Contribution of Visible Surface Mold to Airborne Fungal Concentration as Assessed by Digital Image Quantification
Abstract
:1. Introduction
2. Results
2.1. Temperature, Relative Humidity, and Wind Speed in Indoor Environments
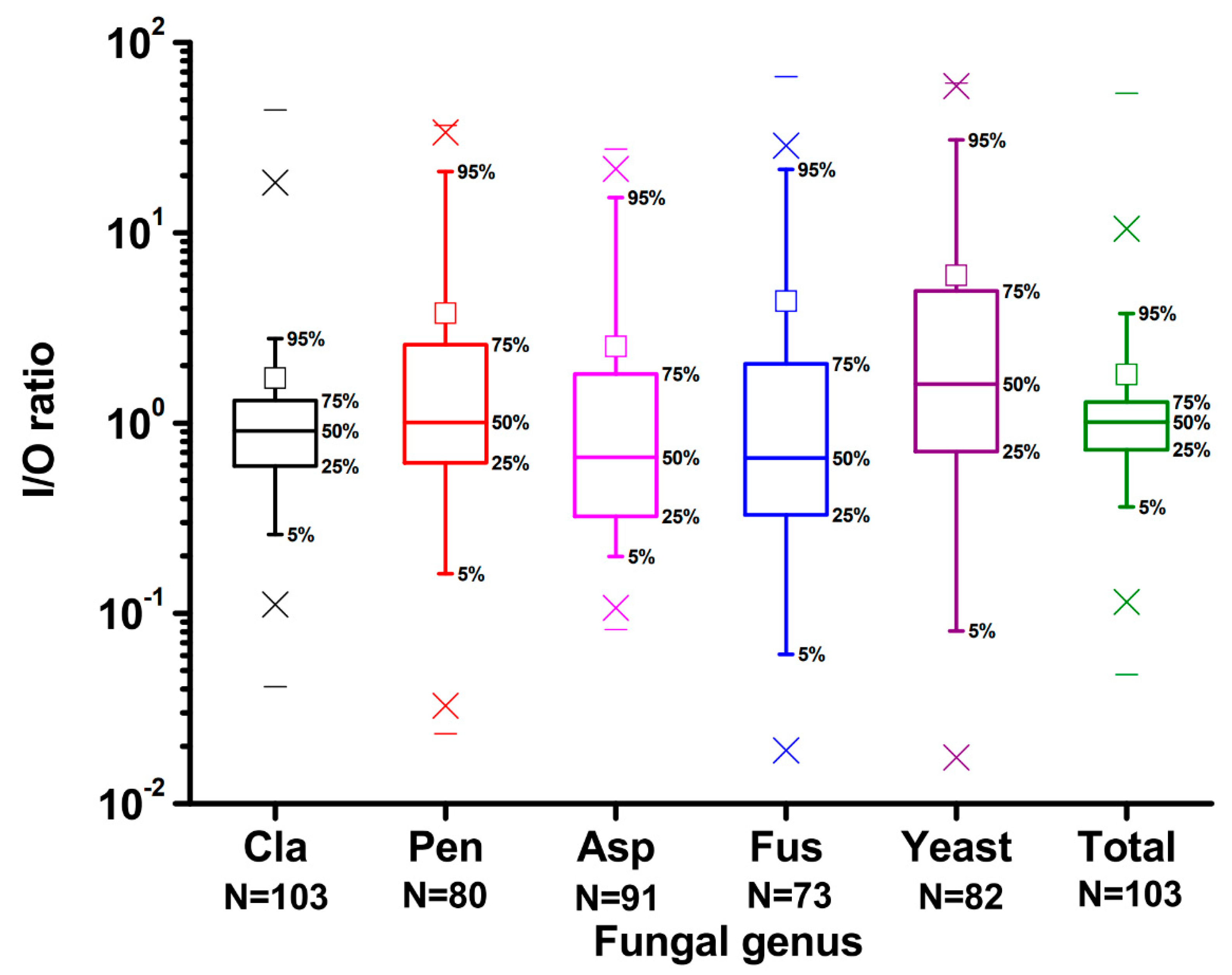
2.2. Concentrations of Airborne Fungi in Indoor and Outdoor Air
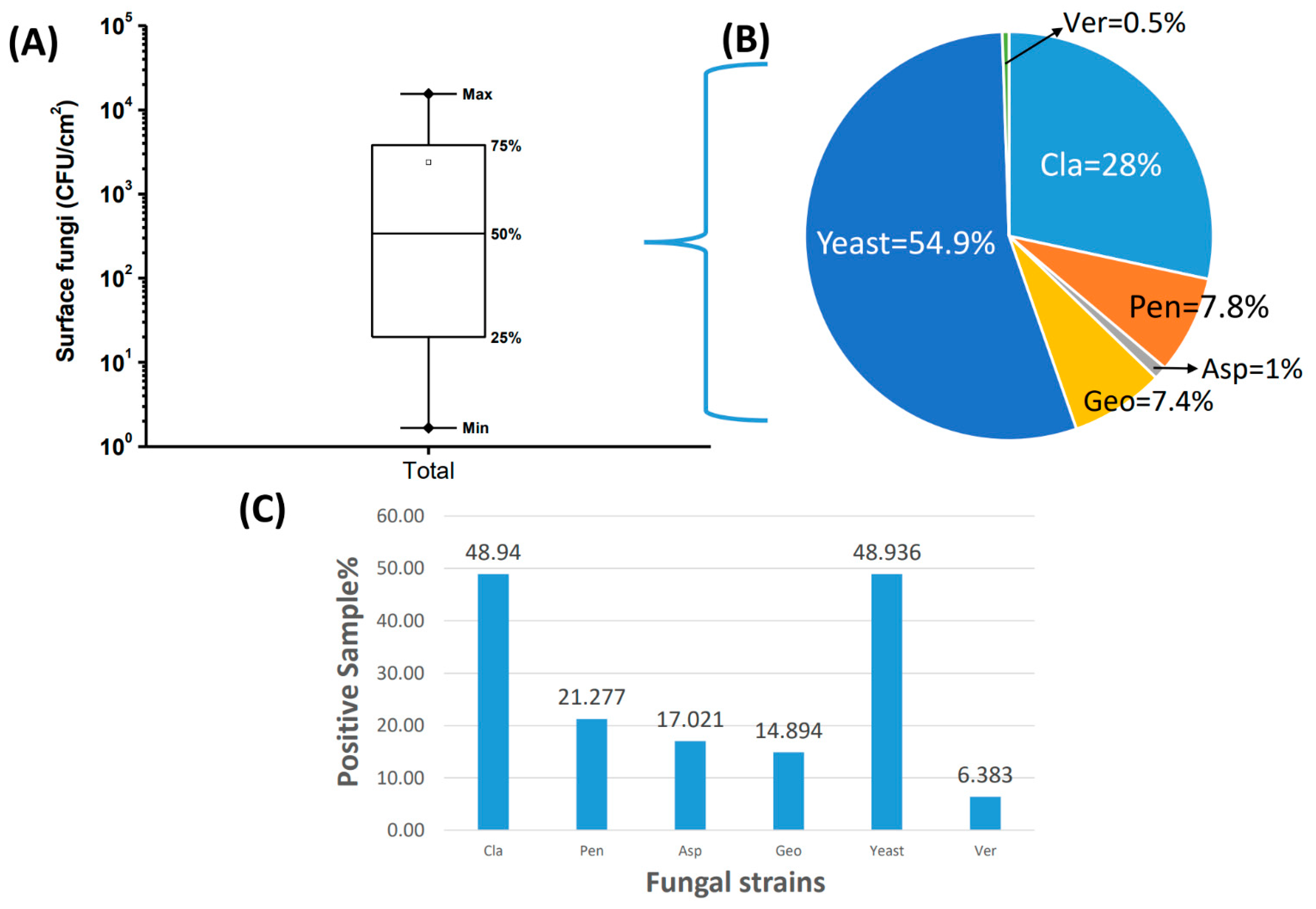
2.3. Concentrations of Surface Fungi in Indoor Environments
2.4. Correlation among Environmental Parameters and Airborne Fungal Concentrations
2.5. Correlation among Environmental Parameters and Surface Fungal Density
2.6. Correlation between the Concentration of Indoor and Outdoor Fungal Aerosols
2.7. Correlation between the Concentrations of Airborne and Surface Fungi in Indoor Environments
2.8. Association among the Airborne Fungal Concentration, Fungal Surface Area Ratio, and Environmental Parameters
3. Discussion
4. Materials and Methods
4.1. Sampling Locations
4.2. Airborne Fungus Measurements
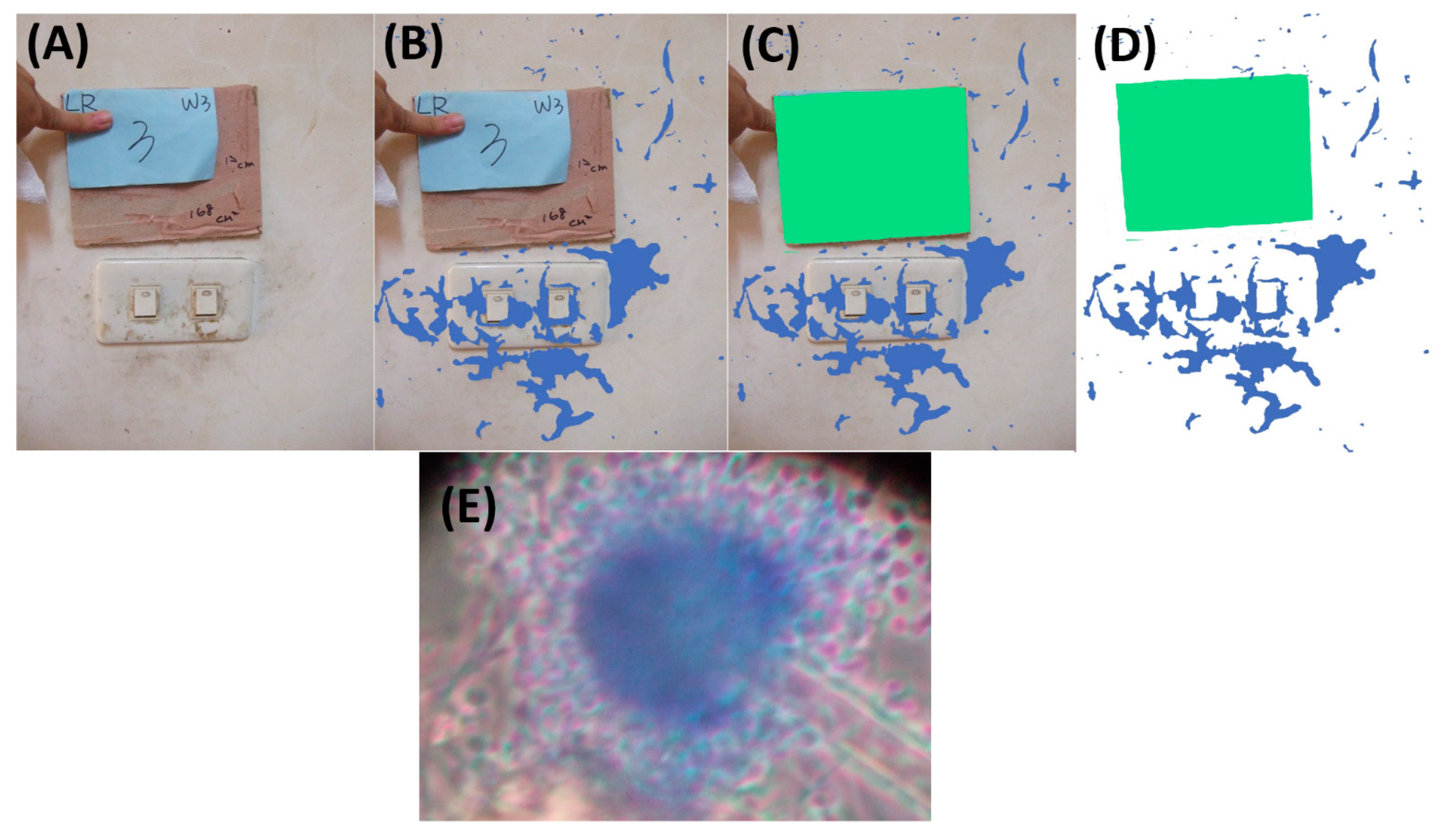
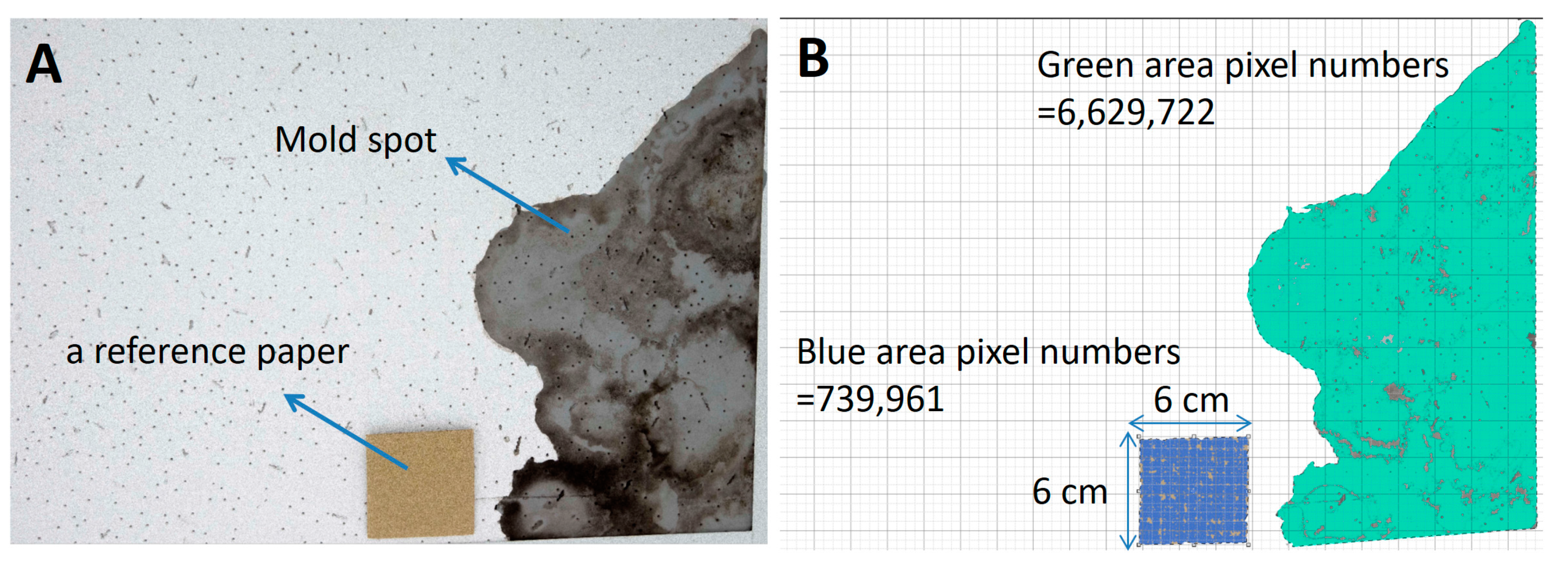
4.3. Surface Sampling and Sampling Area Counting
4.4. Other Environmental Parameter Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bioaerosols, A. Bioaerosols Assessment and Control; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1999. [Google Scholar]
- Mebi, G.; Samuel, W.; Ojogba, M. Relationship Between Fungal Contamination of Indoor Air and Health Problems of Some Residents in Jos. In Air Quality—Monitoring and Modeling; IntechOpen: London, UK, 2012. [Google Scholar]
- Shelton, B.G.; Kirkland, K.H.; Flanders, W.D.; Morris, G.K. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 2002, 68, 1743–1753. [Google Scholar] [CrossRef] [Green Version]
- Duchaine, C.; Mériaux, A. The importance of combining air sampling and surface analysis when studying problematic houses for mold biodiversity determination. Aerobiologia 2001, 17, 121–125. [Google Scholar] [CrossRef]
