Summer Sores Secondary to a Hoof Crack in an Andalusian Stallion
Abstract
:1. Introduction
2. Case Study
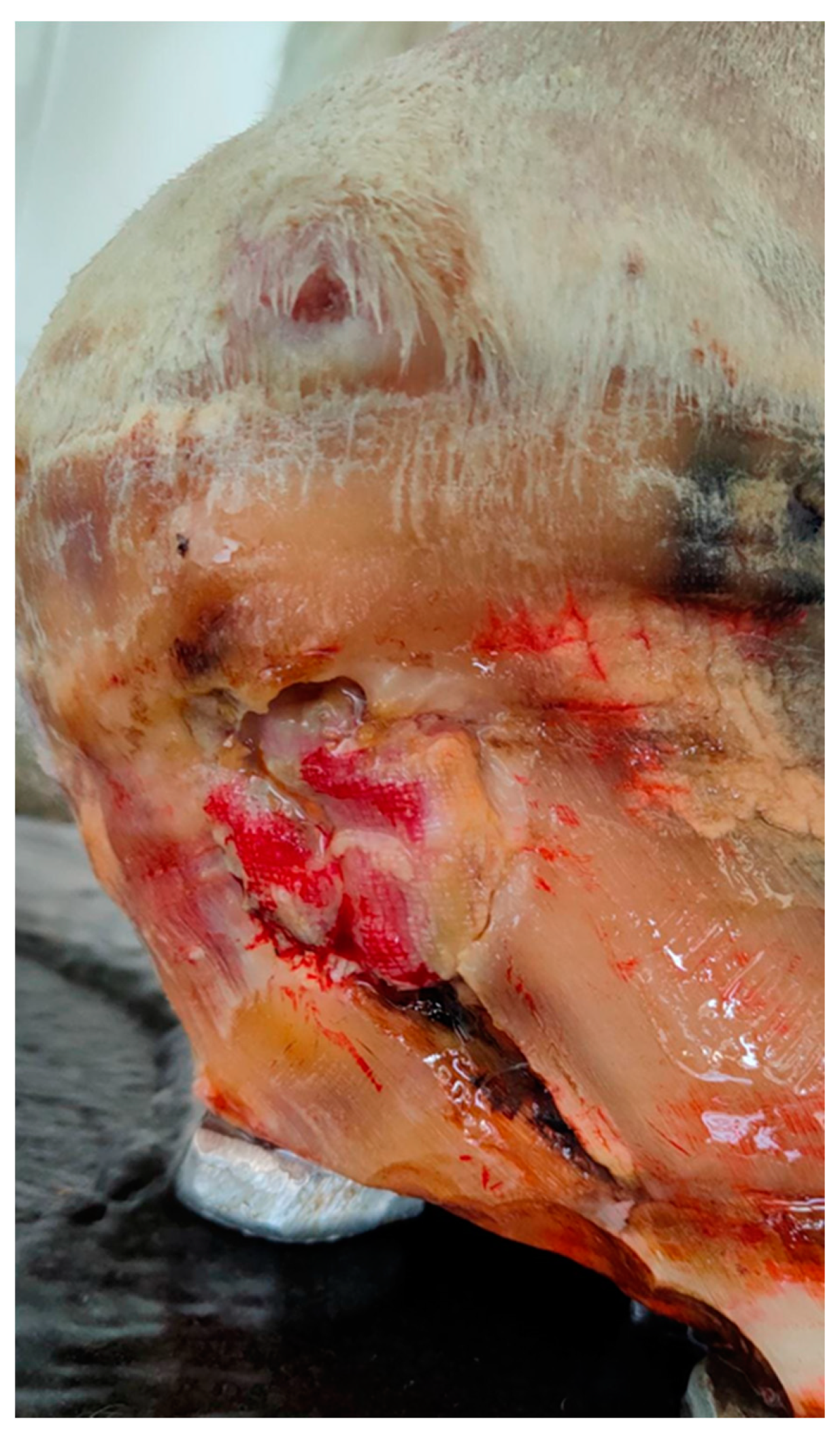
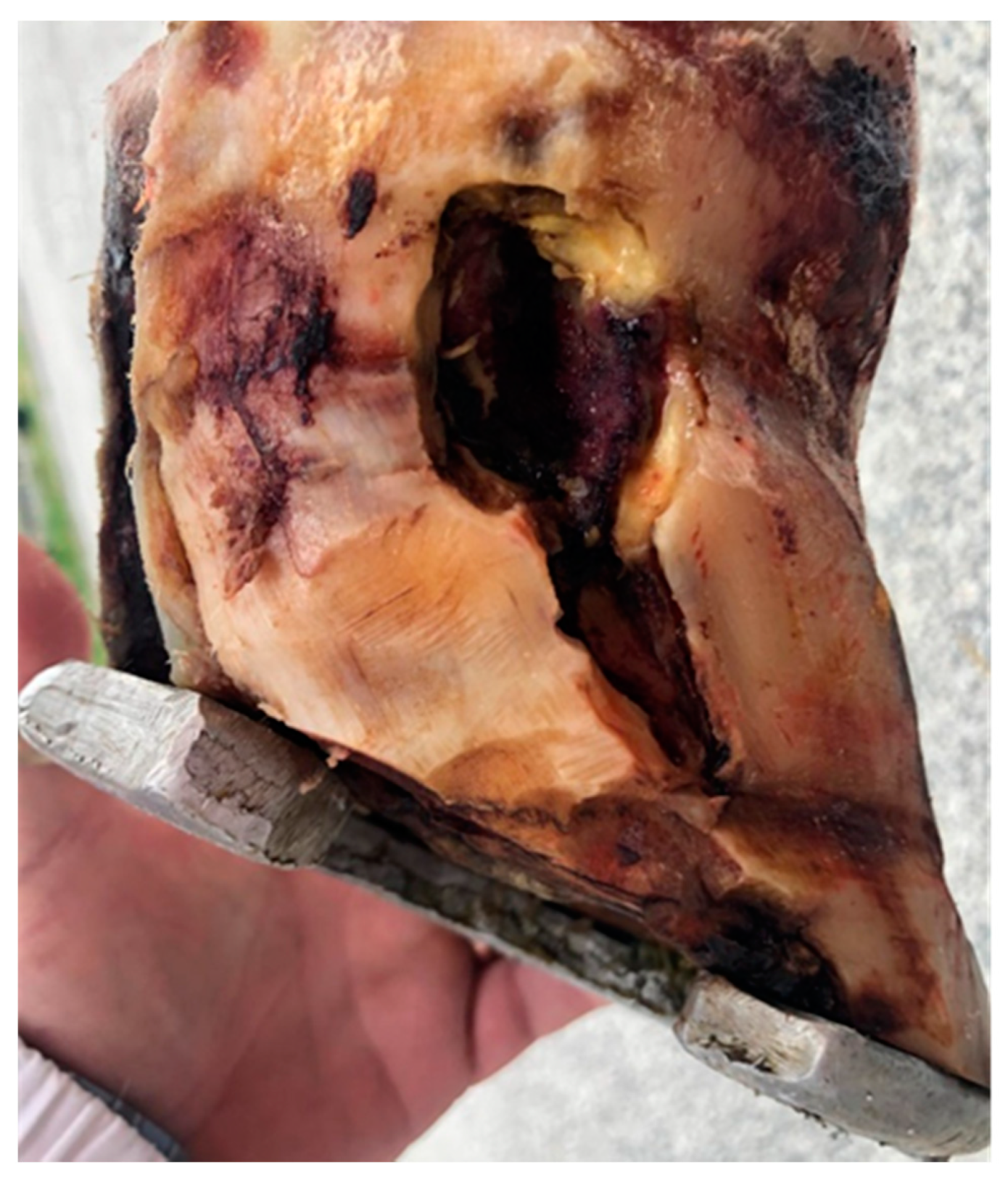
2.1. Case Description
2.2. Parasitological Procedures
2.2.1. Molecular Analysis
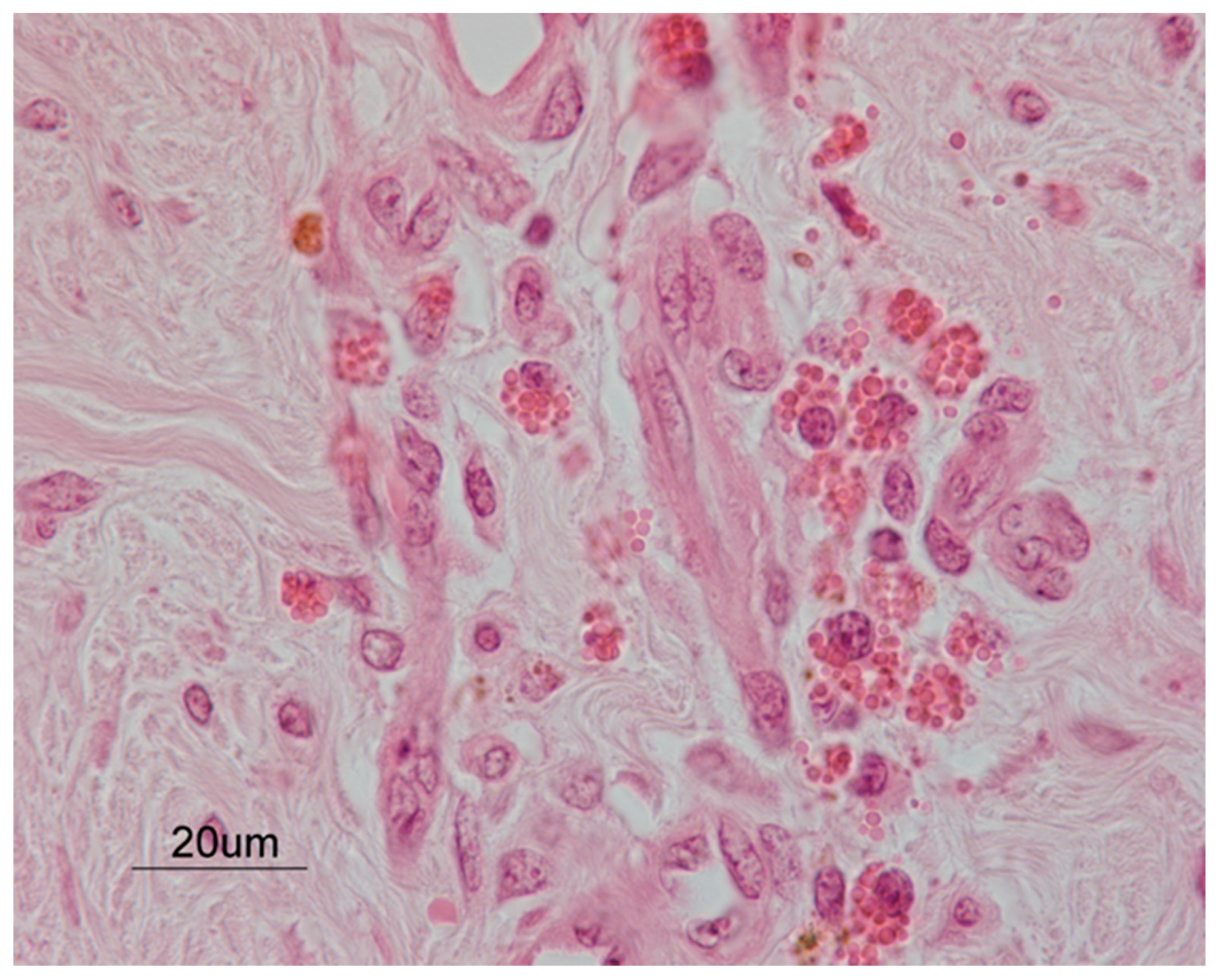
2.3. Histopathology
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barlaam, A.; Traversa, D.; Papini, R.; Giangaspero, A. Habronematidosis in equids: Current status, advances, future challenges. Front. Vet. Sci. 2020, 7, 358. [Google Scholar] [CrossRef]
- Vasey, J.R. Equine cutaneous habronemiasis. Compend. Contin. Educ. Pract. Vet. 1981, 3, 290–298. [Google Scholar]
- Anderson, R.C. The superfamily Habronematoidea. In Nematode Parasites of Vertebrates. Their Development and Transmission; Anderson, R.C., Ed.; CABI Publishing: Guilford, UK, 2000; pp. 428–430. [Google Scholar]
- Pusterla, N.; Watson, J.L.; Wilson, W.D.; Affolter, V.K.; Spier, S.J. Cutaneous and ocular habronemiasis in horses: 63 cases (1988–2002). J. Am. Vet. Med. Assoc. 2003, 222, 978–982. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H. Parasitic diseases. In Equine Dermatology; Saunders: St. Louis, MO, USA, 2003; pp. 357–360. [Google Scholar]
- Murray, D.R.; Ladds, P.W.; Campbell, R.S.F. Granulomatous and neoplastic diseases of the skin of horses. Aust. Vet. J. 1978, 54, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.M. Habronemiasis and Drascheiasis (sic. Draschiasis). In Veterinary Helminthology; W. Heinemann Medical Books Ltd.: London, UK, 1969; pp. 199–200. [Google Scholar]
