Subtyping Cryptosporidium xiaoi, a Common Pathogen in Sheep and Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
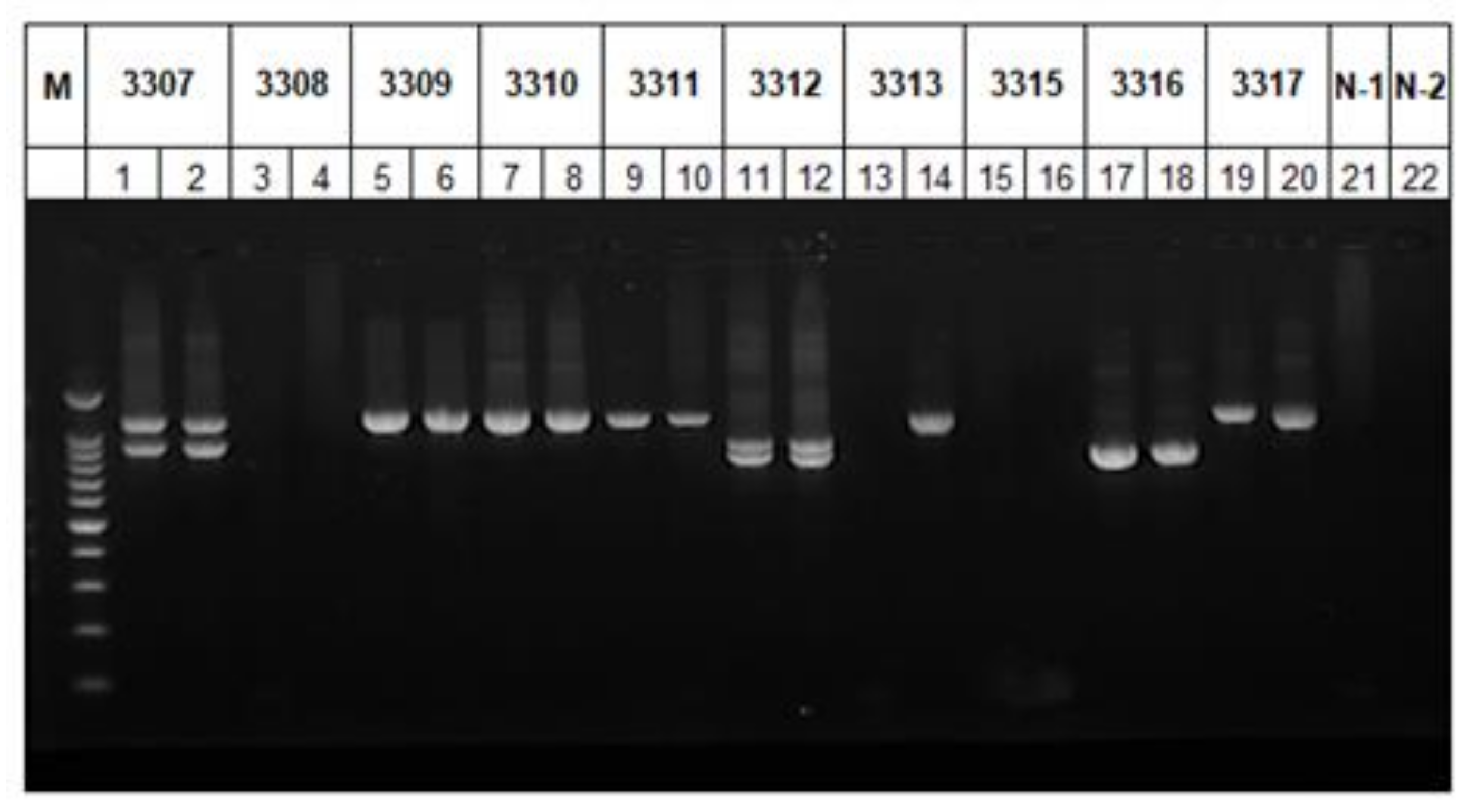
2.2. Identification of the gp60 Gene of C. xiaoi
2.3. Subtyping of C. xiaoi
2.4. DNA Sequence Analysis
2.5. Nucleotide Sequence Accession Numbers
3. Results
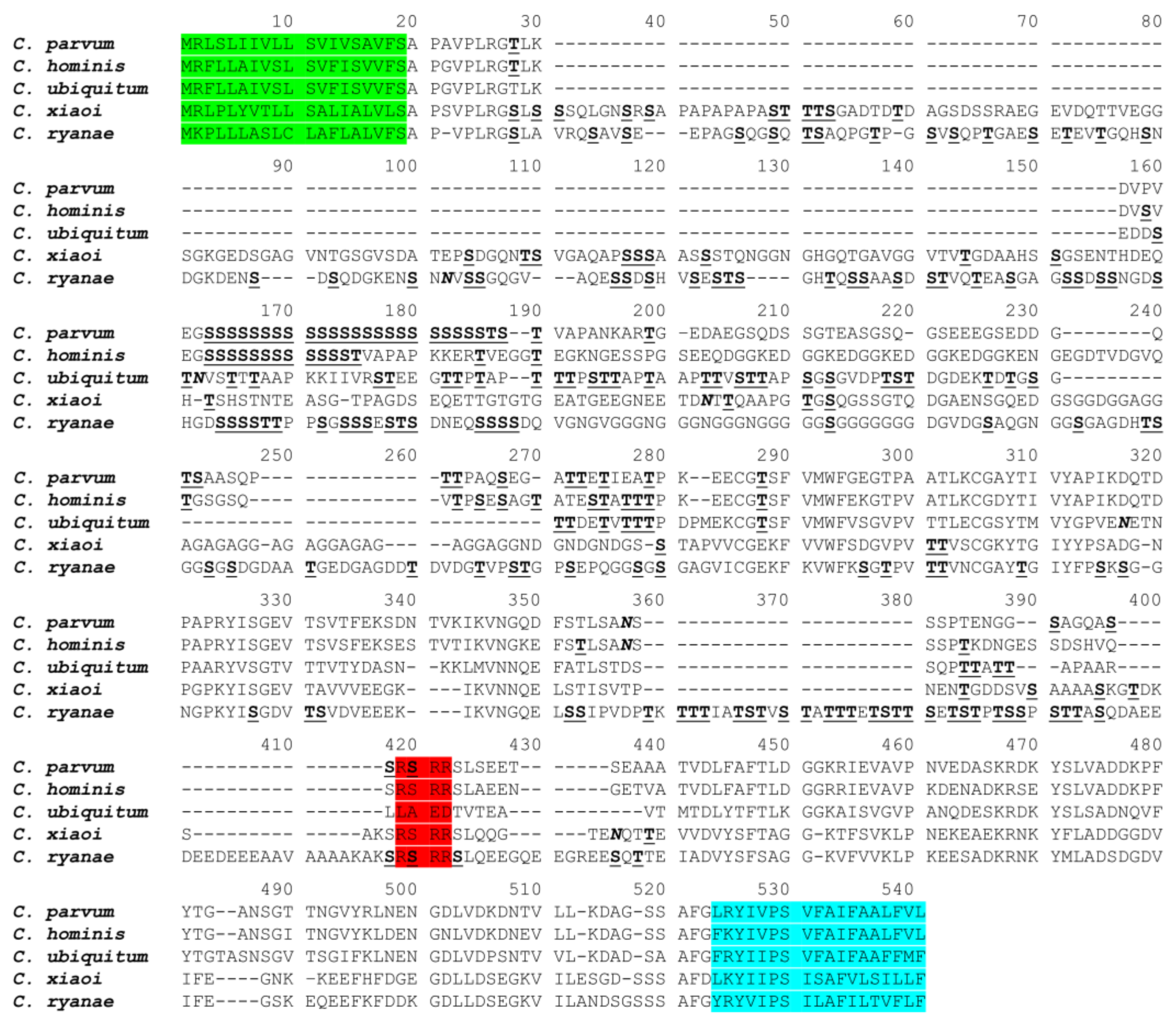
3.1. Features of the gp60 Gene of C. xiaoi
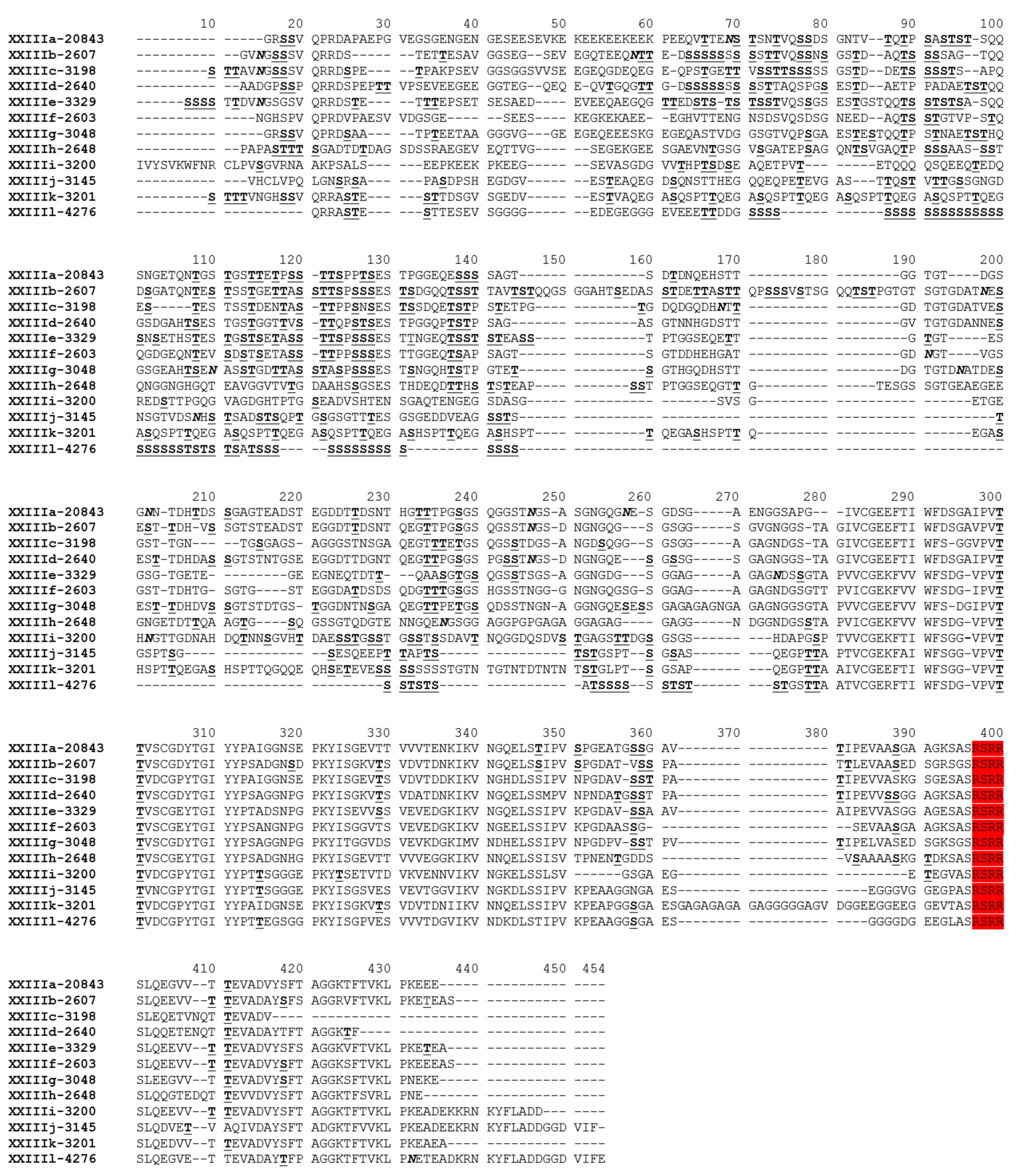
3.2. Sequence Polymorphisms in the gp60 Gene of C. xiaoi
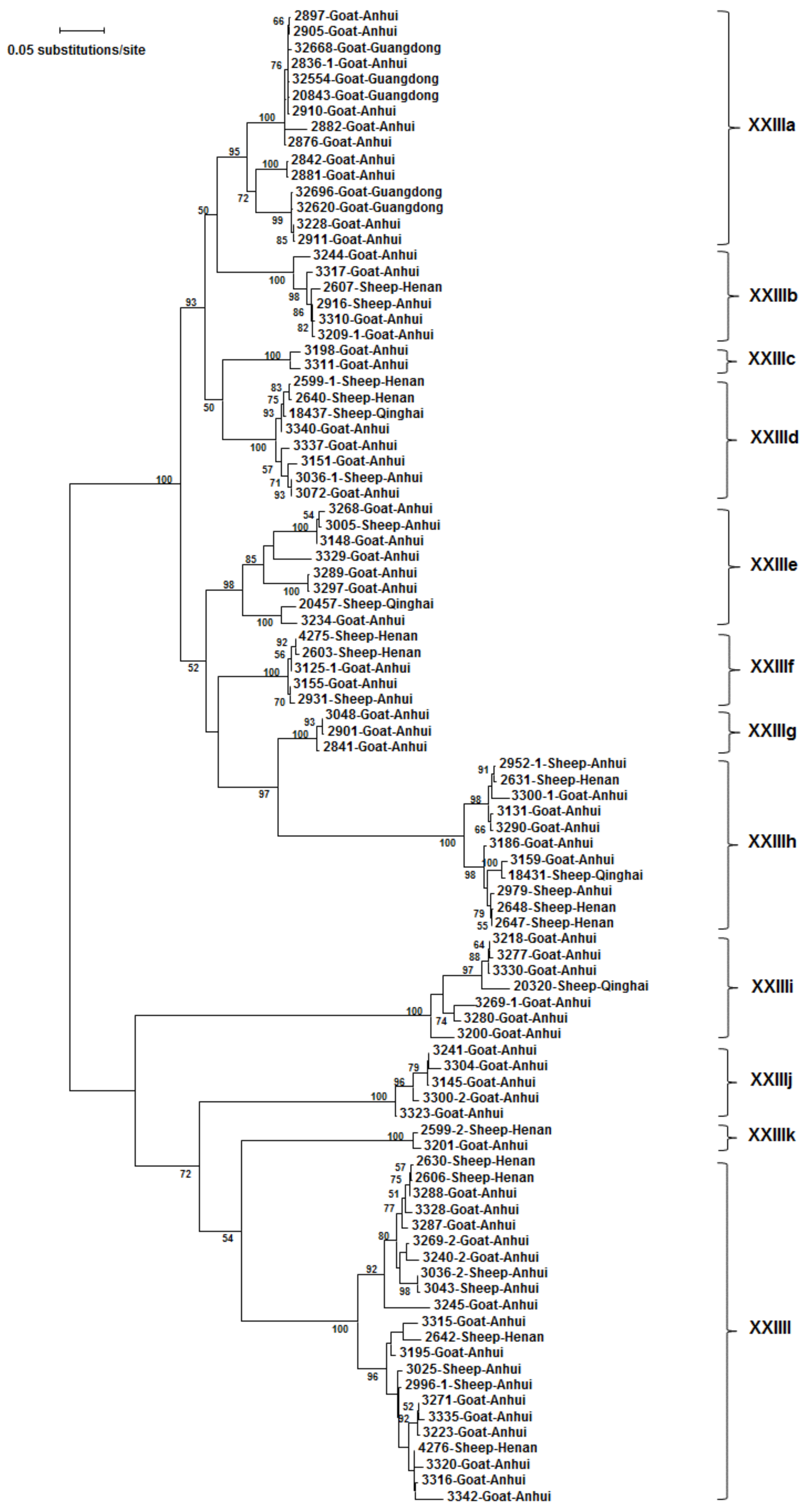
3.3. Subtype Families and Subtypes of C.xiaoi
3.4. Distribution of C. xiaoi Subtype Families by Host
3.5. Distribution of C. xiaoi Subtype Families by Farm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotloff, K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. N. Am. 2017, 64, 799–814. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, L. Molecular epidemiology of cryptosporidiosis in China. Front. Microbiol. 2017, 8, 1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayer, R.; Santin, M. Cryptosporidium xiaoi n. sp. (Apicomplexa: Cryptosporidiidae) in sheep (Ovis aries). Vet. Parasitol. 2009, 164, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kaupke, A.; Michalski, M.M.; Rzezutka, A. Diversity of Cryptosporidium species occurring in sheep and goat breeds reared in Poland. Parasitol. Res. 2017, 116, 871–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Jacobson, C.; Gardner, G.; Carmichael, I.; Campbell, A.J.; Ng-Hublin, J.; Ryan, U. Longitudinal prevalence, oocyst shedding and molecular characterisation of Cryptosporidium species in sheep across four states in Australia. Vet. Parasitol. 