- Park, J.-H. Mold exposure and respiratory health in damp indoor environments. Front. Biosci. 2011, E3, 757–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Su, H.J.; Wu, P.C.; Chen, H.L.; Lee, F.C.; Lin, L.L. Exposure Assessment of Indoor Allergens, Endotoxin, and Airborne Fungi for Homes in Southern Taiwan. Environ. Res. 2001, 85, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. The Diversity and Distribution of Fungi on Residential Surfaces. PLoS ONE 2013, 8, e78866. [Google Scholar] [CrossRef] [PubMed]
- Dallongeville, A.; LE Cann, P.; Zmirou-Navier, D.; Chevrier, C.; Costet, N.; Annesi-Maesano, I.; Blanchard, O. Concentration and determinants of molds and allergens in indoor air and house dust of French dwellings. Sci. Total Environ. 2015, 536, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Aktas, Y.D.; Ioannou, I.; Altamirano, H.; Reeslev, M.; D’Ayala, D.; May, N.; Canales, M. Surface and passive/active air mould sampling: A testing exercise in a North London housing estate. Sci. Total Environ. 2018, 643, 1631–1643. [Google Scholar] [CrossRef]
- Khan, N.N.; Wilson, B.L. An environmental assessment of mold concentrations and potential mycotoxin exposures in the greater Southeast Texas area. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 2759–2772. [Google Scholar] [CrossRef]
- Lee, T.; Grinshpun, S.A.; Martuzevicius, D.; Adhikari, A.; Crawford, C.M.; Reponen, T. Culturability and concentration of indoor and outdoor airborne fungi in six single-family homes. Atmos. Environ. 2006, 40, 2902–2910. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-S.; Kuo, Y.-M. Airborne characterization of fungi indoors and outdoors. J. Aerosol Sci. 1992, 23, 667–670. [Google Scholar] [CrossRef]
- Crawford, J.A.; Rosenbaum, P.F.; Anagnost, S.E.; Hunt, A.; Abraham, J.L. Indicators of airborne fungal concentrations in urban homes: Understanding the conditions that affect indoor fungal exposures. Sci. Total Environ. 2015, 517, 113–124. [Google Scholar] [CrossRef]
- Bamba, I.; Azuma, M.; Hamada, N.; Kubo, H.; Isoda, N. Case Study of Airborne Fungi According to Air Temperature and Relative Humidity in Houses with Semi-Basements Adjacent to a Forested Hillside. Biocontrol Sci. 2014, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.C.; Su, H.J.; Lin, C.Y. Characteristics of indoor and outdoor airborne fungi at suburban and urban homes in two seasons. Sci. Total Environ. 2000, 253, 111–118. [Google Scholar] [CrossRef]