- Soulsby, E.J.L. Helminths. In Arthropods and Protozoa of Domesticated Animals, 6th ed.; Baillière, Tindall and Cassell: Eastbourne, UK, 1982; pp. 285–287. [Google Scholar]
- Schuster, R.K.; Sivakumar, S.; Kinne, J.; Babiker, H.; Traversa, D.; Buzzell, G.R. Cutaneous and pulmonal habronemosis transmitted by Musca domestica in a stable in the United Arab Emirates. Vet. Parasitol. 2010, 174, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.N.; Borthakur, S.K.; Patra, G.; Singh, N.S.; Tolenkhomba, T.C.; Ravindran, R.; Ghosh, S. Incidence of cutaneous habronemosis in Manipuri ponies in India. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100295. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.E. Quarter crack repair: An overview. Equine Vet. Educ. 2001, 3, 280–282. [Google Scholar] [CrossRef]
- Castelijns, H.H. Pathogenesis and treatment of spontaneous quarter cracks—Quantifying vertical mobility of the hoof capsule at the heels. Pferdeheilkunde 2006, 22, 569–576. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, S.E. How to manage a quarter crack. In Proceedings of the Annual Convention of AAEP, Baltimore, MD, USA, 4–8 December 2010; pp. 141–147. [Google Scholar]
- Furst, A.E.; Lischer, C.J. Foot. In Equine Surgery, 4th ed.; Auer, J.A., Stick, J.A., Eds.; Saunders: St. Louis, MO, USA, 2012; pp. 1264–1299. [Google Scholar]
- O’Grady, S.E. How to repair a quarter crack. In Proceedings of the 47th Annual American Association of Equine Practitioners Convention, San Diego, CA, USA, 24–28 November 2001; pp. 287–291. [Google Scholar]