2014, 200, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.B.; Travis, D.; Lonsdorf, E.V.; Lipende, I.; Roellig, D.M.; Kamenya, S.; Zhang, H.; Xiao, L.; Gillespie, T.R. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015, 9, e0003529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, R.; Wang, X.; Huang, Y.; Zhou, P.; Liu, Y.; Chen, Y.; Chen, J.; Zhu, W.; Chen, Z. Prevalence and molecular characterization of Cryptosporidium in goats across four provincial level areas in China. PLoS ONE 2014, 9, e111164. [Google Scholar] [CrossRef]
- Guo, Y.; Cebelinski, E.; Matusevich, C.; Alderisio, K.A.; Lebbad, M.; McEvoy, J.; Roellig, D.M.; Yang, C.; Feng, Y.; Xiao, L. Subtyping novel zoonotic pathogen Cryptosporidium chipmunk genotype I. J. Clin. Microbiol. 2015, 53, 1648–1654. [Google Scholar] [CrossRef] [Green Version]
- Stensvold, C.R.; Beser, J.; Axen, C.; Lebbad, M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J. Clin. Microbiol. 2014, 52, 2311–2319. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Alderisio, K.; Roellig, D.M.; Elwin, K.; Chalmers, R.M.; Yang, F.; Wang, Y.; Feng, Y.; Xiao, L. Subtype analysis of zoonotic pathogen Cryptosporidium skunk genotype. Infect. Genet. Evol. 2017, 55, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Xiao, L.; Alderisio, K.; Elwin, K.; Cebelinski, E.; Chalmers, R.; Santin, M.; Fayer, R.; Kvac, M.; Ryan, U.; et al. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg. Infect. Dis. 2014, 20, 217–224. [Google Scholar] [CrossRef]
