Predicting Lifestyle from Positive Selection Data and Genome Properties in Oomycetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Selection and Functional Annotation
2.2. Phylogeny Inference
2.3. Orthogroup Classification and Positive Selection Analyses
2.4. Enrichment Analyses
2.5. Machine Learning Model
3. Results
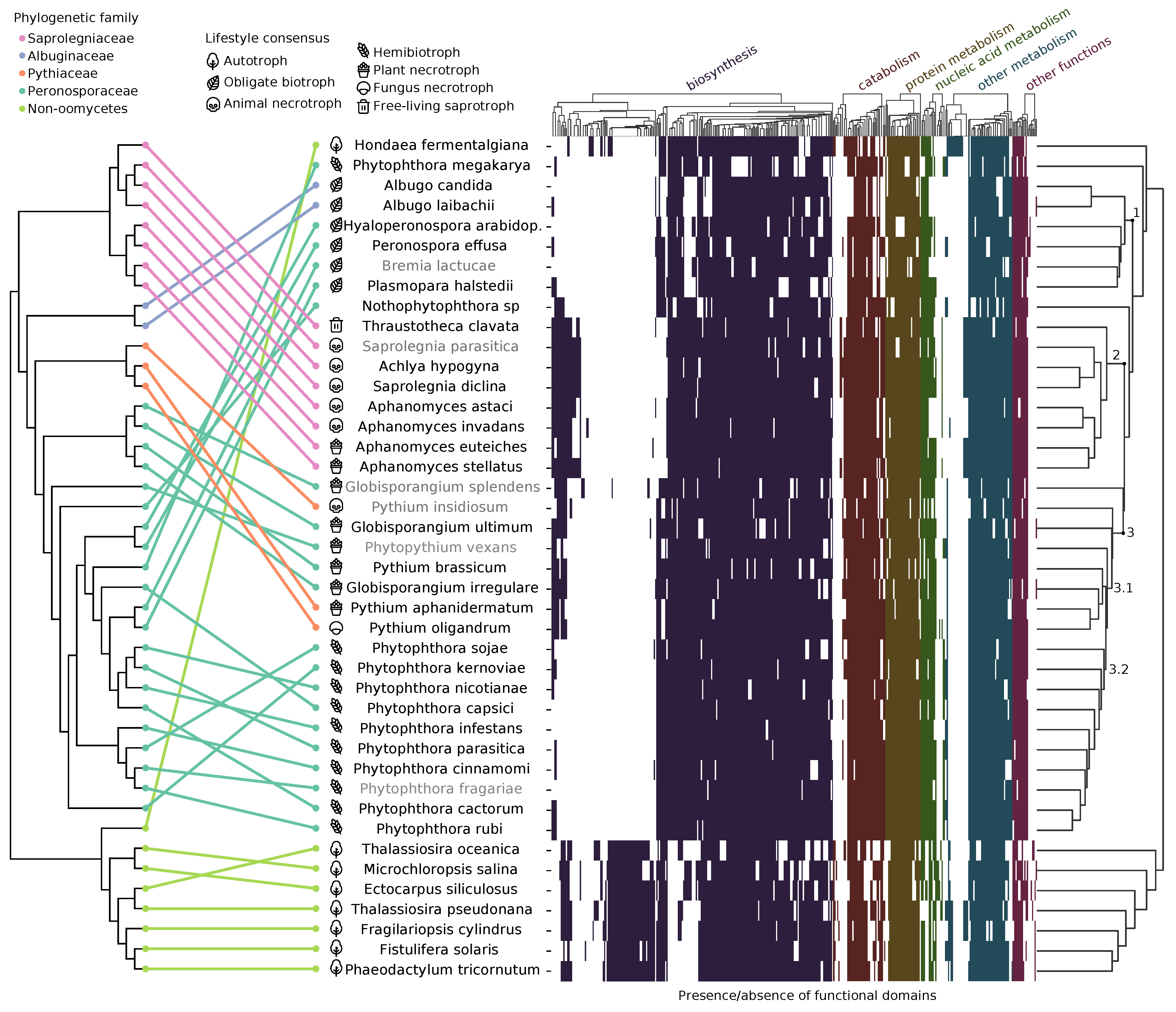
3.1. Proteome Annotation and Clustering
3.2. Ortholog Group Classification
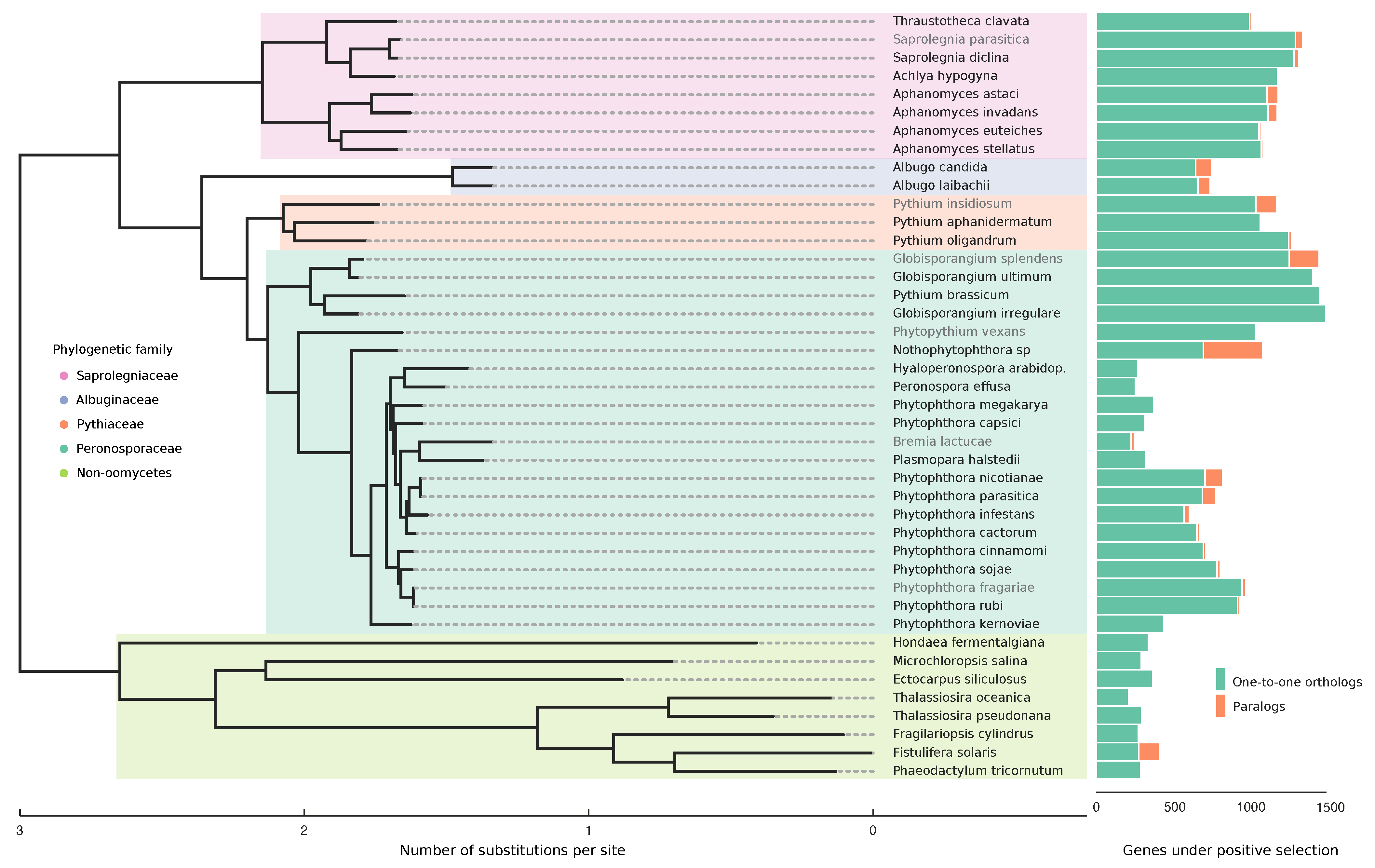
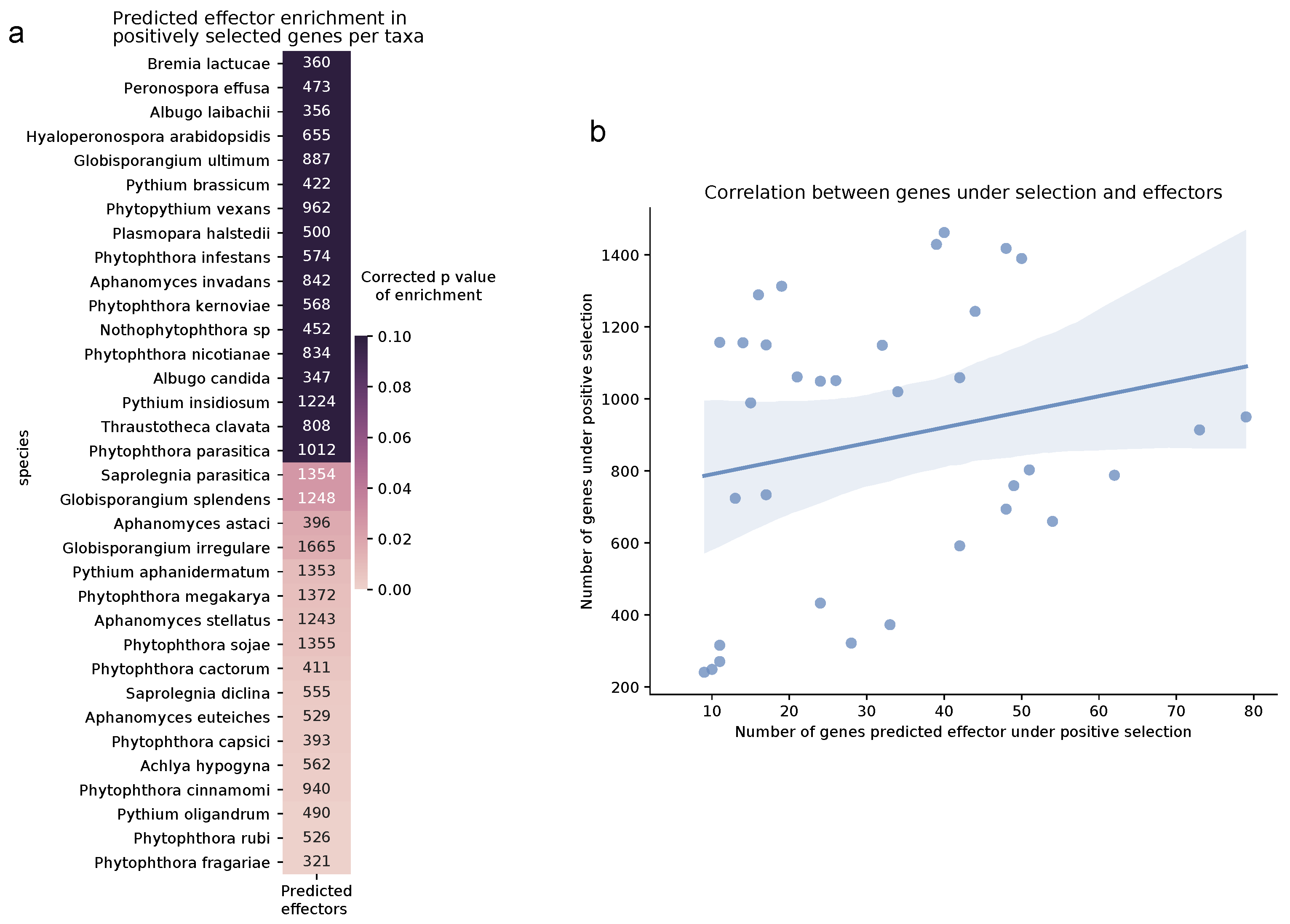
3.3. Positive Selection Analyses
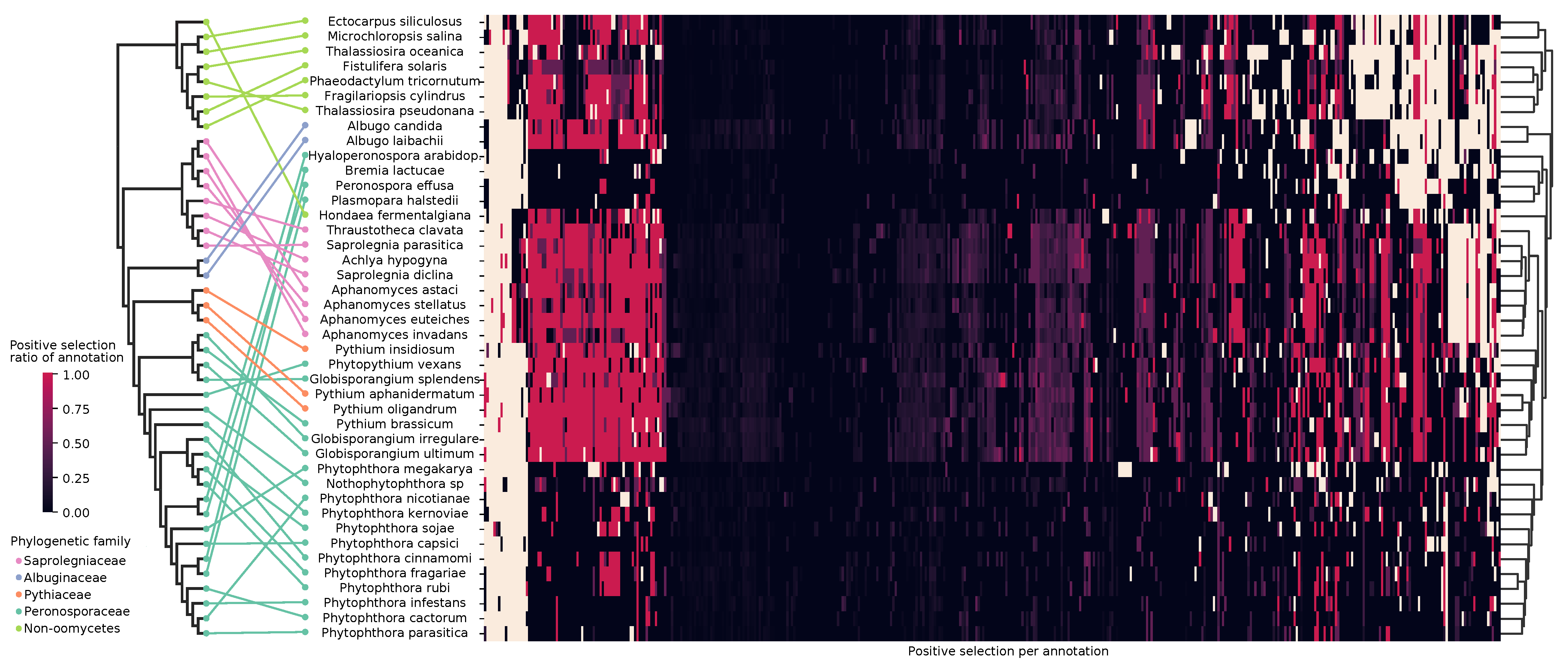
3.4. Enriched Biological Functions under Selection
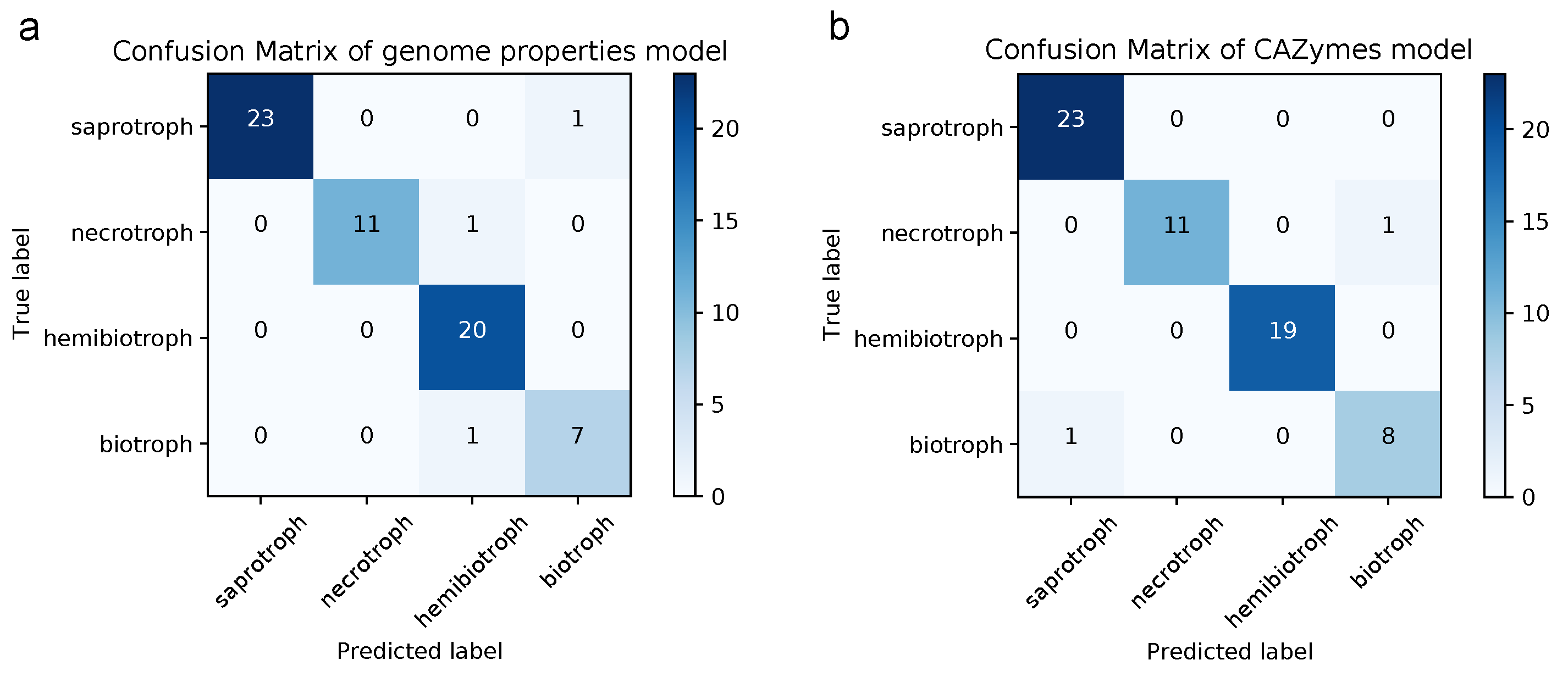
3.5. Lifestyle Prediction
4. Discussion
4.1. Functional Genome Annotations Largely Correlate with Lifestyle
4.2. Generalists Have More Genes under Positive Selection
4.3. Selective Pressures in the Ooomycetes Help Explain Host Adaptation
4.3.1. Selective Pressures Relate to Lifestyles in Oomycetes
4.3.2. Biosynthetic Repertoire Is Important for Lifestyle Adaptation
4.3.3. Protein Family Enrichment Reflects Lifestyle Selective Pressures
4.4. A Model Based on Genome Properties Accurately Predicts Lifestyle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAZyme | Carbohydrate-Active enZyme |
| GO | Gene Ontology |
| THF | tetrahydrofolate |
| UPGMA | Unweighted Pair Group Method with Arithmetic Mean |
Appendix A. Stramenopile Dataset
| Phylogenetic Family | Species Name | Accession | Lifestyle | Complete BUSCOs | Complete and Single-Copy BUSCOs | Complete and Duplicated BUSCOs | Reference |
|---|---|---|---|---|---|---|---|
| Non-oomycete | Ectocarpus siliculosus | GCA_000310025.1 | Autotroph | 97 | 97 | 0 | [98] |
| Fistulifera solaris | GCA_002217885.1 | Autotroph | 97 | 14 | 83 | [62] | |
| Fragilariopsis cylindrus | GCA_001750085.1 | Autotroph | 95 | 95 | 0 | [99] | |
| Hondaea fermentalgiana | GCA_002897355.1 | Autotroph | 95 | 90 | 5 | [100] | |
| Microchloropsis salina | GCA_004565275.1 | Autotroph | 92 | 90 | 2 | [101] | |
| Phaeodactylum tricornutum | GCA_000150955.2 | Autotroph | 97 | 95 | 2 | [102] | |
| Thalassiosira oceanica | GCA_000296195.2 | Autotroph | 90 | 90 | 0 | [103] | |
| Thalassiosira pseudonana | GCA_000149405.2 | Autotroph | 97 | 95 | 2 | [102] | |
| Saprolegniaceae | Achlya hypogyna | GCA_002081595.1 | Animal necrotroph [20] | 99 | 98 | 1 | [20] |
| Aphanomyces astaci | GCA_000520075.1 | Animal necrotroph [104] | 100 | 82 | 18 | ||
| Aphanomyces euteiches | GCA_009835175.1 | Plant necrotroph [19] | 99 | 99 | 0 | ||
| Aphanomyces invadans | GCA_000520115.1 | Animal necrotroph | 100 | 83 | 17 | ||
| Aphanomyces stellatus | GCA_009835185.1 | Plant necrotroph [19] | 97 | 96 | 1 | ||
| Saprolegnia diclina | GCA_000281045.1 | Animal necrotroph [105] | 99 | 98 | 1 | ||
| Saprolegnia parasitica | GCA_000151545.2 | Animal necrotroph [105] | 99 | 99 | 0 | [106] | |
| Thraustotheca clavata | GCA_002081575.1 | Free-living saprotroph [20] | 99 | 98 | 1 | [20] | |
| Albuginaceae | Albugo candida | GCA_001078535.1 | Obligate biotroph [107] | 98 | 86 | 12 | |
| Albugo laibachii | PRJEA53219 | Obligate biotroph [107] | 95 | 82 | 13 | [108] | |
| Peronosporaceae | Bremia lactucae | GCA_004359215.1 | Obligate biotroph [109] | 96 | 90 | 6 | [110] |
| Globisporangium irregulare | GCA_000387425.2 | Plant necrotroph [111] | 98 | 96 | 2 | [112] | |
| Globisporangium splendens | GCA_006386115.1 | Plant necrotroph [113] | 91 | 74 | 17 | [114] | |
| Globisporangium ultimum | GCA_000143045.1 | Plant necrotroph [115] | 94 | 93 | 1 | [112] | |
| Hyaloperonospora arabidopsidis | GCA_000173235.2 | Obligate biotroph [116] | 89 | 82 | 7 | [116] | |
| Nothophytophthora sp. | GCA_001712635.2 | 90 | 28 | 62 | [61] | ||
| Peronospora effusa | GCA_003843895.1 | Obligate biotroph [117] | 94 | 93 | 1 | ||
| Phytophthora cactorum | GCA_003287315.1 | Hemibiotroph [118] | 100 | 98 | 2 | [119] | |
| Phytophthora capsici | GCA_000325885.1 | Hemibiotroph [120] | 98 | 97 | 1 | [121] | |
| Phytophthora cinnamomi | GCA_001314365.1 | Hemibiotroph [122] | 96 | 94 | 2 | [123] | |
| Phytophthora fragariae | GCA_009729455.1 | Hemibiotroph | 94 | 93 | 1 | [124] | |
| Phytophthora infestans | GCA_000142945.1 | Hemibiotroph [125] | 100 | 99 | 1 | ||
| Phytophthora kernoviae | GCA_001712645.2 | Hemibiotroph [126] | 96 | 96 | 0 | [61] | |