- O’Grady, S.E.; Castelijns, H.H. Sheared heels and the correlation to spontaneous quarter cracks. Equine Vet. Educ. 2011, 23, 262–269. [Google Scholar] [CrossRef]
- Sloss, M.W.; Kemp, R.L.; Zajac, A.M. Veterinary Clinical Parasitology, 6th ed.; Iowa State University Press: Ames, IA, USA, 1994. [Google Scholar]
- Traversa, D.; Iorio, R.; Capelli, G.; Paoletti, B.; Bartolini, R.; Otranto, D.; Giangaspero, A. Molecular cross-sectional survey of gastric habronemosis in horses. Vet. Parasitol. 2006, 141, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Kruse, G.O.W.; Pritchard, M.H. Animal Parasite Groups and Their Special Preparations. The Collection and Preservation of Animal Parasites; Technical bulletin No. I; University of Nebraska Press: Lincoln, NE, USA, 1982; p. 78. [Google Scholar]
- Traversa, D.; Iorio, R.; Petrizzi, L.; De Amicis, I.; Brandt, S.; Meana, A.; Giangaspero, A.; Otranto, D. Molecular diagnosis of equid summer sores. Vet. Parasitol. 2007, 150, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Giangaspero, A.; Iorio, R.; Otranto, D.; Paoletti, B.; Gasser, R.B. Semi-nested PCR for the specific detection of Habronema microstoma or Habronema muscae DNA in horse faeces. Parasitology 2004, 129, 733–739. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.E. Strategies for shoeing the horse with palmar foot pain. In Proceedings of the 52nd Annual Convention of the American Association of Equine Practitioners, San Antonio, TX, USA, 2–6 December 2006; pp. 209–212. [Google Scholar]
