Cystic Fibrosis Clinical Isolates of Aspergillus fumigatus Induce Similar Muco-inflammatory Responses in Primary Airway Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. Isolate Clustering and Antifungal Susceptibility
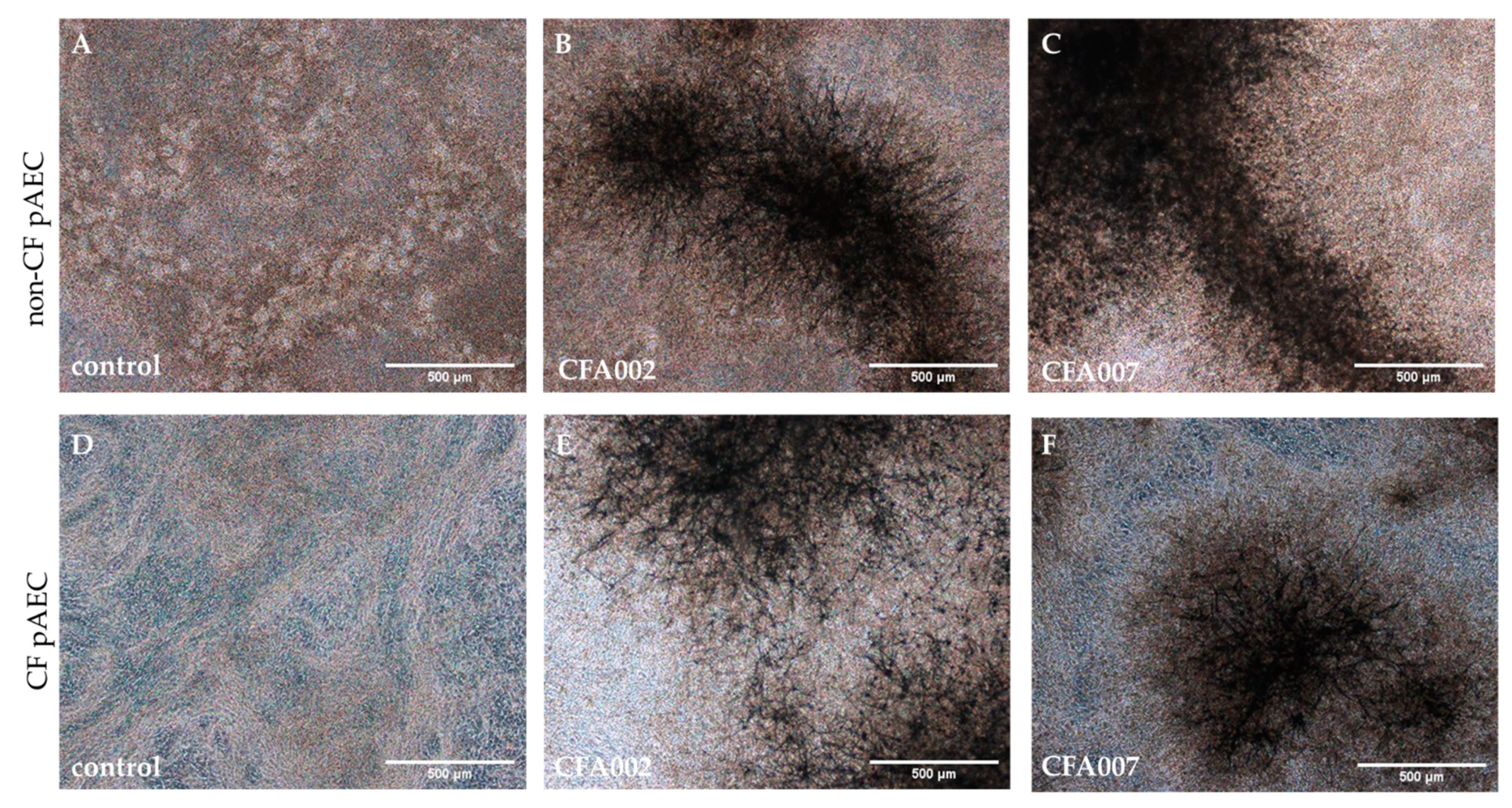
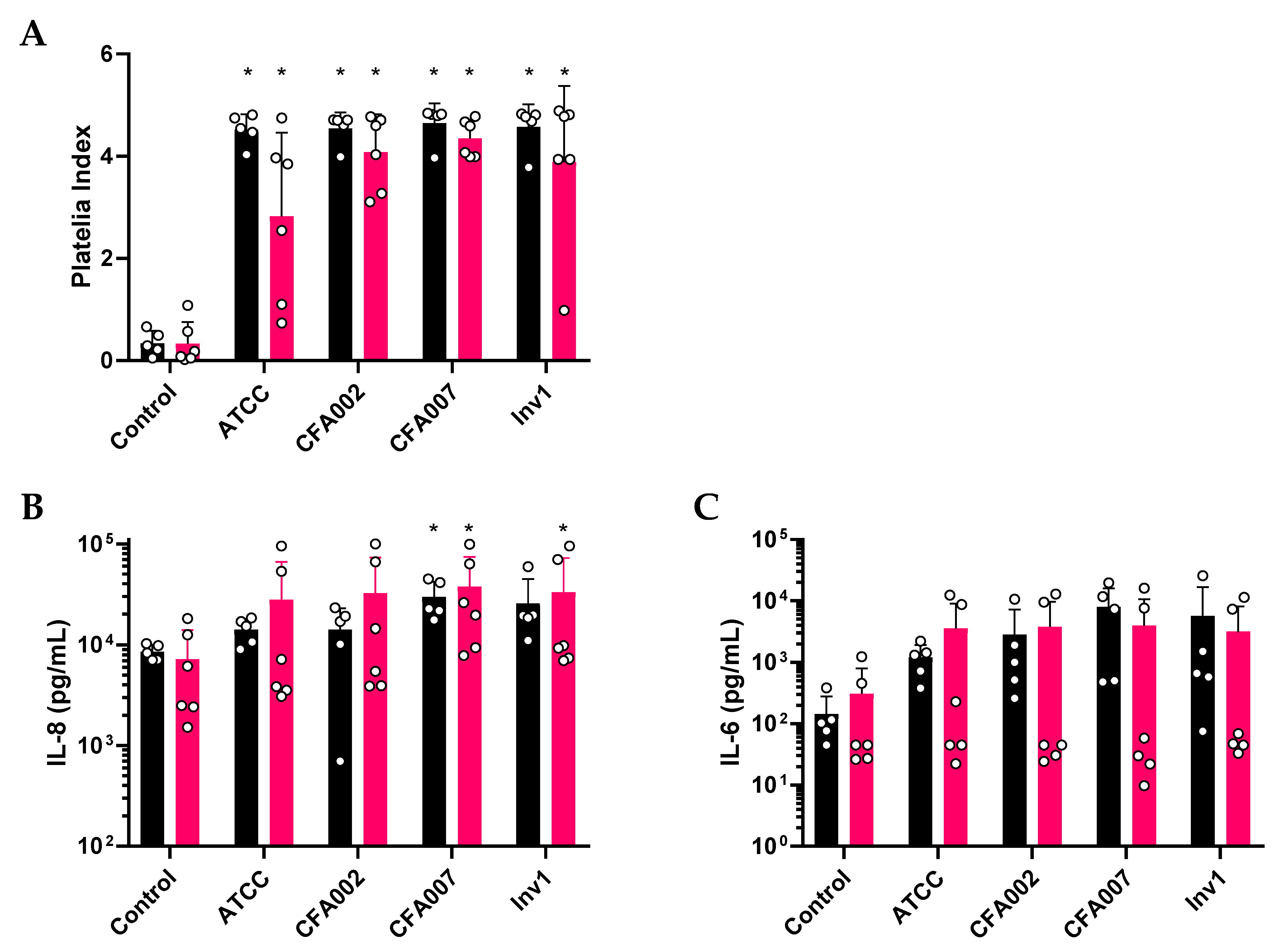
2.2. Monolayer Aspergillus Infections Show no Differences between Responses to Individual Isolates
2.3. Air–Liquid Interface (ALI) Infections also Showed no Inflammatory Differences between Isolates
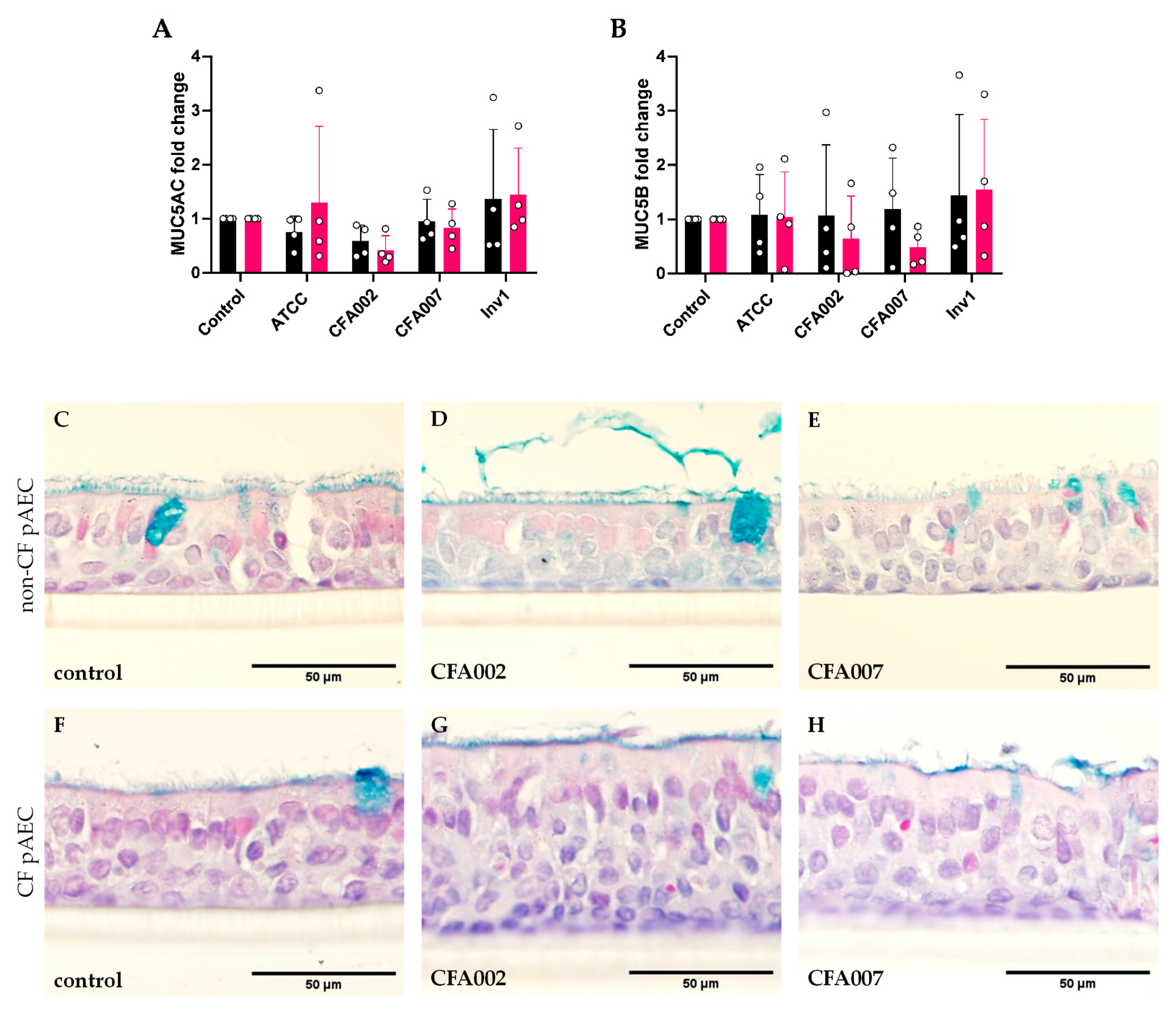
2.4. Aspergillus Isolates Did Not Induce Gene Expression of Secretory Mucins
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Media, and Reagents
4.2. Aspergillus Isolates
4.3. Antifungal Susceptibility
4.4. Isolate Typing
4.5. Aspergillus Epithelial Infection Model
4.6. ELISA Analysis
4.7. Histology and qPCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Breuer, O.; Schultz, A.; Turkovic, L.; de Klerk, N.; Keil, A.D.; Brennan, S.; Harrison, J.; Robertson, C.; Robinson, P.J.; Sly, P.D.; et al. Changing Prevalence of Lower Airway Infections in Young Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 590–599. [Google Scholar] [CrossRef]
- Hong, G.; Psoter, K.J.; Jennings, M.T.; Merlo, C.A.; Boyle, M.P.; Hadjiliadis, D.; Kawut, S.M.; Lechtzin, N. Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus-What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, 1–4. [Google Scholar] [CrossRef]
- Guegan, H.; Chevrier, S.; Belleguic, C.; Deneuville, E.; Robert-Gangneux, F.; Gangneux, J.P. Performance of molecular approaches for Aspergillus detection and azole resistance surveillance in cystic fibrosis. Front. Microbiol. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reece, E.; McClean, S.; Greally, P.; Renwick, J. The prevalence of Aspergillus fumigatus in early cystic fibrosis disease is underestimated by culture-based diagnostic methods. J. Microbiol. Methods 2019, 164, 105683. [Google Scholar] [CrossRef] [PubMed]
- Breuer, O.; Schultz, A.; Garratt, L.W.; Turkovic, L.; Rosenow, T.; Murray, C.P.; Karpievitch, Y.V.; Akesson, L.; Dalton, S.; Sly, P.D.; et al. Aspergillus Infections and Progression of Structural Lung Disease in Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 688–696. [Google Scholar] [CrossRef]