| Phytophthora megakarya | GCA_002215365.1 | Hemibiotroph | 91 | 90 | 1 | [127] | |
| Phytophthora nicotianae | GCA_001483015.1 | Hemibiotroph [128] | 99 | 86 | 13 | [129] | |
| Phytophthora parasitica | GCA_000247585.2 | Hemibiotroph [130] | 98 | 87 | 11 | ||
| Phytophthora rubi | GCA_009733145.1 | Hemibiotroph | 100 | 98 | 2 | [124] | |
| Phytophthora sojae | GCA_000149755.2 | Hemibiotroph [131] | 99 | 98 | 1 | [132] | |
| Phytopythium vexans | GCA_000387545.2 | Plant necrotroph [17] | 94 | 92 | 2 | [17] | |
| Pythium brassicum | GCA_008271595.1 | Plant necrotroph [133] | 100 | 99 | 1 | ||
| Plasmopara halstedii | GCA_900000015.1 | Obligate biotroph [134] | 100 | 100 | 0 | ||
| Pythiaceae | Pythium aphanidermatum | GCA_000387445.2 | Plant necrotroph [135] | 94 | 93 | 1 | [17] |
| Pythium insidiosum | GCA_001029375.1 | Animal necrotroph [136] | 99 | 87 | 12 | [137] | |
| Pythium oligandrum | GCA_005966545.1 | Fungal necrotroph [138] | 100 | 100 | 0 | [139] |
References
- Zhang, W.; Zhang, X.; Li, K.; Wang, C.; Cai, L.; Zhuang, W.; Xiang, M.; Liu, X. Introgression and gene family contraction drive the evolution of lifestyle and host shifts of hypocrealean fungi. Mycology 2018, 9, 176–188. [Google Scholar] [CrossRef]
- Props, R.; Monsieurs, P.; Vandamme, P.; Leys, N.; Denef, V.J.; Boon, N. Gene Expansion and Positive Selection as Bacterial Adaptations to Oligotrophic Conditions. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Scannell, D.R.; Byrne, K.P.; Gordon, J.L.; Wong, S.; Wolfe, K.H. Multiple rounds of speciation associated with reciprocal gene loss in polyploid yeasts. Nature 2006, 440, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Rocha, E.P.C. Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in Prokaryotes. PLoS Genet. 2011, 7, e1001284. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.O.; Raguideau, S.; Quince, C.; Holden, J.; Zhang, L.; Gaze, W.H.; Holden, J.; Mead, A.; Raguideau, S.; Quince, C.; et al. Gene duplication drives genome expansion in a major lineage of Thaumarchaeota. Nat. Commun. 2020, 11, 5494. [Google Scholar] [CrossRef]
- Behe, M. Experimental evolution, loss-of-function mutations, and “the first rule of adaptive evolution”. Q. Rev. Biol. 2010, 85, 419–445. [Google Scholar] [CrossRef][Green Version]
- Yang, Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol. Biol. Evol. 1998, 15, 568–573. [Google Scholar] [CrossRef]
- Hughes, A.L.; Packer, B.; Welch, R.; Bergen, A.W.; Chanock, S.J.; Yeager, M. Widespread purifying selection at polymorphic sites in human protein-coding loci. Proc. Natl. Acad. Sci. USA 2003, 100, 15754–15757. [Google Scholar] [CrossRef] [PubMed]
- Kosiol, C.; Vinař, T.; da Fonseca, R.R.; Hubisz, M.J.; Bustamante, C.D.; Nielsen, R.; Siepel, A. Patterns of Positive Selection in Six Mammalian Genomes. PLoS Genet. 2008, 4, e1000144. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Chao, E.E.Y. Phylogeny and Megasystematics of Phagotrophic Heterokonts (Kingdom Chromista). J. Mol. Evol. 2006, 62, 388–420. [Google Scholar] [CrossRef]
- Beakes, G.W.; Thines, M. Handbook of the Protists; Springer: Cham, Switzerland, 2017; pp. 435–505. [Google Scholar] [CrossRef]
- Matari, N.H.; Blair, J.E. A multilocus timescale for oomycete evolution estimated under three distinct molecular clock models. BMC Evol. Biol. 2014, 14, 101. [Google Scholar] [CrossRef]
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015, 74, 62–64. [Google Scholar] [CrossRef]
- Derevnina, L.; Petre, B.; Kellner, R.; Dagdas, Y.F.; Sarowar, M.N.; Giannakopoulou, A.; De la Concepcion, J.C.; Chaparro-Garcia, A.; Pennington, H.G.; Van West, P.; et al. Emerging oomycete threats to plants and animals. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150459. [Google Scholar] [CrossRef]
- Kemen, E.; Jones, J.D. Obligate biotroph parasitism: Can we link genomes to lifestyles? Trends Plant Sci. 2012, 17, 448–457. [Google Scholar] [CrossRef]
- Lee, S.J.; Rose, J.K.C. Mediation of the transition from biotrophy to necrotrophy in hemibiotrophic plant pathogens by secreted effector proteins. Plant Signal. Behav. 2010, 5, 769–772. [Google Scholar] [CrossRef]
- Adhikari, B.N.; Hamilton, J.P.; Zerillo, M.M.; Tisserat, N.; Lévesque, C.A.; Buell, C.R. Comparative Genomics Reveals Insight into Virulence Strategies of Plant Pathogenic Oomycetes. PLoS ONE 2013, 8, e75072. [Google Scholar] [CrossRef]