- Rojas-Lopez, L.; Elwin, K.; Chalmers, R.M.; Enemark, H.L.; Beser, J.; Troell, K. Development of a gp60-subtyping method for Cryptosporidium felis. Parasites Vectors 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Elwin, K.; Winiecka-Krusnell, J.; Chalmers, R.M.; Xiao, L.; Lebbad, M. Development and application of a gp60-based typing assay for Cryptosporidium viatorum. J. Clin. Microbiol. 2015, 53, 1891–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmouni, I.; Essid, R.; Aoun, K.; Bouratbine, A. Glycoprotein 60 diversity in Cryptosporidium parvum causing human and cattle cryptosporidiosis in the rural region of Northern Tunisia. Am. J. Trop. Med. Hyg. 2014, 90, 346–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, M.L.; Cheung-Kwok-Sang, C.; Slade, M.; Williamson, S. Cryptosporidium fayeri: Diversity within the gp60 locus of isolates from different marsupial hosts. Exp. Parasitol. 2009, 121, 219–223. [Google Scholar] [CrossRef]
- Yang, X.; Huang, N.; Jiang, W.; Wang, X.; Li, N.; Guo, Y.; Kváč, M.; Feng, Y.; Xiao, L. Subtyping Cryptosporidium ryanae: A common pathogen in bovine animals. Microorganisms 2020, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Adamu, H.; Petros, B.; Zhang, G.; Kassa, H.; Amer, S.; Ye, J.; Feng, Y.; Xiao, L. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Negl. Trop. Dis. 2014, 8, e2831. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Cai, J.; Cai, M.; Wu, W.; Li, C.; Lei, M.; Xu, H.; Feng, L.; Ma, J.; Feng, Y.; et al. Distribution of Cryptosporidium species in Tibetan sheep and yaks in Qinghai, China. Vet. Parasitol. 2016, 215, 58–62. [Google Scholar] [CrossRef]
- Xiao, L.; Escalante, L.; Yang, C.; Sulaiman, I.; Escalante, A.A.; Montali, R.J.; Fayer, R.; Lal, A.A. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 1999, 65, 1578–1583. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Li, N.; Lysen, C.; Frace, M.; Tang, K.; Sammons, S.; Roellig, D.M.; Feng, Y.; Xiao, L. Isolation and enrichment of Cryptosporidium DNA and verification of DNA purity for whole genome sequencing. J. Clin. Microbiol. 2015, 53, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Feng, Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017, 8–9, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lal, A.A.; Li, N.; Xiao, L. Subtypes of Cryptosporidium spp. in mice and other small mammals. Exp. Parasitol. 2011, 127, 238–242. [Google Scholar] [CrossRef]
- Jiang, W.; Roellig, D.M.; Guo, Y.; Li, N.; Feng, Y.; Xiao, L. Development of a subtyping tool for zoonotic pathogen Cryptosporidium canis. J. Clin. Microbiol. 2020, 59, e02474-20. [Google Scholar] [CrossRef]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kváč, M.; McEvoy, J.; Loudová, M.; Stenger, B.; Sak, B.; Květoňová, D.; Ditrich, O.; Rašková, V.; Moriarty, E.; Rost, M.; et al. Coevolution of Cryptosporidium tyzzeri and the house mouse (Mus musculus). Int. J. Parasitol. 