- Gangell, C.L.; Gard, S.E.; Douglas, T.A.; Park, J.; de Klerk, N.H.; Keil, T.; Brennan, S.; Ranganathan, S.; Robins-Browne, R.; Sly, P.D.; et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin. Infect. Dis. 2011, 53, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.C.; Turvey, S.E.; Alves, M.P.; Regamey, N.; Tummler, B.; Hartl, D. Current concepts: Host-pathogen interactions in cystic fibrosis airways disease. Eur. Respir. Rev. 2014, 23, 320–332. [Google Scholar] [CrossRef]
- Sabino, R.; Ferreira, J.A.G.; Moss, R.B.; Valente, J.; Veríssimo, C.; Carolino, E.; Clemons, K.V.; Everson, C.; Banaei, N.; Penner, J.; et al. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Verweij, P.E.; Zhang, J.; Debets, A.J.M.; Meis, J.F.; van de Veerdonk, F.L.; Schoustra, S.E.; Zwaan, B.J.; Melchers, W.J.G. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: A dilemma for clinical management. Lancet Infect. Dis. 2016, 16, e251–e260. [Google Scholar] [CrossRef]
- Fothergill, J.L.; Walshaw, M.J.; Winstanley, C. Transmissible strains of Pseudomonas aeruginosa in cystic fibrosis lung infections. Eur. Respir. J. 2012, 40, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, T.G.P.; Slabbers, L.; de Jong, C.; Melchers, W.J.G.; Hagen, F.; Verweij, P.E.; Merkus, P.; Meis, J.F. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients—A Dutch, multicentre study. J. Cyst. Fibros. 2019, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Chazalet, V.; Debeaupuis, J.P.; Sarfati, J.; Lortholary, J.; Ribaud, P.; Shah, P.; Cornet, M.; Vu Thien, H.; Gluckman, E.; Brücker, G.; et al. Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J. Clin. Microbiol. 1998, 36, 1494–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinea, J.; De Viedma, D.G.; Peláez, T.; Escribano, P.; Muñoz, P.; Meis, J.F.; Klaassen, C.H.W.; Bouza, E. Molecular epidemiology of Aspergillus fumigatus: An in-depth genotypic analysis of isolates involved in an outbreak of invasive aspergillosis. J. Clin. Microbiol. 