- Steciow, M.M.; Lara, E.; Paul, C.; Pillonel, A.; Belbahri, L. Multiple barcode assessment within the Saprolegnia-Achlya clade (Saprolegniales, Oomycota, Straminipila) brings order in a neglected group of pathogens. IMA Fungus 2014, 5, 439–448. [Google Scholar] [CrossRef]
- Gaulin, E.; Jacquet, C.; Bottin, A.; Dumas, B. Root rot disease of legumes caused by Aphanomyces euteiches. Mol. Plant Pathol. 2007, 8, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Misner, I.; Blouin, N.; Leonard, G.; Richards, T.A.; Lane, C.E. The Secreted Proteins of Achlya hypogyna and Thraustotheca clavata Identify the Ancestral Oomycete Secretome and Reveal Gene Acquisitions by Horizontal Gene Transfer. Genome Biol. Evol. 2015, 7, 120–135. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; O’Hanlon, R.; Owens, R.A.; Fitzpatrick, D.A. Comparative Genomic and Proteomic Analyses of Three Widespread Phytophthora Species: Phytophthora chlamydospora, Phytophthora gonapodyides and Phytophthora pseudosyringae. Microorganisms 2020, 8, 653. [Google Scholar] [CrossRef] [PubMed]
- Weiblen, C.; Robe, L.J.; de Azevedo, M.I.; Ianiski, L.B.; Stibbe, P.C.; Ribeiro, T.C.; Zanette, R.A.; Pereira, D.I.B.; Santurio, J.M.; Botton, S.D.A. New insights on evolutionary aspects of Pythium insidiosum and other peronosporaleans. Mycoses 2020. [Google Scholar] [CrossRef]
- Thines, M. An evolutionary framework for host shifts—Jumping ships for survival. New Phytol. 2019, 224. [Google Scholar] [CrossRef]
- Haas, B.J.; Kamoun, S.; Zody, M.C.; Jiang, R.H.Y.; Handsaker, R.E.; Cano, L.M.; Grabherr, M.; Kodira, C.D.; Raffaele, S.; Torto-Alalibo, T.; et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 2009, 461, 393–398. [Google Scholar] [CrossRef]
- Richards, T.A.; Soanes, D.M.; Jones, M.D.M.; Vasieva, O.; Leonard, G.; Paszkiewicz, K.; Foster, P.G.; Hall, N.; Talbot, N.J. Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proc. Natl. Acad. Sci. USA 2011, 108, 15258–15263. [Google Scholar] [CrossRef]
- Savory, F.; Leonard, G.; Richards, T.A. The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes. PLoS Pathog. 2015, 11, e1004805. [Google Scholar] [CrossRef]
- Savory, E.A.; Fuller, S.L.; Weisberg, A.J.; Thomas, W.J.; Gordon, M.I.; Stevens, D.M.; Creason, A.L.; Belcher, M.S.; Serdani, M.; Wiseman, M.S.; et al. Evolutionary transitions between beneficial and phytopathogenic Rhodococcus challenge disease management. eLife 2017, 6, e30925. [Google Scholar] [CrossRef] [PubMed]
- Money, N.P.; Davis, C.M.; Ravishankar, J. Biomechanical evidence for convergent evolution of the invasive growth process among fungi and oomycete water molds. Fungal Genet. Biol. 2004, 41, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Latijnhouwers, M.; de Wit, P.J.; Govers, F. Oomycetes and fungi: Similar weaponry to attack plants. Trends Microbiol. 2003, 11, 462–469. [Google Scholar] [CrossRef]
- Richards, T.A.; Dacks, J.B.; Jenkinson, J.M.; Thornton, C.R.; Talbot, N.J. Evolution of Filamentous Plant Pathogens: Gene Exchange across Eukaryotic Kingdoms. Curr. Biol. 2006, 16, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Manni, M.; Zdobnov, E.M. Gene Prediction, Methods and Protocols. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Nur, M.; Wood, K.; Michelmore, R. EffectorO: Motif-independent prediction of effectors in oomycete genomes using machine learning and lineage specificity. BioRxiv 2021. [Google Scholar] [CrossRef]
- Richardson, L.J.; Rawlings, N.D.; Salazar, G.A.; Almeida, A.; Haft, D.R.; Ducq, G.; Sutton, G.G.; Finn, R.D. Genome properties in 2019: A new companion database to InterPro for the inference of complete functional attributes. Nucleic Acids Res. 2018, 47, D564–D572. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Foulds, L. Comparison of phylogenetic trees. Math. Biosci. 1981, 53, 131–147. [Google Scholar] [CrossRef]
- Goluch, T.; Bogdanowicz, D.; Giaro, K. Visual TreeCmp: Comprehensive Comparison of Phylogenetic Trees on the Web. Methods Ecol. Evol. 2020, 11, 494–499. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Köster, J.; Rahmann, S. Snakemake—A scalable bioinformatics workflow engine. Bioinformatics 2012, 28, 2520–2522. [Google Scholar] [CrossRef]