2013, 43, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Roellig, D.M.; Lebbad, M.; Beser, J.; Troell, K.; Guo, Y.Q.; Li, N.; Xiao, L.H.; Feng, Y.Y. Subtype distribution of zoonotic pathogen Cryptosporidium felis in humans and animals in several countries. Emerg. Microbes Infect. 2020, 9, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Caccio, S.M.; de Waele, V.; Widmer, G. Geographical segregation of Cryptosporidium parvum multilocus genotypes in Europe. Infect. Genet. Evol. 2015, 31, 245–249. [Google Scholar] [CrossRef]
| Host | Region | Breed | Farm ID | No. of C. xiaoi-Positive Samples | No. of Samples Positive at the gp60 Locus (%) | No. of Samples with Divergent gp60 PCR Banding Patterns (%) | Subtype Family (no.) | |
|---|---|---|---|---|---|---|---|---|
| One Band | Two Bands | |||||||
| Sheep | Henan | Han | 1 | 71 | 58 (81.7) | 49 (84.5) | 9 (15.5) | XXIIIb (13), XXIIId (3), XXIIIf (1), XXIIIh (13), XXIIIl (22), XXIIId + XXIIIk (1), XXIIId + XXIIIl (1) |
| 2 | 18 | 18 (100.0) | 18 (100.0) | - | XXIIIb (1), XXIIIf (1), XXIIIk (5), XXIIIl (3) | |||
| Anhui | Hu | 3 | 84 | 64 (76.2) | 57 (89.0) | 7 (11.0) | XXIIIb (8), XXIIIe (2), XXIIIf (1), XXIIIh (17), XXIIIk (3), XXIIIl (17), XXIIIh + XXIIIk (2), XXIIIb + XXIIIk (1), XXIIId + XXIIIl (1) | |
| Qinghai * | Tibetan | 4 | 39 | 8 (20.5) | 8 (100.0) | - | XXIIId (1), XXIIIe (1), XXIIIh (1), XXIIIi (3) | |
