Pathogenesis of Two Western Mediterranean West Nile Virus Lineage 1 Isolates in Experimentally Infected Red-Legged Partridges (Alectoris rufa)
Abstract
1. Introduction
2. Results
2.1. Clinical Signs and Gross Pathology
2.2. Virus Genome Detection
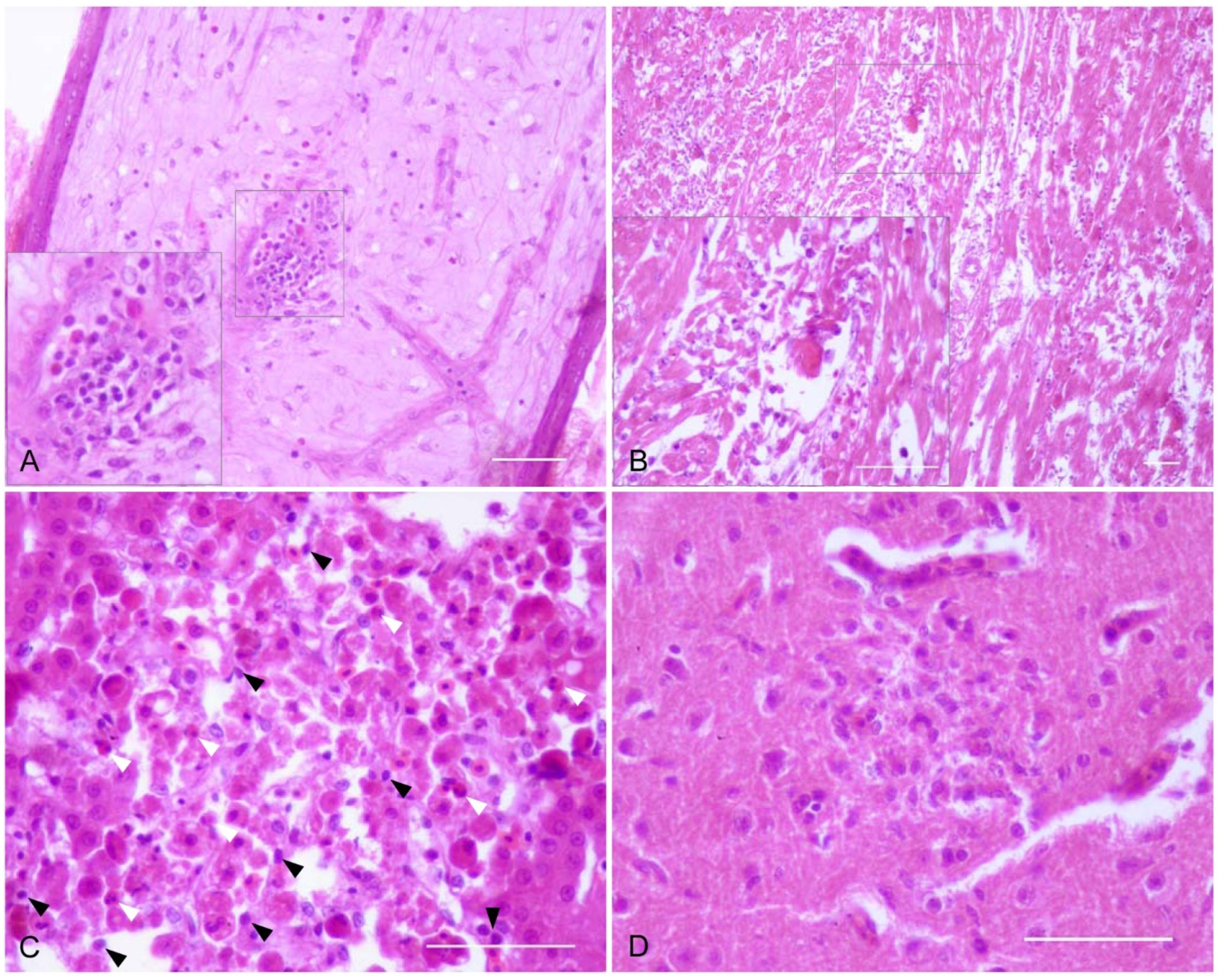
2.3. Histopathology
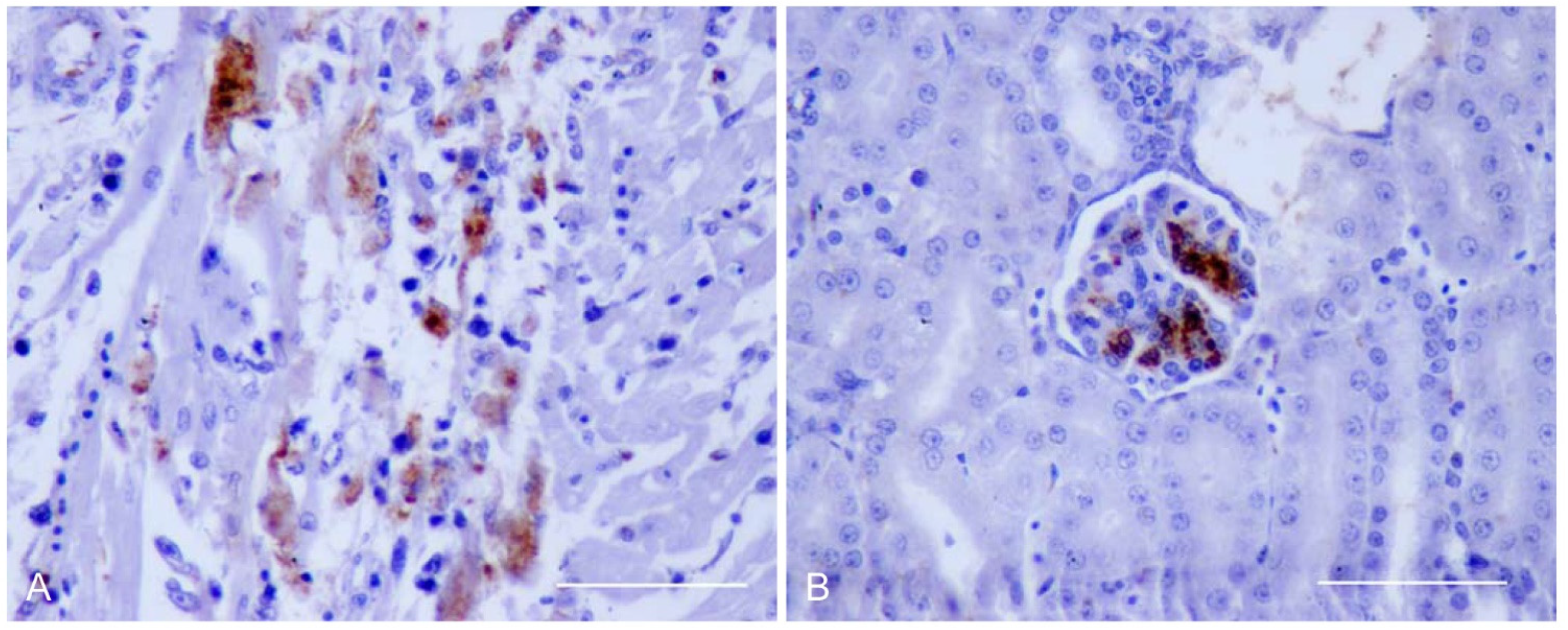