- Katoh, K.; Asimenos, G.; Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 2009, 537, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Findeiß, S.; Steiner, L.; Marz, M.; Stadler, P.F.; Prohaska, S.J. Proteinortho: Detection of (Co-)orthologs in large-scale analysis. BMC Bioinform. 2011, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Wertheim, J.O.; Weaver, S.; Murrell, B.; Scheffler, K.; Pond, S.L.K. Less Is More: An Adaptive Branch-Site Random Effects Model for Efficient Detection of Episodic Diversifying Selection. Mol. Biol. Evol. 2015, 32, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Pond, S.L.K.; Scheffler, K. FUBAR: A Fast, Unconstrained Bayesian AppRoximation for Inferring Selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Carbon, S.; Douglass, E.; Dunn, N.; Good, B.; Harris, N.L.; Lewis, S.E.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; et al. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2018, 47, gky1055. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Vesztrocy, A.W.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L.; et al. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37, D211–D215. [Google Scholar] [CrossRef]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-Scale Machine Learning on Heterogeneous Systems. 2015. Available online: tensorflow.org (accessed on 24 June 2021).
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, gky418. [Google Scholar] [CrossRef]
- O’Malley, T.; Bursztein, E.; Long, J.; Chollet, F.; Jin, H.; Invernizzi, L.; et al. Keras Tuner. 2019. Available online: https://github.com/keras-team/keras-tuner (accessed on 24 June 2021).
- Li, L.; Jamieson, K.; DeSalvo, G.; Rostamizadeh, A.; Talwalkar, A. Hyperband: A Novel Bandit-Based Approach to Hyperparameter Optimization. J. Mach. Learn. Res. 2018, 18, 6765–6816. [Google Scholar]
- Basenko, E.; Pulman, J.; Shanmugasundram, A.; Harb, O.; Crouch, K.; Starns, D.; Warrenfeltz, S.; Aurrecoechea, C.; Stoeckert, C.; Kissinger, J.; et al. FungiDB: An Integrated Bioinformatic Resource for Fungi and Oomycetes. J. Fungi 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- De Cock, A.; Lodhi, A.; Rintoul, T.; Bala, K.; Robideau, G.; Abad, Z.G.; Coffey, M.; Shahzad, S.; Lévesque, C. Phytopythium: Molecular phylogeny and systematics. Persoonia Mol. Phylogeny Evol. Fungi 2015, 34, 25–39. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Fitzpatrick, D.A. Recent advances in oomycete genomics. Adv. Genet. 2020, 105, 175–228. [Google Scholar] [CrossRef]
- Martens, C.; Van de Peer, Y. The hidden duplication past of the plant pathogen Phytophthora and its consequences for infection. BMC Genom. 2010, 11, 353. [Google Scholar] [CrossRef]
- Studholme, D.J.; Panda, P.; Stowasser, E.S.V.; González, M.; Hill, R.; Sambles, C.; Grant, M.; Williams, N.M.; McDougal, R.L. Genome sequencing of oomycete isolates from Chile supports the New Zealand origin of Phytophthora kernoviae and makes available the first Nothophytophthora sp. genome: Comparative genomics of Chilean oomycete isolates. Mol. Plant Pathol. 2018, 20, 423–431. [Google Scholar] [CrossRef]
- Tanaka, T.; Maeda, Y.; Veluchamy, A.; Tanaka, M.; Abida, H.; Maréchal, E.; Bowler, C.; Muto, M.; Sunaga, Y.; Tanaka, M.; et al. Oil accumulation by the oleaginous diatom Fistulifera solaris as revealed by the genome and transcriptome. Plant Cell 2015, 27, 162–176. [Google Scholar] [CrossRef]
- Gaulin, E.; Pel, M.J.C.; Camborde, L.; San-Clemente, H.; Courbier, S.; Dupouy, M.A.; Lengellé, J.; Veyssiere, M.; Ru, A.L.; Grandjean, F.; et al. Genomics analysis of Aphanomyces spp. identifies a new class of oomycete effector associated with host adaptation. BMC Biol. 2018, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.A.; Green, J.R.; Manners, J.M.; Maclean, D.J. Cloning and characterisation of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis. Curr. Genet. 1997, 31, 447–454. [Google Scholar] [CrossRef]
- Hallen, H.E.; Huebner, M.; Shiu, S.H.; Güldener, U.; Trail, F. Gene expression shifts during perithecium development in Gibberella zeae (anamorph Fusarium graminearum), with particular emphasis on ion transport proteins. Fungal Genet. Biol. 2007, 44, 1146–1156. [Google Scholar] [CrossRef]
- Walley, J.W.; Kliebenstein, D.J.; Bostock, R.M.; Dehesh, K. Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 2013, 16, 520–526. [Google Scholar] [CrossRef]
- McCutcheon, J.P.; Moran, N.A. Functional Convergence in Reduced Genomes of Bacterial Symbionts Spanning 200 My of Evolution. Genome Biol. Evol. 2010, 2, 708–718. [Google Scholar] [CrossRef]