| Goats | Anhui | Huanghuai | 5 | 77 | 77 (100.0) | 66 (84.7) | 11 (15.3) | XXIIIa (6), XXIIIb (4), XXIIIc (1), XXIIId (2), XXIIIe (5), XXIIIh (7), XXIIIi (4), XXIIIj (4), XXIIIk (5), XXIIIl (18), XXIIIb + XXIIIl (1), XXIIIh + XXIIIi (1), XXIIIh + XXIIIj (1), XXIIIh + XXIIIk (1), XXIIIi + XXIIIl (1) |
| 6 | 51 | 46 (90.2) | 43 (93.5) | 3 (6.5) | XXIIIb (3), XXIIIc (1), XXIIId (3), XXIIIe (5), XXIIIf (7), XXIIIh (4), XXIIIi (3), XXIIIj (1), XXIIIk (7), XXIIIl (4), XXIIIh +XXIIIk (1) | |||
| 7 | 33 | 33 (100.0) | 31 (93.9) | 2 (6.1) | XXIIIa (20), XXIIIg (5), XXIIIa + XXIIIg (1) | |||
| 8 | 20 | 16 (80.0) | 16 (100.0) | - | XXIIId (1), XXIIIg (14) | |||
| Guangdong | Black | 9 | 11 | 8 (72.7) | 8 (100.0) | - | XXIIIa (8) | |
| 10 | 18 | 16 (88.9) | 16 (100.0) | - | XXIIIa (16) | |||
| 11 | 12 | 11 (91.7) | 11 (100.0) | - | XXIIIa (11) | |||
| Total | - | - | - | 434 | 355 (81.8%) | 323 (90.1) | 32 (9.9) | XXIIIa (61), XXIIIb (29), XXIIIc (2), XXIIId (10), XXIIIe (13), XXIIIf (10), XXIIIg (19), XXIIIh (42), XXIIIi (10), XXIIIj (5), XXIIIk (20), XXIIIl (64), XXIIIa + XXIIIg (1), XXIIIb + XXIIIk (1), XXIIIb + XXIIIl (1), XXIIId + XXIIIk (1), XXIIId + XXIIIl (2), XXIIIh + XXIIIi (1), XXIIIh + XXIIIj (1), XXIIIh + XXIIIk (4), XXIIIi + XXIIIl (1) |
| XXIIIa | XXIIIb | XXIIIc | XXIIId | XXIIIe | XXIIIf | XXIIIg | XXIIIh | XXIIIi | XXIIIj | XXIIIk | XXIIIl | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XXIIIa | ||||||||||||
| XXIIIb | 76.0% | |||||||||||
| XXIIIc | 71.2% | 74.0% | ||||||||||
| XXIIId | 70.1% | 72.7% | 68.8% | |||||||||
| XXIIIe | 71.8% | 72.7% | 68.3% | 71.6% | ||||||||
| XXIIIf | 74.3% | 69.0% | 66.3% | 70.1% | 75.8% | |||||||