2.4. Virus Antigen
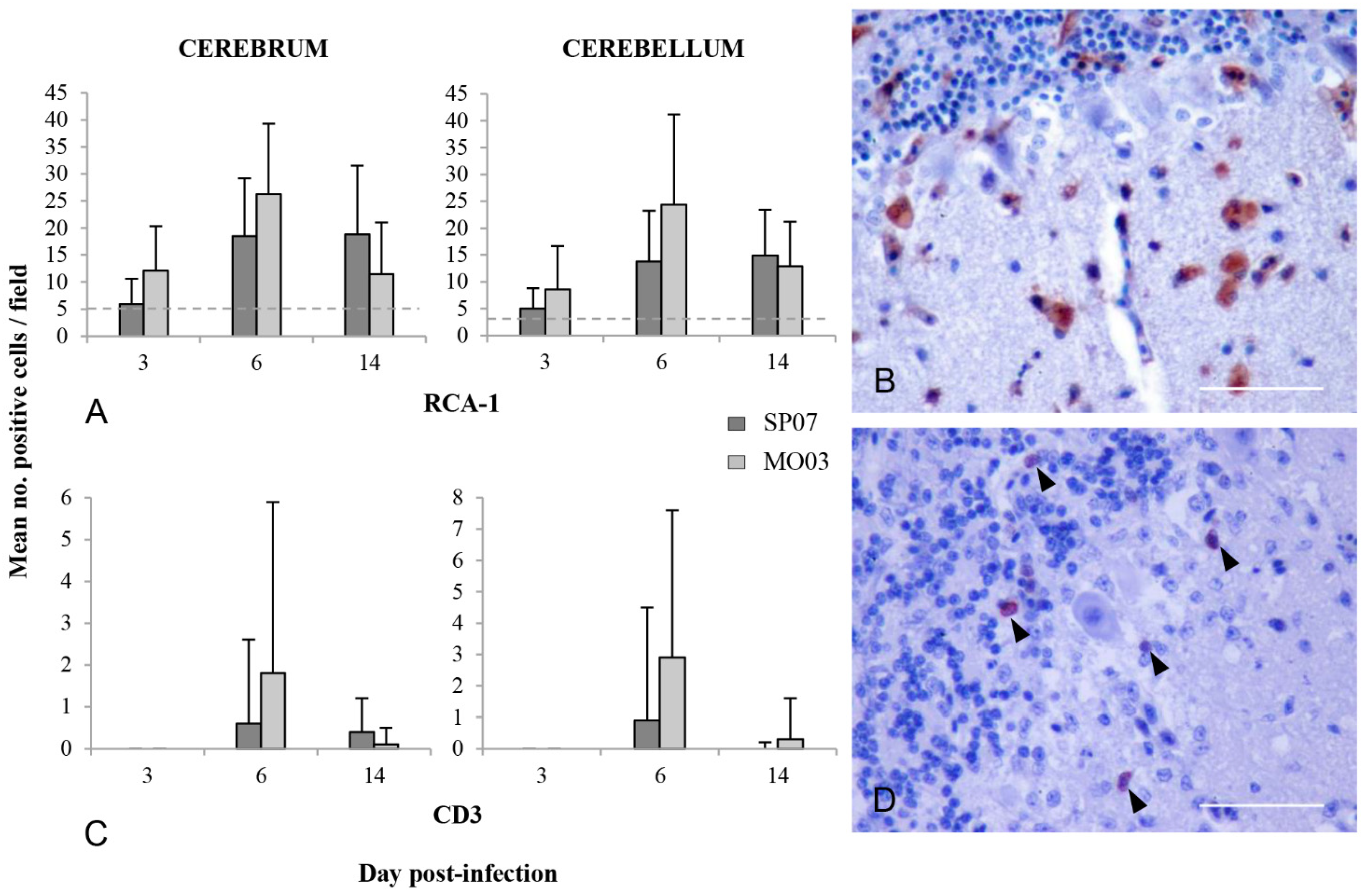
2.5. Inflammatory Cells in the Brain
2.5.1. Microglia Activation/Macrophage Infiltration
2.5.2. Astrocyte Activation
2.5.3. T Cell Infiltration
3. Discussion
4. Materials and Methods
4.1. Viruses
4.2. Experimental Infection
4.3. Sample Collection
4.4. Virus Genome Detection
4.5. Histopathology
4.6. Immunohistochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Bakonyi, T.; Haussig, J.M. West Nile virus keeps on moving up in Europe. Euro. Surveill. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Available online: http://www.ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/pages/index.aspx (accessed on 10 December 2020).