- Shang, Y.; Xiao, G.; Zheng, P.; Cen, K.; Zhan, S.; Wang, C. Divergent and Convergent Evolution of Fungal Pathogenicity. Genome Biol. Evol. 2016, 8, 1374–1387. [Google Scholar] [CrossRef]
- Rodenburg, S.Y.; De Ridder, D.; Govers, F.; Seidl, M.F. Oomycete metabolism is highly dynamic and reflects lifestyle adaptations. BioRxiv 2020. [Google Scholar] [CrossRef]
- Lewis, D.H. Concepts in Fungal Nutrition and the Origin of Biotrophy. Biol. Rev. 1973, 48, 261–277. [Google Scholar] [CrossRef]
- Svoboda, J.; Mrugała, A.; Kozubíková-Balcarová, E.; Petrusek, A. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. J. Fish Dis. 2016, 40, 127–140. [Google Scholar] [CrossRef]
- Mélida, H.; Sandoval-Sierra, J.V.; Diéguez-Uribeondo, J.; Bulone, V. Analyses of Extracellular Carbohydrates in Oomycetes Unveil the Existence of Three Different Cell Wall Types. Eukaryot. Cell 2013, 12, 194–203. [Google Scholar] [CrossRef]
- Wang, E.; Schornack, S.; Marsh, J.; Gobbato, E.; Schwessinger, B.; Eastmond, P.; Schultze, M.; Kamoun, S.; Oldroyd, G. A Common Signaling Process that Promotes Mycorrhizal and Oomycete Colonization of Plants. Curr. Biol. 2012, 22, 2242–2246. [Google Scholar] [CrossRef]
- Zheng, L.; Mackrill, J.J. Calcium Signaling in Oomycetes: An Evolutionary Perspective. Front. Physiol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Judelson, H.S. Dynamics and Innovations within Oomycete Genomes: Insights into Biology, Pathology, and Evolution. Eukaryot. Cell 2012, 11, 1304–1312. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.M.; Yvin, J.C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 Glucans Are Elicitors of Defense Responses in Tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Précigout, P.A.; Claessen, D.; Makowski, D.; Robert, C. Does the Latent Period of Leaf Fungal Pathogens Reflect Their Trophic Type? A Meta-Analysis of Biotrophs, Hemibiotrophs, and Necrotrophs. Phytopathology 2020, 110, 345–361. [Google Scholar] [CrossRef]
- Xiang, Q.; Judelson, H.S. Myb transcription factors in the oomycete Phytophthora with novel diversified DNA-binding domains and developmental stage-specific expression. Gene 2010, 453, 1–8. [Google Scholar] [CrossRef]
- Xiang, Q.; Judelson, H.S. Myb Transcription Factors and Light Regulate Sporulation in the Oomycete Phytophthora infestans. PLoS ONE 2014, 9, e92086. [Google Scholar] [CrossRef]
- Rocafort, M.; Fudal, I.; Mesarich, C.H. Apoplastic effector proteins of plant-associated fungi and oomycetes. Curr. Opin. Plant Biol. 2020, 56, 9–19. [Google Scholar] [CrossRef]
- Wang, Y.; Tyler, B.M.; Wang, Y. Defense and Counterdefense during Plant-Pathogenic Oomycete Infection. Annu. Rev. Microbiol. 2019, 73, 667–696. [Google Scholar] [CrossRef]
- Raffaele, S.; Win, J.; Cano, L.M.; Kamoun, S. Analyses of genome architecture and gene expression reveal novel candidate virulence factors in the secretome of Phytophthora infestans. BMC Genom. 2010, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Idänheimo, N.; Gauthier, A.; Salojärvi, J.; Siligato, R.; Brosché, M.; Kollist, H.; Mähönen, A.P.; Kangasjärvi, J.; Wrzaczek, M. The Arabidopsis thaliana cysteine-rich receptor-like kinases CRK6 and CRK7 protect against apoplastic oxidative stress. Biochem. Biophys. Res. Commun. 2014, 445, 457–462. [Google Scholar] [CrossRef]
- Grenville-Briggs, L.J.; Avrova, A.O.; Bruce, C.R.; Williams, A.; Whisson, S.C.; Birch, P.R.; van West, P. Elevated amino acid biosynthesis in Phytophthora infestans during appressorium formation and potato infection. Fungal Genet. Biol. 2005, 42, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, P.; Srivastava, V.; Ekengren, S.; McKee, L.S.; Bulone, V. Comparative analysis of sterol acquisition in the oomycetes Saprolegnia parasitica and Phytophthora infestans. PLoS ONE 2017, 12, e0170873. [Google Scholar] [CrossRef]
- Daumann, M.; Fischer, M.; Niopek-Witz, S.; Girke, C.; Möhlmann, T. Apoplastic Nucleoside Accumulation in Arabidopsis Leads to Reduced Photosynthetic Performance and Increased Susceptibility against Botrytis cinerea. Front. Plant Sci. 2015, 6, 1158. [Google Scholar] [CrossRef]
- Fones, H.; Preston, G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol. Lett. 2012, 327, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D. Differences in the Sterol Synthesizing Pathways of Sterol-Producing and Non-Sterol-Producing Fungi. Phytopathology 1978, 68, 1168. [Google Scholar] [CrossRef]