- McGlinchey, L.; Robinson, P.; Porter, B.; Sidhu, A.B.S.; Rosonowski, S.M. Quarter cracks in Thoroughbred racehorses trained in Hong Kong over a 9-year period (2007–2015): Incidence, clinical presentation, and future racing performance. Equine Vet. Educ. 2019, 32, 18–24. [Google Scholar] [CrossRef]
- Moyer, W. Hoof wall defects: Chronic hoof wall separations and hoof wall cracks. Vet. Clin. N. Am. Equine Pract. 2003, 19, 463–477. [Google Scholar] [CrossRef]
- Dabareiner, R.M.; Moyer, W.A.; Carter, G.K. Trauma to the sole and wall. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders: St Louis, MO, USA, 2011; pp. 309–315. [Google Scholar]
- Pleasant, R.S.; O’Grady, S.E.; McKinlay, I. Farriery for hoof wall defects: Quarter cracks and toe cracks. Vet. Clin. N. Am. Equine Pract. 2012, 28, 393–406. [Google Scholar] [CrossRef]
- Knottenbelt, D.C.; Snalune, K.; Kane, J.P. Tumours of the skin. In Clinical Equine Oncology; Elsevier Health Sciences: New York, NY, USA, 2015; pp. 582–583. [Google Scholar]
- Berry, C.R.; O’Brien, T.R.; Pool, R.R. Squamous cell carcinoma of the hoof wall in a stallion. J. Am. Vet. Med. Assoc. 1991, 199, 90–92. [Google Scholar] [PubMed]
- Honnas, C.M.; Liskey, C.C.; Meagher, D.M.; Brown, D.; Luck, E.E. Malignant melanoma in the foot of a horse. J. Am. Vet. Med. Assoc. 1990, 197, 756–758. [Google Scholar] [PubMed]
- Parks, A.H. Wounds of the equine foot: Principles of healing and treatment. Equine Vet. Educ. 1997, 9, 317–327. [Google Scholar] [CrossRef]
- Wilmink, J.M.; Van Weeren, P.R. Treatment of exuberant granulation tissue. Clin. Tech. Equine Pract. 2004, 3, 141–147. [Google Scholar] [CrossRef]
- Waddell, A.H. A survey of Habronema spp and the identification of third-stage larvae of Habronema megastoma and Habronema muscae in section. Aust. Vet. J. 1969, 45, 20–21. [Google Scholar] [CrossRef]