2011, 49, 3498–3503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravelat, F.N.; Doedt, T.; Chiang, L.Y.; Liu, H.; Filler, S.G.; Patterson, T.F.; Sheppard, D.C. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect. Immun. 2008, 76, 3632–3639. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Debets, A.J.M.; Verweij, P.E.; Melchers, W.J.G.; Zwaan, B.J.; Schoustra, S.E. Asexual sporulation facilitates adaptation: The emergence of azole resistance in Aspergillus fumigatus. Evolution 2015, 69, 2573–2586. [Google Scholar] [CrossRef]
- Singh, A.; Ralhan, A.; Schwarz, C.; Hartl, D.; Hector, A. Fungal Pathogens in CF Airways: Leave or Treat? Mycopathologia 2018, 183, 119–137. [Google Scholar] [CrossRef]
- Jordan, C.L.; Noah, T.L.; Henry, M.M. Therapeutic challenges posed by critical drug-drug interactions in cystic fibrosis. Pediatr. Pulmonol. 2016, 51, S61–S70. [Google Scholar] [CrossRef] [Green Version]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [Green Version]
- Margalit, A.; Kavanagh, K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol. Rev. 2015, 39, 670–687. [Google Scholar] [CrossRef] [Green Version]
- Balloy, V.; Sallenave, J.M.; Wu, Y.; Touqui, L.; Latgé, J.P.; Si-Tahar, M.; Chignard, M. Aspergillus fumigatus-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 MAPK, and ERK1/2 pathways and not by the toll-like receptor-MyD88 pathway. J. Biol. Chem. 2008, 283, 30513–30521. [Google Scholar] [CrossRef] [Green Version]
- Reihill, J.A.; Moore, J.E.; Elborn, J.S.; Ennis, M. Effect of Aspergillus fumigatus and Candida albicans on pro-inflammatory response in cystic fibrosis epithelium. J. Cyst. Fibros. 2011, 10, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Oguma, T.; Asano, K.; Tomomatsu, K.; Kodama, M.; Fukunaga, K.; Shiomi, T.; Ohmori, N.; Ueda, S.; Takihara, T.; Shiraishi, Y.; et al. Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J. Immunol. 2011, 187, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Reece, E.; Doyle, S.; Greally, P.; Renwick, J.; McClean, S. Aspergillus fumigatus inhibits Pseudomonas aeruginosa in co-culture: Implications of a mutually antagonistic relationship on virulence and inflammation in the CF airway. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toor, A.; Culibrk, L.; Singhera, G.K.; Moon, K.; Prudova, A.; Foster, L.J.; Moore, M.M.; Dorscheid, D.R.; Tebbutt, S.J. Transcriptomic and proteomic host response to Aspergillus fumigatus conidia in an air-liquid interface model of human bronchial epithelium. PLoS ONE 2018, 13, e0209652. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M59 Epidemiological Cutoff Values for Antifungal Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Marshall, L.J.; Perks, B.; Ferkol, T.; Shute, J.K. IL-8 released constitutively by primary bronchial epithelial cells in culture forms an inactive complex with secretory component. J. Immunol. 2001, 167, 2816–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutanto, E.N.; Kicic, A.; Foo, C.J.; Stevens, P.T.; Mullane, D.; Knight, D.A.; Stick, S.M.; Arest, C.F. Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: Effects of nonviral and viral stimulation. Am. J. Respir Cell Mol. Biol. 2011, 44, 761–767. [Google Scholar] [CrossRef]
- Montgomery, S.T.; Frey, D.L.; Mall, M.A.; Stick, S.M.; Kicic, A.; Arest, C.F. Rhinovirus Infection Is Associated with Airway Epithelial Cell Necrosis and Inflammation via Interleukin-1 in Young Children with Cystic Fibrosis. Front. Immunol. 2020, 11, 596. [Google Scholar] [CrossRef]
- Botterel, F.; Cordonnier, C.; Barbier, V.; Wingerstmann, L.; Liance, M.; Coste, A.; Escudier, E.; Bretagne, S. Aspergillus fumigatus causes in vitro electrophysiological and morphological modifications in human nasal epithelial cells. Histol. Histopathol. 2002, 17, 1095–1101. [Google Scholar] [CrossRef]
- Botterel, F.; Gross, K.; Ibrahim-Granet, O.; Khoufache, K.; Escabasse, V.; Coste, A.; Cordonnier, C.; Escudier, E.; Bretagne, S. Phagocytosis of Aspergillus fumigatus conidia by primary nasal epithelial cells in vitro. BMC Microbiol. 2008, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.; Hamidi, F.; Leborgne, R.; Beau, R.; Castier, Y.; Mordant, P.; Boukkerou, A.; Latgé, J.P.; Pretolani, M. Penetration of the Human Pulmonary Epithelium by Aspergillus fumigatus Hyphae. J. Infect. Dis. 2018, 218, 1306–1313. [Google Scholar] [CrossRef]