- Ho, H.M.; Rao, C.; Hsu, H.H.; Chiu, Y.H.; Liu, C.M.; Chao, H.J. Characteristics and determinants of ambient fungal spores in Hualien, Taiwan. Atmos. Environ. 2005, 39, 5839–5850. [Google Scholar] [CrossRef]
- Mui, K.W.; Chan, W.Y.; Wong, L.T.; Hui, P.S. Scoping indoor airborne fungi in an excellent indoor air quality office building in Hong Kong. Build. Serv. Eng. Res. Technol. 2010, 31, 191–199. [Google Scholar] [CrossRef]
- Ziaee, A.; Zia, M.; Goli, M. Identification of saprophytic and allergenic fungi in indoor and outdoor environments. Environ. Monit. Assess. 2018, 190, 574. [Google Scholar] [CrossRef]
- Beguin, H.; Nolard, N. Mould biodiversity in homes I. Air and surface analysis of 130 dwellings. Aerobiologia 1994, 10, 157–166. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Pettersson, M.; Bååth, E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol. Ecol. 2005, 52, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Frankel, M.; Bekö, G.; Timm, M.; Gustavsen, S.; Hansen, E.W.; Madsen, A.M. Seasonal Variations of Indoor Microbial Exposures and Their Relation to Temperature, Relative Humidity, and Air Exchange Rate. Appl. Environ. Microbiol. 2012, 78, 8289–8297. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, Y.; Tian, Y.; Li, A.; Li, Y.; Xiong, J.; Gao, R. On-site investigation of the concentration and size distribution characteristics of airborne fungi in a university library. Environ. Pollut. 2020, 261, 114138. [Google Scholar] [CrossRef]
- van Rhijn, N.; Bromley, M. The Consequences of Our Changing Environment on Life Threatening and Debilitating Fungal Diseases in Humans. J. Fungi 2021, 7, 367. [Google Scholar] [CrossRef] [PubMed]
- Heseltine, E.; Rosen, J. WHO Guidelines for Indoor Air Quality: Dampness and Mould; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2009. [Google Scholar]
- Viitanen, H.; Ritschkoff, A.-C. Mould Growth in Pine and Spruce Sapwood in Relation to Air Humidity and Temperature; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1991. [Google Scholar]
