The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
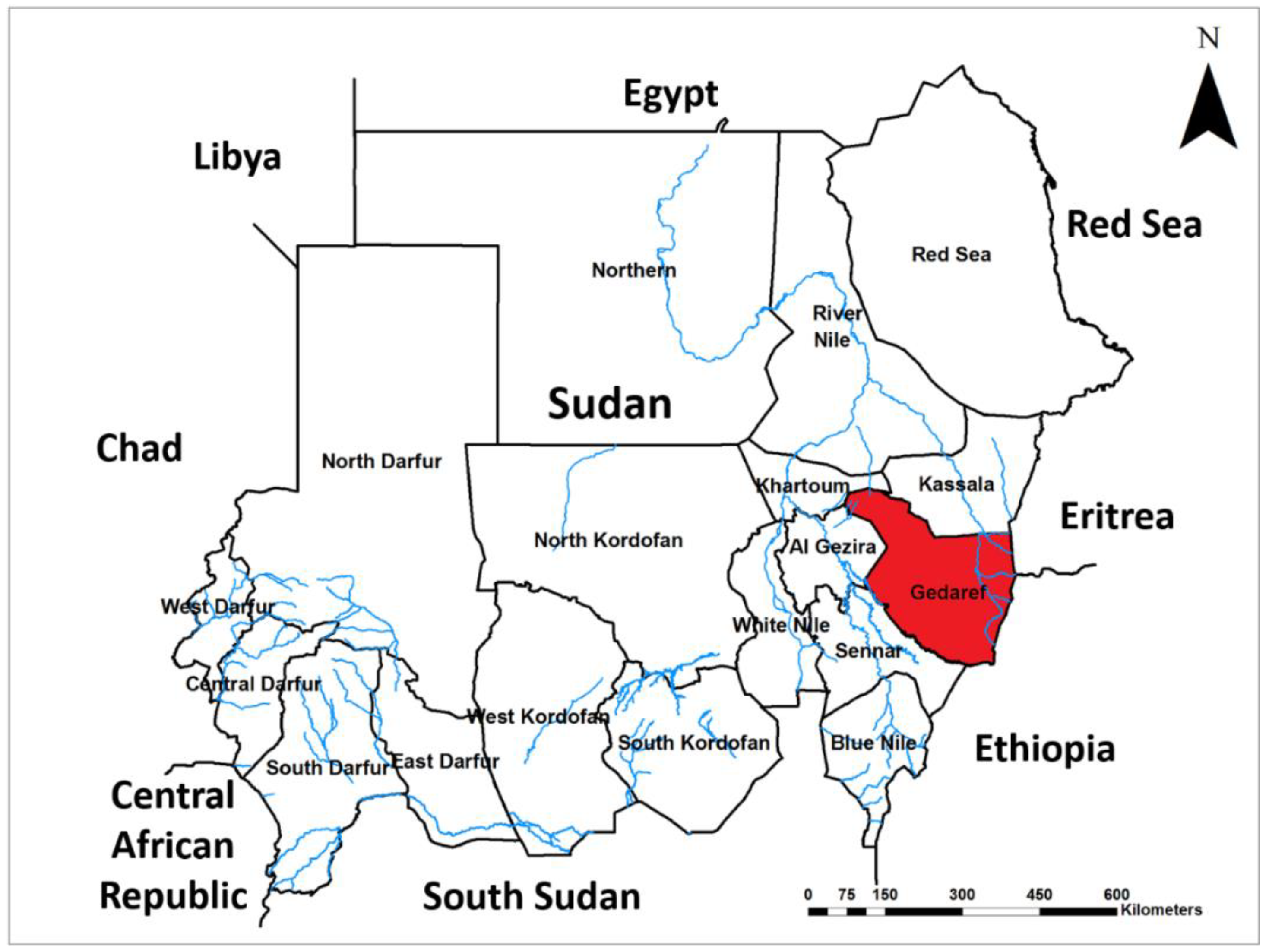
4.2. Study Area
4.3. The Outbreak Investigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.; Ali, Y.; Elduma, A.; Eldigail, M.H.; Mhmoud, R.A.; Mohamed, N.S.; Ksiazek, T.G.; Dietrich, I.; Weaver, S.C. Unique Out-break of Rift Valley Fever in Sudan, 2019. Emerg. Infect. Dis. 2020, 26, 3030–3033. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Indran, S.V.; Balaraman, V.; Wilson, W.C.; Richt, J.A. Molecular aspects of Rift Valley fever virus and the emergence of reassortants. Virus Genes 2018, 55, 1–11. [Google Scholar] [CrossRef]
- Ahmed, A.; Dietrich, I.; LaBeaud, A.D.; Lindsay, S.W.; Musa, A.; Weaver, S.C. Risks and Challenges of Arboviral Diseases in Sudan: The Urgent Need for Actions. Viruses 2020, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.S.; Ali, Y.; Muneer, M.S.; Siddig, E.E.; Sibley, C.H.; Ahmed, A. Malaria epidemic in humanitarian crisis settings the case of South Kordofan state, Sudan. J. Infect. Dev. Ctries. 2021, 15, 168–171. [Google Scholar] [CrossRef]
- Duong, V.; Lambrechts, L.; Paul, R.E.; Ly, S.; Lay, R.S.; Long, K.C.; Huy, R.; Tarantola, A.; Scott, T.W.; Sakuntabhai, A.; et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 14688–14693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Elbashir, A.; Mohamed, A.A.; Alim, A.A.; Mubarak, A.; Abdelrahman, D.; Mohammed, E.; Mohamed, N.S.; Elaagip, A.H.; Zarroug, I.M.A.; et al. Socioeconomic impacts of elimination of onchocerciasis in Abu-Hamed focus, northern Sudan: Lessons after elimination. BMC Res. Notes 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Mohamed, N.S. Arboviral diseases: The emergence of a major yet ignored public health threat in Africa. Lancet Planet. Health 2020, 4, e555. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, Y.; Elmagboul, B.; Mohamed, O.; Elduma, A.; Bashab, H.; Mahamoud, A.; Khogali, H.; Elaagip, A.; Higazi, T. Dengue Fever in the Darfur Area, Western Sudan. Emerg. Infect. Dis. 2019, 25, 2126. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Elduma, A.; Magboul, B.; Higazi, T.; Ali, Y. The First Outbreak of Dengue Fever in Greater Darfur, Western Sudan. Trop. Med. Infect. Dis. 2019, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Eldigail, M.; Elduma, A.; Breima, T.; Dietrich, I.; Ali, Y.; Weaver, S.C. First report of epidemic dengue fever and malaria co-infections among internally displaced persons in humanitarian camps of North Darfur, Sudan. Int. J. Infect. Dis. 2021, 108, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A. Urgent call for a global enforcement of the public sharing of health emergencies data: Lesson learned from serious arboviral disease epidemics in Sudan. Int. Health 2020, 12, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Elduma, A.H.; LaBeaud, A.D.; APlante, J.; Plante, K.S.; Ahmed, A. High Seroprevalence of Dengue Virus Infection in Sudan: Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2020, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Baudin, M.; Jumaa, A.M.; Jomma, H.J.E.; Karsany, M.S.; Bucht, G.; Näslund, J.; Ahlm, C.; Evander, M.; Mohamed, N. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: A cross-sectional study. Lancet Glob. Health 2016, 4, e864–e871. [Google Scholar] [CrossRef] [Green Version]
- Hassan, O.A.; Ahlm, C.; Sang, R.; Evander, M. The 2007 Rift Valley Fever Outbreak in Sudan. PLoS Negl. Trop. Dis. 2011, 5, e1229. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A. Current Status of Mosquito-Borne Arboviruses in Sudan, and Challenges of Surveillance and Responses [Internet]. Online. 2021. Available online: https://www.malariaconsortium.org/pages/webinars/webinar-mosquito-borne-arboviruses-the-rising-global-threat.htm (accessed on 11 March 2021).