- Hong, G.; Lechtzin, N. Cystic fibrosis: We see fungus among us, but should we care? J. Cyst. Fibros. 2019, 4–5. [Google Scholar] [CrossRef]
- Shanthikumar, S.; Neeland, M.N.; Saffery, R.; Ranganathan, S. Gene modifiers of cystic fibrosis lung disease: A systematic review. Pediatr. Pulmonol. 2019, 54, 1356–1366. [Google Scholar] [CrossRef]
- Hong, G.; Alby, K.; Ng, S.C.W.; Fleck, V.; Kubrak, C.; Rubenstein, R.C.; Dorgan, D.J.; Kawut, S.M.; Hadjiliadis, D. The presence of Aspergillus fumigatus is associated with worse respiratory quality of life in cystic fibrosis. J. Cyst. Fibros. 2019, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.D.; Bae, C.H.; Song, S.Y.; Choi, Y.S. Effect of β-glucan on MUC4 and MUC5B expression in human airway epithelial cells. Int. Forum Allergy Rhinol. 2015, 5, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.S.; Gao, Y.Y.; Liu, M.J.; Liu, Y.Q. Chronic Aspergillus fumigatus exposure upregulates the expression of mucin 5AC in the airways of asthmatic rats. Exp. Lung Res. 2012, 38, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Slesiona, S.; Gressler, M.; Mihlan, M.; Zaehle, C.; Schaller, M.; Barz, D.; Hube, B.; Jacobsen, I.D.; Brock, M. Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Keown, K.; Reid, A.; Moore, J.E.; Taggart, C.C.; Downey, D.G. Coinfection with Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. Eur Respir Rev. 2020, 29. [Google Scholar] [CrossRef]
- Martinovich, K.M.; Iosifidis, T.; Buckley, A.G.; Looi, K.; Ling, K.-M.; Sutanto, E.N.; Kicic-Starcevich, E.; Garratt, L.W.; Shaw, N.C.; Montgomery, S.; et al. Conditionally reprogrammed primary airway epithelial cells maintain morphology, lineage and disease specific functional characteristics. Sci. Rep. 2017, 7, 17971. [Google Scholar] [CrossRef]
- Schneider, D.; Ganesan, S.; Comstock, A.T.; Meldrum, C.A.; Mahidhara, R.; Goldsmith, A.M.; Curtis, J.L.; Martinez, F.J.; Hershenson, M.B.; Sajjan, U. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 332–340. [Google Scholar] [CrossRef]