- Chao, H.J.; Schwartz, J.; Milton, D.K.; A Burge, H. Populations and determinants of airborne fungi in large office buildings. Environ. Health Perspect. 2002, 110, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Lang-Yona, N.; Levin, Y.; Dannemiller, K.; Yarden, O.; Peccia, J.; Rudich, Y. Changes in atmospheric CO2influence the allergenicity ofAspergillus fumigatus. Glob. Chang. Biol. 2013, 19, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Levetin, E.; Dorsey, K. Contribution of leaf surface fungi to the air spora. Aerobiologia 2006, 22, 3–12. [Google Scholar] [CrossRef]
- Debode, J.; Van Poucke, K.; França, S.D.C.; Maes, M.; Höfte, M.; Heungens, K. Detection of Multiple Verticillium Species in Soil Using Density Flotation and Real-Time Polymerase Chain Reaction. Plant Dis. 2011, 95, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Pottier, I.; Gente, S.; Vernoux, J.-P.; Guéguen, M. Safety assessment of dairy microorganisms: Geotrichum candidum. Int. J. Food Microbiol. 2007, 126, 327–332. [Google Scholar] [CrossRef]
- Bandler, R.; Stack, M.; Koch, H.; Tournas, V.; Mislivec, P. Chapter 18 Yeasts, molds, and mycotoxins. In Bacteriological Analytical Manual; Food and Drug Administration: Washington, DC, USA, 1998. [Google Scholar]
- Belizán, M.M.; Gomez, A.D.L.A.; Baptista, Z.P.T.; Jimenez, C.M.; Matías, M.D.H.S.; Catalán, C.A.; Sampietro, D.A. Influence of water activity and temperature on growth and production of trichothecenes by Fusarium graminearum sensu stricto and related species in maize grains. Int. J. Food Microbiol. 2019, 305, 108242. [Google Scholar] [CrossRef]
- Garbe, L.-A.; Schwarz, P.; Ehmer, A. Beer gushing. In Beer; Bamforth, C.W., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 185–212. [Google Scholar]
- Khan, A.H.; Karuppayil, S.M. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, S.; Devassikutty, A.K.; Mathew, M.; Ayyappan, D.; Thiagarajan, S.; Raghunathan, R. Passive Release of Fungal Spores from Synthetic Solid Waste Surfaces. Aerosol Air Qual. Res. 2016, 16, 1441–1451. [Google Scholar] [CrossRef] [Green Version]
- Hsu, N.Y.; Chen, P.Y.; Chang, H.W.; Su, H.J. Changes in profiles of airborne fungi in flooded homes in southern Taiwan after Typhoon Morakot. Sci. Total Environ. 2011, 409, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Hansen, V.M.; Meyling, N.; Eilenberg, J. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann. Agric. Environ. Med. 2007, 14, 5–24. [Google Scholar]