- Gaulin, E.; Bottin, A.; Dumas, B. Sterol biosynthesis in oomycete pathogens. Plant Signal. Behav. 2010, 5, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Ah-Fong, A.M.V.; Kagda, M.S.; Abrahamian, M.; Judelson, H.S. Niche-specific metabolic adaptation in biotrophic and necrotrophic oomycetes is manifested in differential use of nutrients, variation in gene content, and enzyme evolution. PLoS Pathog. 2019, 15, e1007729. [Google Scholar] [CrossRef]
- Huennekens, F. Folic Acid Coenzymes in the Biosynthesis of Purines and Pyrimidines. Vitam. Horm. 1969, 26, 375–394. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R. Molybdenum Cofactor Biosynthesis and Molybdenum Enzymes. Plant Biol. 2006, 57, 623–647. [Google Scholar] [CrossRef] [PubMed]
- Judelson, H.S. Metabolic Diversity and Novelties in the Oomycetes. Annu. Rev. Microbiol. 2016, 71, 21–39. [Google Scholar] [CrossRef]
- Zhang, J.; Rosenberg, H.F.; Nei, M. Positive Darwinian selection after gene duplication in primate ribonuclease genes. Proc. Natl. Acad. Sci. USA 1998, 95, 3708–3713. [Google Scholar] [CrossRef]
- King, B.C.; Waxman, K.D.; Nenni, N.V.; Walker, L.P.; Bergstrom, G.C.; Gibson, D.M. Arsenal of plant cell wall degrading enzymes reflects host preference among plant pathogenic fungi. Biotechnol. Biofuels 2011, 4, 4. [Google Scholar] [CrossRef]
- Hane, J.K.; Paxman, J.; Jones, D.A.B.; Oliver, R.P.; de Wit, P. “CATAStrophy”, a Genome-Informed Trophic Classification of Filamentous Plant Pathogens—How Many Different Types of Filamentous Plant Pathogens Are There? Front. Microbiol. 2020, 10, 3088. [Google Scholar] [CrossRef]
- Torruella, G.; de Mendoza, A.; Grau-Bové, X.; Antó, M.; Chaplin, M.; del Campo, J.; Eme, L.; Pérez-Cordón, G.; Whipps, C.; Nichols, K.; et al. Phylogenomics Reveals Convergent Evolution of Lifestyles in Close Relatives of Animals and Fungi. Curr. Biol. 2015, 25, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef]
- Mock, T. Extensive genetic diversity and differential bi-allelic expression in a Southern Ocean diatom. Eur. J. Phycol. 2015, 50, 75. [Google Scholar]
- Seddiki, K.; Godart, F.; Cigliano, R.A.; Sanseverino, W.; Barakat, M.; Ortet, P.; Rébeillé, F.; Maréchal, E.; Cagnac, O.; Amato, A. Sequencing, De Novo Assembly, and Annotation of the Complete Genome of a New Thraustochytrid Species, Strain CCAP_4062/3. Genome Announc. 2018, 6, e01335-17. [Google Scholar] [CrossRef]
- Ohan, J.A.; Hovde, B.T.; Zhang, X.L.; Davenport, K.W.; Chertkov, O.; Han, C.; Twary, S.N.; Starkenburg, S.R. Nuclear Genome Assembly of the Microalga Nannochloropsis salina CCMP1776. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Lommer, M.; Specht, M.; Roy, A.S.; Kraemer, L.; Andreson, R.; Gutowska, M.A.; Wolf, J.; Bergner, S.V.; Schilhabel, M.B.; Klostermeier, U.C.; et al. Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genome Biol. 2012, 13, R66. [Google Scholar] [CrossRef]
- Alderman, D.J.; Polglase, J.L.; Frayling, M. Aphanomyces astaci pathogenicity under laboratory and field conditions. J. Fish Dis. 1987, 10, 385–393. [Google Scholar] [CrossRef]
- Willoughby, L.G. Saprolegnias of salmonid fish in Windermere: A critical analysis. J. Fish Dis. 1978, 1, 51–67. [Google Scholar] [CrossRef]
- Jiang, R.H.Y.; de Bruijn, I.; Haas, B.J.; Belmonte, R.; Löbach, L.; Christie, J.; van den Ackerveken, G.; Bottin, A.; Bulone, V.; Díaz-Moreno, S.M.; et al. Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen Saprolegnia parasitica. PLoS Genet. 2013, 9, e1003272. [Google Scholar] [CrossRef]
- Ruhe, J.; Agler, M.T.; Placzek, A.; Kramer, K.; Finkemeier, I.; Kemen, E.M. Obligate Biotroph Pathogens of the Genus Albugo Are Better Adapted to Active Host Defense Compared to Niche Competitors. Front. Plant Sci. 2016, 7, 820. [Google Scholar] [CrossRef]
- Kemen, E.; Gardiner, A.; Schultz-Larsen, T.; Kemen, A.C.; Balmuth, A.L.; Robert-Seilaniantz, A.; Bailey, K.; Holub, E.; Studholme, D.J.; MacLean, D.; et al. Gene Gain and Loss during Evolution of Obligate Parasitism in the White Rust Pathogen of Arabidopsis thaliana. PLoS Biol. 2011, 9, e1001094. [Google Scholar] [CrossRef]
- Francis, D.M.; Hulbert, S.H.; Michelmore, R.W. Genome size and complexity of the obligate fungal pathogen, Bremia lactucae. Exp. Mycol. 1990, 14, 299–309. [Google Scholar] [CrossRef]
- Fletcher, K.; Gil, J.; Bertier, L.D.; Kenefick, A.; Wood, K.J.; Zhang, L.; Reyes-Chin-Wo, S.; Cavanaugh, K.; Tsuchida, C.; Wong, J.; et al. Genomic signatures of somatic hybrid vigor due to heterokaryosis in the oomycete pathogen, Bremia lactucae. BioRxiv 2019, 516526. [Google Scholar] [CrossRef]