- Shoemaker, T.; Boulianne, C.; Vincent, M.J.; Pezzanite, L.; Al-Qahtani, M.M.; Al-Mazrou, Y.; Khan, A.S.; Rollin, P.E.; Swanepoel, R.; Ksiazek, T.G.; et al. Genetic Analysis of Viruses Associated with Emergence of Rift Valley Fever in Saudi Arabia and Yemen, 2000–2001. Emerg. Infect. Dis. 2002, 8, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, V.; Pépin, M.; Plée, L.; Lancelot, R. Rift Valley fever—A threat for Europe? Eurosurveillance 2010, 15, 19506. [Google Scholar] [CrossRef]
- Elaagip, A.; Alsedig, K.; Altahir, O.; Ageep, T.; Ahmed, A.; Siam, H.A.; Samy, A.M.; Mohamed, W.; Khalid, F.; Gumaa, S.; et al. Seroprevalence and associated risk factors of Dengue fever in Kassala state, eastern Sudan. PLoS Negl. Trop. Dis. 2020, 14, e0008918. [Google Scholar] [CrossRef]
- Whiteman, A.; Loaiza, J.R.; Yee, D.A.; Poh, K.C.; Watkins, A.S.; Lucas, K.J.; Rapp, T.J.; Kline, L.; Ahmed, A.; Chen, S.; et al. Do socioeconomic factors drive Aedes mosquito vectors and their arboviral diseases? A systematic review of dengue, chikungunya, yellow fever, and Zika Virus. One Health 2020, 11, 100188. [Google Scholar] [CrossRef]
- Pigott, D.M.; Deshpande, A.; Letourneau, I.; Morozoff, C.; Reiner, R.C.; Kraemer, M.U.G.; Brent, S.E.; Bogoch, I.I.; Khan, K.; Biehl, M.H.; et al. Local, national, and regional viral haemorrhagic fever pandemic potential in Africa: A multistage analysis. Lancet 2017, 390, 2662–2672. [Google Scholar] [CrossRef] [Green Version]
- Drosten, C.; Göttig, S.; Schilling, S.; Asper, M.; Panning, M.; Schmitz, H.; Günther, S. Rapid Detection and Quantification of RNA of Ebola and Marburg Viruses, Lassa Virus, Crimean-Congo Hemorrhagic Fever Virus, Rift Valley Fever Virus, Dengue Virus, and Yellow Fever Virus by Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2002, 40, 2323–2330. [Google Scholar] [CrossRef] [Green Version]
- GAVI TGA for V and I. The Next Pandemic: Rift Valley Fever? 2021. Available online: https://www.gavi.org/vaccineswork/next-pandemic/rift-valley-fever (accessed on 30 May 2021).
- The World Health Organization. Prioritizing Diseases for Research and Development in Emergency Contexts. 2021. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 30 June 2021).
- Pedarrieu, A.; Mellouli, F.E.; Khallouki, H.; Zro, K.; Sebbar, G.; Sghaier, S.; Madani, H.; Bouayed, N.; Lo, M.M.; Diop, M.; et al. External quality assessment of Rift Valley fever diagnosis in countries at risk of the disease: African, Indian Ocean and Middle-East regions. PLoS ONE 2021, 16, e0251263. [Google Scholar] [CrossRef]
- Clark, M.H.A.; Warimwe, G.M.; Nardo, A.D.; Lyons, N.A.; Gubbins, S. Systematic literature review of Rift Valley fever virus se-roprevalence in livestock, wildlife and humans in Africa from 1968 to 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.-J.; Reusken, C. Come fly with me: Review of clinically important arboviruses for global travelers. J. Clin. Virol. 2012, 55, 191–203. [Google Scholar] [CrossRef] [PubMed]
- EUTF for Africa TEUETF for Stability and Addressing Root Causes of Irregular Migration and Displaced Persons in A. Sudan and Ethiopia Met for the First Time to Discuss Improvements to Seasonal Labour Migration. EU Emergency Trust Fund for Africa—European Commission. 2018. Available online: https://ec.europa.eu/trustfundforafrica/all-news-and-stories/sudan-and-ethiopia-met-first-time-discuss-improvements-seasonal-labour_en (accessed on 30 April 2021).