- Hameed, A.A.A.; Yasser, I.H.; Khoder, M. Indoor air quality during renovation actions: A case study. J. Environ. Monit. 2004, 6, 740–744. [Google Scholar] [CrossRef]
- Chao, H.J.; Chan, C.C.; Rao, C.Y.; Lee, C.T.; Chuang, Y.C.; Chiu, Y.H.; Hsu, H.H.; Wu, Y.H. The effects of transported Asian dust on the composition and concentration of ambient fungi in Taiwan. Int. J. Biometeorol. 2011, 56, 211–219. [Google Scholar] [CrossRef]
- Adams, R.I.; Lymperopoulou, D.S.; Misztal, P.K.; Pessotti, R.D.C.; Behie, S.W.; Tian, Y.; Goldstein, A.H.; Lindow, S.E.; Nazaroff, W.W.; Taylor, J.W.; et al. Microbes and associated soluble and volatile chemicals on periodically wet household surfaces. Microbiome 2017, 5, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in Food: Ecophysiology, Mycotoxin Production and Toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Fu, Z.; Pan, Y.; Wang, J.; Zeng, Z. Single Leaf Area Measurement Using Digital Camera Image. In Computer and Computing Technologies in Agriculture IV; Li, D., Liu, Y., Chen, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 345, pp. 525–530. [Google Scholar]
- Chaudary, P.; Godara, S. Fast and Accurate Method for Leaf Area Measurement. Int. J. Comput. Appl. 2012, 49, 22–25. [Google Scholar] [CrossRef]
- St-Germain, G.; Summerbell, R. Identifying Filamentous Fungi: A Clinical Laboratory Handbook; Star Publishing Company: Singapore, 1996. [Google Scholar]
- Ho, Y.H.; Wang, L.S.; Jiang, H.L.; Chang, C.H.; Hsieh, C.-J.; Chang, D.C.; Tu, H.Y.; Chiu, T.Y.; Chao, H.J.; Tseng, C.C. Use of a Sampling Area-Adjusted Adenosine Triphosphate Bioluminescence Assay Based on Digital Image Quantification to Assess the Cleanliness of Hospital Surfaces. Int. J. Environ. Res. Public Health 2016, 13, 576. [Google Scholar] [CrossRef] [PubMed]

| Temperature # | Atmospheric RH # | Wind Speed | CO2 | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Cladosporium spp. | 0.195 * | 0.048 | −0.149 | 0.133 | 0.094 | 0.345 | −0.200 * | 0.042 |
| Penicillium spp. | −0.064 | 0.524 | 0.397 ** | <0.0001 | −0.020 | 0.842 | 0.023 | 0.819 |