- Pauli, G.; Bauerfeind, U.; Blumel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; et al. West Nile virus. Transfus Med. Hemother. 2013, 40, 265–284. [Google Scholar] [CrossRef]
- Sotelo, E.; Fernández-Pinero, J.; Llorente, F.; Vázquez, A.; Moreno, A.; Agüero, M.; Cordioli, P.; Tenorio, A.; Jiménez-Clavero, M.A. Phylogenetic relationships of Western Mediterranean West Nile virus strains (1996–2010) using full-length genome sequences: Single or multiple introductions? J. Gen Virol. 2011, 92, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg. Infect Dis. 2000, 8, 392–397. [Google Scholar] [CrossRef]
- Höfle, U.; Blanco, J.M.; Crespo, E.; Naranjo, V.; Jiménez-Clavero, M.A.; Sánchez, A.; de la Fuente, J.; Gortazar, C. West Nile virus in the endangered Spanish imperial eagle. Vet. Microbiol. 2008, 129, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Clavero, M.A.; Sotelo, E.; Fernández-Pinero, J.; Llorente, F.; Blanco, J.M.; Rodriguez-Ramos, J.; Perez-Ramirez, E.; Höfle, U. West Nile virus in golden eagles, Spain, 2007. Emerg. Infect. Dis. 2008, 14, 1489–1491. [Google Scholar] [CrossRef]
- Jourdain, E.; Schuffenecker, I.; Korimbocus, J.; Reynard, S.; Murri, S.; Kayser, Y.; Gauthier-Clerc, M.; Sabatier, P.; Zeller, H.G. West Nile virus in wild resident birds, Southern France, 2004. Vector Borne Zoonotic Dis. 2007, 7, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Monaco, F.; Lelli, R.; Teodori, L.; Pinoni, C.; Di Gennaro, A.; Polci, A.; Calistri, P.; Savini, G. Re-emergence of West Nile virus in Italy. Zoonoses Public Health 2010, 57, 476–486. [Google Scholar] [CrossRef]
- Busquets, N.; Laranjo-González, M.; Soler, M.; Nicolás, O.; Rivas, R.; Talavera, S.; Villalba, R.; San Miguel, E.; Torner, N.; Aranda, C.; et al. Detection of West Nile virus lineage 2 in North-Eastern Spain (Catalonia). Transbound. Emerg. Dis. 2019, 66, 617–621. [Google Scholar] [CrossRef]
- Valiakos, G.; Plavos, K.; Vontas, A.; Sofia, M.; Giannakopoulos, A.; Giannoulis, T.; Spyrou, V.; Tsokana, C.N.; Chatzopoulos, D.; Kantere, M.; et al. Phylogenetic Analysis of Bird-Virulent West Nile Virus Strain, Greece. Emerg. Infect. Dis. 2019, 25, 2323–2325. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z.; Kosina, M.; Rudolf, I.; Mendel, J.; Straková, P.; Tomešek, M. Mortality of Goshawks (Accipiter gentilis) Due to West Nile Virus Lineage 2. Vector Borne Zoonotic Dis. 2018, 18, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, J.; Llorente, F.; Perez-Ramirez, E.; Soriguer, R.C.; Figuerola, J.; Nowotny, N.; Jiménez-Clavero, M.A. Experimental infection of house sparrows (Passer domesticus) with West Nile virus strains of lineages 1 and 2. Vet. Microbiol. 2014, 172, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Dridi, M.; Vangeluwe, D.; Lecollinet, S.; van den Berg, T.; Lambrecht, B. Experimental infection of Carrion crows (Corvus corone) with two European West Nile virus (WNV) strains. Vet. Microbiol. 2013, 165, 160–166. [Google Scholar] [CrossRef]
- Lim, S.M.; Brault, A.C.; van Amerongen, G.; Sewbalaksing, V.D.; Osterhaus, A.D.; Martina, B.E.E.; Koraka, P. Susceptibility of European jackdaws (Corvus monedula) to experimental infection with lineage 1 and 2 West Nile viruses. J. Gen. Virol. 2014, 95, 1320–1329. [Google Scholar] [CrossRef]