- Fadok, V.A.; Mullowney, P.C. Dermatologic diseases of horses Part, I. Parasitic dermatoses of the horse. Compend. Contin. Educ. 1983, 5, 615–622. [Google Scholar]
- Howard, J.J. Nuisance flies around a landfill: Patterns of abundance and distribution. Waste Manag. Res. 2001, 19, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Derwent, L.C.; Hanley, M.E.; Dunn, D.W.; Abolins, S.R. Predicting calyptrate fly populations from the weather, and probable consequences of climate change. J. App. Ecol. 2005, 42, 795–804. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palozzo, A.; Traversa, D.; Marruchella, G.; Celani, G.; Morelli, S.; Petrizzi, L. Summer Sores Secondary to a Hoof Crack in an Andalusian Stallion. Pathogens 2021, 10, 1038. https://doi.org/10.3390/pathogens10081038
Palozzo A, Traversa D, Marruchella G, Celani G, Morelli S, Petrizzi L. Summer Sores Secondary to a Hoof Crack in an Andalusian Stallion. Pathogens. 2021; 10(8):1038. https://doi.org/10.3390/pathogens10081038
Chicago/Turabian StylePalozzo, Adriana, Donato Traversa, Giuseppe Marruchella, Gianluca Celani, Simone Morelli, and Lucio Petrizzi. 2021. "Summer Sores Secondary to a Hoof Crack in an Andalusian Stallion" Pathogens 10, no. 8: 1038. https://doi.org/10.3390/pathogens10081038
APA StylePalozzo, A., Traversa, D., Marruchella, G., Celani, G., Morelli, S., & Petrizzi, L. (2021). Summer Sores Secondary to a Hoof Crack in an Andalusian Stallion. Pathogens, 10(8), 1038. https://doi.org/10.3390/pathogens10081038