| Aspergillus spp. | 0.063 | 0.525 | 0.118 | 0.235 | −0.156 | 0.116 | −0.279 ** | 0.004 |
| Fusarium spp. | 0.205 * | 0.038 | −0.274 ** | 0.005 | −0.001 | 0.992 | −0.419 ** | <0.0001 |
| Geotrichum spp. | 0.028 | 0.783 | 0.062 | 0.532 | −0.054 | 0.586 | 0.010 | 0.922 |
| Yeast | −0.016 | 0.874 | −0.157 | 0.114 | 0.070 | 0.485 | 0.038 | 0.702 |
| Verticillium spp. | 0.247 * | 0.012 | −0.269 ** | 0.006 | 0.237 * | 0.016 | −0.246 * | 0.012 |
| Total fungi | 0.144 | 0.148 | −0.026 | 0.798 | 0.156 | 0.115 | −0.273 ** | 0.005 |
| Surface Tem # | Surface RH # | Wind Speed | CO2 | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Cladosporium spp. | 0.1571 | 0.113 | 0.0344 | 0.730 | −0.1073 | 0.281 | −0.0050 | 0.960 |
| Penicillium spp. | 0.1573 | 0.112 | 0.1802 | 0.069 | −0.0636 | 0.523 | −0.0130 | 0.896 |
| Aspergillus spp. | 0.0807 | 0.418 | 0.0435 | 0.663 | −0.0915 | 0.358 | 0.0610 | 0.541 |
| Geotrichum spp. | 0.1050 | 0.291 | 0.2325 * | 0.018 | −0.0275 | 0.783 | 0.0117 | 0.907 |
| Yeast | −0.073 | 0.462 | 0.1467 | 0.091 | −0.143 | 0.149 | 0.335 ** | 0.001 |
| Verticillium spp. | 0.0355 | 0.722 | 0.2425 * | 0.014 | −0.0729 | 0.464 | −0.0088 | 0.930 |
| Total fungi | 0.0934 | 0.348 | 0.1594 | 0.108 | −0.2302 * | 0.019 | 0.3021 ** | 0.002 |
| ID # cla | ID pen | ID asp | ID fus | ID geo | ID Yeast | ID ver | ID Total | OD # cla | OD pen | OD asp | OD fus | OD geo | OD Yeast | OD ver | OD Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID cla | 1.000 | |||||||||||||||
| ID pen | −0.231 * | 1.000 | ||||||||||||||
| ID asp | 0.235 * | 0.110 | 1.000 | |||||||||||||
| ID fus | 0.397 ** | −0.247 * | 0.242 * | 1.000 | ||||||||||||
| ID geo | −0.264 ** | 0.141 | −0.160 | −0.061 | 1.000 | |||||||||||
| ID yeast | 0.043 | −0.411 ** | −0.085 | 0.077 | −0.136 | 1.000 | ||||||||||
| ID ver | 0.294 ** | −0.115 | −0.115 | 0.265 ** | −0.083 | −0.137 | 1.000 | |||||||||
| ID total | 0.737 ** | −0.096 | 0.186 | 0.298 ** | −0.260 ** | 0.312 ** | 0.242 * | 1.000 | ||||||||
| OD cla | 0.716 ** | −0.258 ** | 0.275 ** | 0.513 ** | −0.207 * | −0.007 | 0.301 ** | 0.451 ** | 1.000 | |||||||
| OD pen | −0.328 ** | 0.810 ** | 0.093 | −0.117 | 0.314 ** | −0.456 ** | −0.134 | −0.291 ** | −0.151 | 1.000 | ||||||
