of SARS-CoV-2 that may be natural targets of CD8+) T cell recognition in vivo. J. Virol. 2020, 95, e01837-20. [Google Scholar] [CrossRef]
- Kotturi, M.F.; Assarsson, E.; Peters, B.; Grey, H.; Oseroff, C.; Pasquetto, V.; Sette, A. Of mice and humans: How good are HLA transgenic mice as a model of human immune responses? Immunome Res. 2009, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascolo, S. HLA class I transgenic mice: Development, utilisation and improvement. Expert Opin. Biol. Ther. 2005, 5, 919–938. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Marchesini, D.; Furze, J.; Sherman, L.A.; Chesnut, R.W. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J. Exp. Med. 1991, 173, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Bar-Haim, E.; Rotem, S.; Elia, U.; Bercovich-Kinori, A.; Israeli, M.; Cohen-Gihon, I.; Israeli, O.; Erez, N.; Achdout, H.; Zauberman, A.; et al. Early diagnosis of pathogen infection by cell-based activation immunoassay. Cells 2019, 8, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitlaru, T.; Bar-Haim, E.; Bar-On, L.; Rotem, S.; Cohen, H.; Elia, U.; Gur, D.; Aftalion, M.; Alkalay, R.; Makdasi, E.; et al. Implementation of Adenovirus-Mediated Pulmonary Expression of Human ACE2 in HLA Transgenic Mice Enables Establishment of a COVID-19 Murine Model for Assessment of Immune Responses to SARS-CoV-2 Infection. Pathogens 2021, 10, 940. https://doi.org/10.3390/pathogens10080940
Chitlaru T, Bar-Haim E, Bar-On L, Rotem S, Cohen H, Elia U, Gur D, Aftalion M, Alkalay R, Makdasi E, et al. Implementation of Adenovirus-Mediated Pulmonary Expression of Human ACE2 in HLA Transgenic Mice Enables Establishment of a COVID-19 Murine Model for Assessment of Immune Responses to SARS-CoV-2 Infection. Pathogens. 2021; 10(8):940. https://doi.org/10.3390/pathogens10080940
Chicago/Turabian StyleChitlaru, Theodor, Erez Bar-Haim, Liat Bar-On, Shahar Rotem, Hila Cohen, Uri Elia, David Gur, Moshe Aftalion, Ron Alkalay, Efi Makdasi, and et al. 2021. "Implementation of Adenovirus-Mediated Pulmonary Expression of Human ACE2 in HLA Transgenic Mice Enables Establishment of a COVID-19 Murine Model for Assessment of Immune Responses to SARS-CoV-2 Infection" Pathogens 10, no. 8: 940. https://doi.org/10.3390/pathogens10080940
APA StyleChitlaru, T., Bar-Haim, E., Bar-On, L., Rotem, S., Cohen, H., Elia, U., Gur, D., Aftalion, M., Alkalay, R., Makdasi, E., Evgy, Y., Falach, R., Israeli, M., Bercovich-Kinori, A., Achdout, H., Yahalom-Ronen, Y., Rosenfeld, R., & Cohen, O. (2021). Implementation of Adenovirus-Mediated Pulmonary Expression of Human ACE2 in HLA Transgenic Mice Enables Establishment of a COVID-19 Murine Model for Assessment of Immune Responses to SARS-CoV-2 Infection. Pathogens, 10(8), 940. https://doi.org/10.3390/pathogens10080940