- Hancock, J.G. Seedling and Rootlet Diseases of Forage Alfalfa Caused by Pythium irregulare. Plant Dis. 1991, 75, 691. [Google Scholar] [CrossRef]
- Lévesque, C.A.; Brouwer, H.; Cano, L.; Hamilton, J.P.; Holt, C.; Huitema, E.; Raffaele, S.; Robideau, G.P.; Thines, M.; Win, J.; et al. Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol. 2010, 11, R73. [Google Scholar] [CrossRef] [PubMed]
- Linde, C. Root and Root Collar Disease of Eucalyptus grandis Caused by Pythium splendens. Plant Dis. 1994, 78, 10061. [Google Scholar] [CrossRef]
- Reghu, R.J.; Chellappan, B.V.; Beena, S.H.; Sasi, A.; Vasudevan, S.E.; Nair, A.S. Draft Genome Sequence of the Oomycete Globisporangium splendens Strain rgcb-1. Microbiol. Resour. Announc. 2020, 9. [Google Scholar] [CrossRef]
- Howell, C.R. Suppression of Pythium ultimum-Induced Damping-Off of Cotton Seedlings by Pseudomonas fluorescens and its Antibiotic, Pyoluteorin. Phytopathology 1980, 70, 712. [Google Scholar] [CrossRef]
- Baxter, L.; Tripathy, S.; Ishaque, N.; Boot, N.; Cabral, A.; Kemen, E.; Thines, M.; Ah-Fong, A.; Anderson, R.; Badejoko, W.; et al. Signatures of Adaptation to Obligate Biotrophy in the Hyaloperonospora arabidopsidis Genome. Science 2010, 330, 1549–1551. [Google Scholar] [CrossRef]
- Lyon, R.; Correll, J.; Feng, C.; Bluhm, B.; Shrestha, S.; Shi, A.; Lamour, K. Population Structure of Peronospora effusa in the Southwestern United States. PLoS ONE 2016, 11, e0148385. [Google Scholar] [CrossRef]
- Chen, X.R.; Zhang, B.Y.; Xing, Y.P.; Li, Q.Y.; Li, Y.P.; Tong, Y.H.; Xu, J.Y. Transcriptomic analysis of the phytopathogenic oomycete Phytophthora cactorum provides insights into infection-related effectors. BMC Genom. 2014, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.D.; Lysøe, E.; Nellist, C.F.; Lewis, L.A.; Cano, L.M.; Harrison, R.J.; Brurberg, M.B. Bioinformatic characterisation of the effector repertoire of the strawberry pathogen Phytophthora cactorum. PLoS ONE 2018, 13, e0202305. [Google Scholar] [CrossRef]
- Chen, X.R.; Huang, S.X.; Zhang, Y.; Sheng, G.L.; Li, Y.P.; Zhu, F. Identification and functional analysis of the NLP-encoding genes from the phytopathogenic oomycete Phytophthora capsici. Mol. Genet. Genom. 2018, 293, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Lamour, K.H.; Mudge, J.; Gobena, D.; Hurtado-Gonzales, O.P.; Schmutz, J.; Kuo, A.; Miller, N.A.; Rice, B.J.; Raffaele, S.; Cano, L.M.; et al. Genome Sequencing and Mapping Reveal Loss of Heterozygosity as a Mechanism for Rapid Adaptation in the Vegetable Pathogen Phytophthora capsici. Mol. Plant-Microbe Interact. 2012, 25, 1350–1360. [Google Scholar] [CrossRef]
- Santos, C.; Duarte, S.; Tedesco, S.; Fevereiro, P.; Costa, R.L. Expression Profiling of Castanea Genes during Resistant and Susceptible Interactions with the Oomycete Pathogen Phytophthora cinnamomi Reveal Possible Mechanisms of Immunity. Front. Plant Sci. 2017, 8, 515. [Google Scholar] [CrossRef]
- Studholme, D.; McDougal, R.; Sambles, C.; Hansen, E.; Hardy, G.; Grant, M.; Ganley, R.; Williams, N. Genome sequences of six Phytophthora species associated with forests in New Zealand. Genom. Data 2016, 7, 54–56. [Google Scholar] [CrossRef]
- Adams, T.M.; Armitage, A.D.; Sobczyk, M.K.; Bates, H.J.; Tabima, J.F.; Kronmiller, B.A.; Tyler, B.M.; Grünwald, N.J.; Dunwell, J.M.; Nellist, C.F.; et al. Genomic Investigation of the Strawberry Pathogen Phytophthora fragariae Indicates Pathogenicity Is Associated with Transcriptional Variation in Three Key Races. Front. Microbiol. 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, A.P.; Vega-Arreguín, J.C.; Fei, Z.; Ponnala, L.; Lee, S.J.; Matas, A.J.; Patev, S.; Fry, W.E.; Rose, J.K.C. Transcriptome of P. infestans in tomato. Mol. Plant Pathol. 2016, 17, 29–41. [Google Scholar] [CrossRef]
- Denman, S.; Kirk, S.A.; Moralejo, E.; Webber, J.F. Phytophthora ramorum and Phytophthora kernoviae on naturally infected asymptomatic foliage. EPPO Bull. 2009, 39, 105–111. [Google Scholar] [CrossRef]