| OD asp | 0.340 ** | −0.056 | 0.484 ** | 0.289 ** | −0.250 * | −0.144 | 0.105 | 0.173 | 0.469 ** | −0.002 | 1.000 | |||||
| OD fus | 0.475 ** | −0.205 * | 0.147 | 0.585 ** | −0.082 | −0.040 | 0.222 * | 0.221 * | 0.640 ** | −0.028 | 0.272 ** | 1.000 | ||||
| OD geo | −0.317 ** | 0.140 | −0.090 | −0.054 | 0.793 ** | −0.024 | −0.073 | −0.223 * | −0.198 * | 0.256 ** | −0.289 ** | −0.126 | 1.000 | |||
| OD yeast | 0.169 | −0.466 ** | −0.091 | 0.113 | −0.173 | 0.697 ** | −0.071 | 0.320 ** | 0.219 * | −0.467 ** | 0.049 | 0.149 | −0.214 * | 1.000 | ||
| OD ver | 0.294 ** | −0.116 | −0.114 | 0.265 ** | −0.083 | −0.137 | 1.000 ** | 0.242 * | 0.301 ** | −0.134 | 0.105 | 0.222 * | −0.073 | −0.071 | 1.000 | |
| OD total | 0.670 ** | −0.210 * | 0.321 ** | 0.525 ** | −0.112 | 0.131 | 0.231 * | 0.583 ** | 0.856 ** | −0.149 | 0.504 ** | 0.571 ** | −0.124 | 0.430 ** | 0.231 * | 1.000 |
| ID # cla | ID pen | ID asp | ID geo | ID yeast | ID ver | ID Total | SF # cla | SF pen | SF asp | SF geo | SF Yeast | SF ver | SF Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID cla | 1.000 | |||||||||||||
| ID pen | −0.231 * | 1.000 | ||||||||||||
| ID asp | 0.235 * | 0.110 | 1.000 | |||||||||||
| ID geo | −0.264 ** | 0.141 | −0.160 | 1.000 | ||||||||||
| ID yeast | 0.043 | −0.411 ** | −0.085 | −0.136 | 1.000 | |||||||||
| ID ver | 0.294 ** | −0.115 | −0.115 | −0.083 | −0.137 | 1.000 | ||||||||
| ID total | 0.737 ** | −0.096 | 0.186 | −0.260 ** | 0.312 ** | 0.242 * | 1.000 | |||||||
| SF cla | 0.063 | 0.109 | 0.295 ** | 0.181 | 0.002 | −0.010 | 0.197 * | 1.000 | ||||||
| SF pen | −0.088 | 0.185 | 0.178 | 0.322 ** | 0.022 | 0.288 ** | 0.022 | 0.381 ** | 1.000 | |||||
| SF asp | −0.013 | −0.087 | 0.081 | 0.184 | −0.043 | 0.137 | −0.047 | 0.300 ** | 0.424 ** | 1.000 | ||||
| SF geo | −0.303 ** | 0.282 ** | 0.095 | 0.420 ** | 0.014 | −0.054 | −0.054 | 0.504 ** | 0.704 ** | 0.389 ** | 1.000 | |||
| SF yeast | 0.061 | 0.003 | −0.057 | 0.101 | 0.051 | 0.009 | 0.052 | −0.176 | 0.053 | −0.154 | −0.143 | 1.000 | ||
| SF ver | −0.198 * | 0.271 ** | 0.105 | 0.361 ** | 0.065 | −0.035 | −0.029 | 0.389 ** | 0.563 ** | 0.402 ** | 0.655 ** | −0.089 | 1.000 | |