| XXIIIg | 68.3% | 66.2% | 66.0% | 66.4% | 69.8% | 71.4% | ||||||
| XXIIIh | 54.1% | 56.0% | 50.4% | 55.0% | 56.4% | 57.1% | 57.6% | |||||
| XXIIIi | 41.4% | 38.2% | 37.9% | 42.6% | 40.9% | 41.4% | 39.4% | 38.5% | ||||
| XXIIIj | 47.6% | 45.3% | 43.6% | 46.1% | 44.8% | 45.2% | 44.3% | 42.4% | 47.1% | |||
| XXIIIk | 37.9% | 35.7% | 35.5% | 37.3% | 37.9% | 39.4% | 37.6% | 34.7% | 49.8% | 50.5% | ||
| XXIIIl | 33.9% | 31.7% | 32.2% | 35.4% | 33.6% | 35.3% | 33.9% | 31.8% | 42.3% | 42.5% | 58.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Huang, X.; Guo, S.; Yang, F.; Yang, X.; Guo, Y.; Feng, Y.; Xiao, L.; Li, N. Subtyping Cryptosporidium xiaoi, a Common Pathogen in Sheep and Goats. Pathogens 2021, 10, 800. https://doi.org/10.3390/pathogens10070800
Fan Y, Huang X, Guo S, Yang F, Yang X, Guo Y, Feng Y, Xiao L, Li N. Subtyping Cryptosporidium xiaoi, a Common Pathogen in Sheep and Goats. Pathogens. 2021; 10(7):800. https://doi.org/10.3390/pathogens10070800
Chicago/Turabian StyleFan, Yingying, Xitong Huang, Sheng Guo, Fang Yang, Xin Yang, Yaqiong Guo, Yaoyu Feng, Lihua Xiao, and Na Li. 2021. "Subtyping Cryptosporidium xiaoi, a Common Pathogen in Sheep and Goats" Pathogens 10, no. 7: 800. https://doi.org/10.3390/pathogens10070800
APA StyleFan, Y., Huang, X., Guo, S., Yang, F., Yang, X., Guo, Y., Feng, Y., Xiao, L., & Li, N. (2021). Subtyping Cryptosporidium xiaoi, a Common Pathogen in Sheep and Goats. Pathogens, 10(7), 800. https://doi.org/10.3390/pathogens10070800