- Ueki, I.F.; Min-Oo, G.; Kalinowski, A.; Ballon-Landa, E.; Lanier, L.L.; Nadel, J.A.; Koff, J.L. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon λ and antiviral defense in airway epithelium. J. Exp. Med. 2013, 210, 1929–1936. [Google Scholar] [CrossRef]
- Pryce, T.M.; Palladino, S.; Price, D.M.; Gardam, D.J.; Campbell, P.B.; Christiansen, K.J.; Murray, R.J. Rapid identification of fungal pathogens in BacT/ALERT, BACTEC, and BBL MGIT media using polymerase chain reaction and DNA sequencing of the internal transcribed spacer regions. Diagn. Microbiol. Infect. Dis. 2006, 54, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manavathu, E.K.; Cutright, J.; Chandrasekar, P.H. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J. Clin. Microbiol. 1999, 37, 858–861. [Google Scholar] [CrossRef] [Green Version]
- Buckley, A.G.; Looi, K.; Iosifidis, T.; Ling, K.-M.; Sutanto, E.N.; Martinovich, K.M.; Kicic-Starcevich, E.; Garratt, L.W.; Shaw, N.C.; Lannigan, F.J.; et al. Visualisation of Multiple Tight Junctional Complexes in Human Airway Epithelial Cells. Biol. Proced. Online 2018, 20, 3. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.L.; Hale, J.; Hales, B.J.; Dunstan, J.A.; Thomas, W.R.; Prescott, S.L. FOXP3 mRNA expression at 6 months of age is higher in infants who develop atopic dermatitis, but is not affected by giving probiotics from birth. Pediatr. Allergy Immunol. 2007, 18, 10–19. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Lin, W.; Wan, C.; Li, Q.; Jiang, Y. Liquid-based cytopathology test as a novel method to identify Aspergillus in patients with pulmonary aspergillosis. Sci. Rep. 2017, 7, 7528. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ITS Cluster | Isolate ID | MIC Endpoint (µg/mL) | ||
|---|---|---|---|---|
| PZ ND | VOR 1 | IZ 1 | ||
| A—A. fumigatus S10 | CFA 001 | 0.12 | 0.25 | 0.25 |
| CFA 002 | 0.03 | 0.25 | 0.06 | |
| CFA 004 | 0.03 | 0.25 | 0.06 | |
| CFA 005 | 0.015 | 0.25 | <0.015 | |
| CFA 006 | 0.015 | 0.25 | 0.03 | |
| CFA 010 | 0.015 | 0.25 | 0.03 | |
| Inv 1 | 0.25 | 0.5 | 0.5 | |
| Inv 2 | 0.12 | 0.5 | 0.25 | |
| B—Aspergillus spp. isolate CK392 | CFA 007 | 0.03 | 0.5 | 0.06 |
| CFA 008 | 0.015 | 0.25 | 0.12 | |
| CFA 009 | 0.015 | 0.12 | <0.015 | |
| C—A. fumigatus strain I | CFA 003 | 0.015 | 0.25 | 0.03 |