- Ali, S.S.; Shao, J.; Lary, D.J.; Kronmiller, B.A.; Shen, D.; Strem, M.D.; Amoako-Attah, I.; Akrofi, A.Y.; Begoude, B.D.; Ten Hoopen, G.M.; et al. Phytophthora megakarya and Phytophthora palmivora, Closely Related Causal Agents of Cacao Black Pod Rot, Underwent Increases in Genome Sizes and Gene Numbers by Different Mechanisms. Genome Biol. Evol. 2017, 9, 536–557. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C.; Melser, S.; DeBoer, K.; Thatcher, L.F.; Kamphuis, L.G.; Foley, R.C.; Singh, K.B. Narrow-Leafed Lupin (Lupinus angustifolius) β1- and β6-Conglutin Proteins Exhibit Antifungal Activity, Protecting Plants against Necrotrophic Pathogen Induced Damage from Sclerotinia sclerotiorum and Phytophthora nicotianae. Front. Plant Sci. 2016, 7, 1856. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Yu, H.; Fang, D.; Li, Y.; Wang, X.; Wang, W.; Dong, Y.; Xiao, B. Genomes and virulence difference between two physiological races of Phytophthora nicotianae. GigaScience 2016, 5, 3. [Google Scholar] [CrossRef]
- Cho, K.; Kim, Y.; Wi, S.J.; Seo, J.B.; Kwon, J.; Chung, J.H.; Park, K.Y.; Nam, M.H. Metabolic Survey of Defense Responses to a Compatible Hemibiotroph, Phytophthora parasitica var. nicotianae, in Ethylene Signaling-Impaired Tobacco. J. Agric. Food Chem. 2013, 61, 8477–8489. [Google Scholar] [CrossRef]
- Moy, P.; Qutob, D.; Chapman, B.P.; Atkinson, I.; Gijzen, M. Patterns of Gene Expression Upon Infection of Soybean Plants by Phytophthora sojae. Mol. Plant-Microbe Interact. 2004, 17, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.M.; Tripathy, S.; Zhang, X.; Dehal, P.; Jiang, R.H.Y.; Aerts, A.; Arredondo, F.D.; Baxter, L.; Bensasson, D.; Beynon, J.L.; et al. Phytophthora Genome Sequences Uncover Evolutionary Origins and Mechanisms of Pathogenesis. Science 2006, 313, 1261–1266. [Google Scholar] [CrossRef]
- Stanghellini, M.E.; Mohammadi, M.; Förster, H.; Adaskaveg, J.E. Pythium brassicum sp. nov.: A Novel Plant Family-Specific Root Pathogen. Plant Dis. 2014, 98, 1619–1625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delmotte, F.; Giresse, X.; Richard-Cervera, S.; M’Baya, J.; Vear, F.; Tourvieille, J.; Walser, P.; de Labrouhe, D.T. Single nucleotide polymorphisms reveal multiple introductions into France of Plasmopara halstedii, the plant pathogen causing sunflower downy mildew. Infect. Genet. Evol. 2008, 8, 534–540. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.; Nassar, A.; Hardy, G.; Sivasithamparam, K. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 2009, 106, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Gaastra, W.; Lipman, L.J.; Cock, A.W.D.; Exel, T.K.; Pegge, R.B.; Scheurwater, J.; Vilela, R.; Mendoza, L. Pythium insidiosum: An overview. Vet. Microbiol. 2010, 146, 1–16. [Google Scholar] [CrossRef]
- Rujirawat, T.; Patumcharoenpol, P.; Lohnoo, T.; Yingyong, W.; Lerksuthirat, T.; Tangphatsornruang, S.; Suriyaphol, P.; Grenville-Briggs, L.J.; Garg, G.; Kittichotirat, W.; et al. Draft Genome Sequence of the Pathogenic Oomycete Pythium insidiosum Strain Pi-S, Isolated from a Patient with Pythiosis. Genome Announc. 2015, 3, e00574-15. [Google Scholar] [CrossRef]
- Deacon, J. Studies on Pythium oligandrum, an aggressive parasite of other fungi. Trans. Br. Mycol. Soc. 1976, 66, 383–391. [Google Scholar] [CrossRef]
- Faure, C.; Veyssière, M.; Boëlle, B.; San Clemente, H.; Bouchez, O.; Lopez-Roques, C.; Chaubet, A.; Martinez, Y.; Bezouška, K.; Suchánek, M.; et al. Long-Read Genome Sequence of the Sugar Beet Rhizosphere Mycoparasite Pythium oligandrum. G3 Genes Genomes Genet. 2020, 10, 431–436. [Google Scholar] [CrossRef]


| Clustering 1 | Clustering 2 | Robison–Foulds Distance Metric |
|---|---|---|
| Phylogenetic | Genome properties | 28 |
| Phylogenetic | Positive selection | 30 |
| Genome properties | Positive selection | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Pérez, D.; Kemen, E. Predicting Lifestyle from Positive Selection Data and Genome Properties in Oomycetes. Pathogens 2021, 10, 807. https://doi.org/10.3390/pathogens10070807
Gómez-Pérez D, Kemen E. Predicting Lifestyle from Positive Selection Data and Genome Properties in Oomycetes. Pathogens. 2021; 10(7):807. https://doi.org/10.3390/pathogens10070807
Chicago/Turabian StyleGómez-Pérez, Daniel, and Eric Kemen. 2021. "Predicting Lifestyle from Positive Selection Data and Genome Properties in Oomycetes" Pathogens 10, no. 7: 807. https://doi.org/10.3390/pathogens10070807
APA StyleGómez-Pérez, D., & Kemen, E. (2021). Predicting Lifestyle from Positive Selection Data and Genome Properties in Oomycetes. Pathogens, 10(7), 807. https://doi.org/10.3390/pathogens10070807