| SF total | 0.108 | 0.032 | 0.131 | 0.269 ** | 0.078 | 0.020 | 0.176 | 0.537 ** | 0.401 ** | 0.291 ** | 0.312 ** | 0.623 ** | 0.303 ** | 1.000 |
| N | ID # cla | ID pen | ID asp | ID geo | ID Yeast | ID ver | ID Total | |
|---|---|---|---|---|---|---|---|---|
| Percent Changes (β ± SE) | ||||||||
| Surface area ratio | ||||||||
| Level 1:0 | 56 | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Level 2: ≤Median (>0–0.00031) | 24 | −8.11 ± 37.14 | −55.42 ± 58.10 | 61.0 ± 34.94 | 23.63 ± 27.49 | 10.86 ± 76.8 | 39.8 ± 22.92 | 7.5 ± 22.73 |
| Level 3: >Median (0.00031–1) | 23 | 33.02 ± 38.02 | 112.19 ± 59.57 | 138.8 ± 35.7 ** | 85.5 ± 28.12 * | 1.39 ± 78.88 | 2.53 ± 23.44 | 39.03 ± 23.24 |
| Atmospheric Temperature | 103 | 11.68 ± 7.22 | 5.62 ± 10.63 | 4.37 ± 6.83 | 1.24 ± 5.5 | −4.42 ± 13.40 | 4.61 ± 4.66 | 10.33 ± 4.62 * |
| Atmospheric humidity | 103 | −0.33 ± 1.46 | 7.76 ± 2.13 ** | 1.31 ± 1.39 | 0.19 ± 1.12 | −3.81 ± 2.65 | −1.46 ± 0.95 | 1.21 ± 0.94 |
| CO2 | 103 | −0.05 ± 0.07 | −0.04 ± 0.10 | −0.23 ± 0.06 ** | −0.03 ± 0.05 | 0.03 ± 0.12 | −0.08 ± 0.04 | −0.12 ± 0.04 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-C.; Huang, N.; Hsieh, C.-J.; Hung, C.-C.; Guo, Y.-L.L. Contribution of Visible Surface Mold to Airborne Fungal Concentration as Assessed by Digital Image Quantification. Pathogens 2021, 10, 1032. https://doi.org/10.3390/pathogens10081032
Tseng C-C, Huang N, Hsieh C-J, Hung C-C, Guo Y-LL. Contribution of Visible Surface Mold to Airborne Fungal Concentration as Assessed by Digital Image Quantification. Pathogens. 2021; 10(8):1032. https://doi.org/10.3390/pathogens10081032
Chicago/Turabian StyleTseng, Chun-Chieh, Ning Huang, Chia-Jung Hsieh, Chien-Che Hung, and Yue-Liang Leon Guo. 2021. "Contribution of Visible Surface Mold to Airborne Fungal Concentration as Assessed by Digital Image Quantification" Pathogens 10, no. 8: 1032. https://doi.org/10.3390/pathogens10081032
APA StyleTseng, C.-C., Huang, N., Hsieh, C.-J., Hung, C.-C., & Guo, Y.-L. L. (2021). Contribution of Visible Surface Mold to Airborne Fungal Concentration as Assessed by Digital Image Quantification. Pathogens, 10(8), 1032. https://doi.org/10.3390/pathogens10081032