| Non-CF | CF | |
|---|---|---|
| Number (n) | 3 | 3 |
| Age (years) (mean ± SD) | 2.8 ± 0.7 | 2.8 ± 2.9 |
| Gender | 1/3 male | 2/3 male |
| Current Aspergillus BAL culture | Not tested | 0/3 |
| Previous Aspergillus BAL culture | Not tested | 2/3 (A. fumigatus; A. niger) |
| Non-CF | CF | |
|---|---|---|
| Number (n) | 5 | 6 |
| Age (years) (mean ± SD) | 2.9 ± 0.8 | 2.8 ± 2.5 |
| Gender | 3/5 male | 1/6 male |
| Current Aspergillus BAL culture | Not tested | 0/6 |
| Previous Aspergillus BAL culture | Not tested | 2/6 (A. fumigatus; A. niger) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLean, S.A.; Cullen, L.; Gardam, D.J.; Schofield, C.J.; Laucirica, D.R.; Sutanto, E.N.; Ling, K.-M.; Stick, S.M.; Peacock, C.S.; Kicic, A.; et al. Cystic Fibrosis Clinical Isolates of Aspergillus fumigatus Induce Similar Muco-inflammatory Responses in Primary Airway Epithelial Cells. Pathogens 2021, 10, 1020. https://doi.org/10.3390/pathogens10081020
McLean SA, Cullen L, Gardam DJ, Schofield CJ, Laucirica DR, Sutanto EN, Ling K-M, Stick SM, Peacock CS, Kicic A, et al. Cystic Fibrosis Clinical Isolates of Aspergillus fumigatus Induce Similar Muco-inflammatory Responses in Primary Airway Epithelial Cells. Pathogens. 2021; 10(8):1020. https://doi.org/10.3390/pathogens10081020
Chicago/Turabian StyleMcLean, Samantha A., Leilani Cullen, Dianne J. Gardam, Craig J. Schofield, Daniel R. Laucirica, Erika N. Sutanto, Kak-Ming Ling, Stephen M. Stick, Christopher S. Peacock, Anthony Kicic, and et al. 2021. "Cystic Fibrosis Clinical Isolates of Aspergillus fumigatus Induce Similar Muco-inflammatory Responses in Primary Airway Epithelial Cells" Pathogens 10, no. 8: 1020. https://doi.org/10.3390/pathogens10081020
APA StyleMcLean, S. A., Cullen, L., Gardam, D. J., Schofield, C. J., Laucirica, D. R., Sutanto, E. N., Ling, K.-M., Stick, S. M., Peacock, C. S., Kicic, A., Garratt, L. W., on behalf of AREST CF, & WAERP. (2021). Cystic Fibrosis Clinical Isolates of Aspergillus fumigatus Induce Similar Muco-inflammatory Responses in Primary Airway Epithelial Cells. Pathogens, 10(8), 1020. https://doi.org/10.3390/pathogens10081020