- Hassan, O.A.; Affognon, H.; Rocklöv, J.; Mburu, P.; Sang, R.; Ahlm, C.; Evander, M. The One Health approach to identify knowledge, attitudes and practices that affect community involvement in the control of Rift Valley fever outbreaks. PLoS Negl. Trop. Dis. 2017, 11, e0005383. [Google Scholar] [CrossRef] [Green Version]
- Hassan, O.A.; Ahlm, C.; Evander, M. A need for One Health approach—Lessons learned from outbreaks of Rift Valley fever in Saudi Arabia and Sudan. Infect. Ecol. Epidemiol. 2014, 4, 20710. [Google Scholar] [CrossRef]
- Bashir, R.S.E.; Hassan, O.A. A One Health perspective to identify environmental factors that affect Rift Valley fever transmission in Gezira state, Central Sudan. Trop. Med. Health 2019, 47, 54. [Google Scholar] [CrossRef]
- Petrova, V.; Kristiansen, P.; Norheim, G.; Yimer, S.A. Rift valley fever: Diagnostic challenges and investment needs for vaccine development. BMJ Glob. Health 2020, 5, e002694. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Mohamed, N.S.; El-Sadig, S.M.; Fahal, L.A.; Abelrahim, Z.B.; Ahmed, E.S.; Siddig, E.E. COVID-19 in Sudan. J. Infect. Dev. Ctries. 2021, 15, 204–208. [Google Scholar] [CrossRef]
- Zarroug, I.M.A.; Elaagip, A.; Gumaa, S.G.; Ali, A.K.; Ahmed, A.; Siam, H.A.M.; Abdelgadir, D.M.; Surakat, O.A.; Olamiju, O.J.; Boakye, D.A.; et al. Notes on distribution of Simulium damnosum s. l. along Atbara River in Galabat sub-focus, eastern Sudan. BMC Infect. Dis. 2019, 19, 477. [Google Scholar] [CrossRef] [Green Version]
| Character | Frequency | Percentage/Average | |
|---|---|---|---|
| Sex | Male | 6 | 100% |
| Female | 0 | 0 | |
| Age range | 20–48 years | 6 | 30 years |
| Symptoms | Fever | 6 | 100% |
| Bleeding | 6 | 100% | |
| Headache | 4 | 67% | |
| Laboratory results for RVFV | Positive | 3 | 50% |
| Negative | 3 | 50% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Mahmoud, I.; Eldigail, M.; Elhassan, R.M.; Weaver, S.C. The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations. Pathogens 2021, 10, 885. https://doi.org/10.3390/pathogens10070885
Ahmed A, Mahmoud I, Eldigail M, Elhassan RM, Weaver SC. The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations. Pathogens. 2021; 10(7):885. https://doi.org/10.3390/pathogens10070885
Chicago/Turabian StyleAhmed, Ayman, Iman Mahmoud, Mawahib Eldigail, Rehab M. Elhassan, and Scott C. Weaver. 2021. "The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations" Pathogens 10, no. 7: 885. https://doi.org/10.3390/pathogens10070885
APA StyleAhmed, A., Mahmoud, I., Eldigail, M., Elhassan, R. M., & Weaver, S. C. (2021). The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations. Pathogens, 10(7), 885. https://doi.org/10.3390/pathogens10070885