- Sotelo, E.; Gutiérrez-Guzmán, A.V.; del Amo, J.; Llorente, F.; El-Harrak, M.; Pérez-Ramírez, E.; Blanco, J.M.; Höfle, U.; Jiménez-Clavero, M.A. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to Southern Europe: The red-legged partridge. Vet. Res. 2011, 42, 11. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M.Á. Experimental infections of wild birds with West Nile virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef]
- Blanco-Aguiar, J.A.; Virgós, E.; Villafuerte, R. Perdiz roja (Alectoris rufa). In Atlas de las Aves Reproductoras de España; Martí, R., del Moral, J.C., Eds.; DGCoNa-SEO: Madrid, Spain, 2003; pp. 212–213. [Google Scholar]
- Schuffenecker, I.; Peyrefitte, C.N.; el Harrak, M.; Murri, S.; Leblond, A.; Zeller, H.G. West Nile virus in Morocco, 2003. Emerg. Infect. Dis. 2005, 11, 306–309. [Google Scholar] [CrossRef]
- Sotelo, E.; Fernández-Pinero, J.; Llorente, F.; Agüero, M.; Hoefle, U.; Blanco, J.M.; Jiménze-Clavero, M.A. Characterization of West Nile virus isolates from Spain: New insights into the distinct West Nile virus eco-epidemiology in the Western Mediterranean. Virology 2009, 395, 289–297. [Google Scholar] [CrossRef]
- Lim, S.M.; Koraka, P.; van Boheemen, S.; Roose, J.M.; Jaarsma, D.; van de Vijver, D.A.; Osterhaus, A.D.; Martina, B.E. Characterization of the mouse neuroinvasiveness of selected European strains of West Nile virus. PLoS ONE 2013, 8, e74575. [Google Scholar] [CrossRef]
- Reisen, W.K.; Fang, Y.; Lothrop, H.D.; Martinez, V.M.; Wilson, J.; Oconnor, P.; Carney, R.; Cahoon-Young, B.; Shafii, M.; Brault, A.C. Overwintering of West Nile virus in Southern California. J. Med. Entomol. 2006, 43, 344–355. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Langevin, S.A.; Brault, A.C.; Woods, L.; Carroll, B.D.; Reisen, W.K. Detection of persistent West Nile virus RNA in experimentally and naturally infected avian hosts. Am. J. Trop. Med. Hyg. 2012, 87, 559–564. [Google Scholar] [CrossRef]
- Steele, K.E.; Linn, M.J.; Schoepp, R.J.; Komar, N.; Geisbert, T.W.; Manduca, R.M.; Calle, P.P.; Raphael, B.L.; Clippinger, T.L.; Larsen, T.; et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 2000, 37, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Wünschmann, A.; Ziegler, A. West Nile virus-associated mortality events in domestic chukar partridges (Alectoris chukar) and domestic Impeyan pheasants (Lophophorus impeyanus). Avian Dis. 2006, 50, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wilson, F.; Read, R.; Pace, L.; Zhang, S. Detection and characterization of naturally acquired West Nile virus infection in a female wild turkey. J. Vet. Diagn Invest. 2006, 18, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Eckstrand, C.D.; Woods, L.W.; Diab, S.S.; Crossley, B.M.; Giannitti, F. Diagnostic Exercise: High Mortality in a Flock of Chukar Partridge Chicks (Alectoris chukar) in California. Vet. Pathol. 2015, 52, 189–192. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Bosco-Lauth, A.M.; Williams, L.M.; Bowen, R.A.; Brown, J.D. West Nile Virus Infection in Ruffed Grouse (Bonasa umbellus): Experimental Infection and Protective Effects of Vaccination. Vet. Pathol. 2017, 54, 901–911. [Google Scholar] [CrossRef]
- Byas, A.D.; Ebel, G.D. Comparative Pathology of West Nile Virus in Humans and Non-Human Animals. Pathogens 2020, 9, 48. [Google Scholar] [CrossRef]
- Gancz, A.Y.; Smith, D.A.; Barker, I.K.; Lindsay, R.; Hunter, B. Pathology and tissue distribution of West Nile virus in North American owls (family: Strigidae). Avian Pathol. 2006, 35, 17–29. [Google Scholar] [CrossRef]
- Shirafuji, H.; Kanehira, K.; Kubo, M.; Shibahara, T.; Kamio, T. Experimental West Nile virus infection in jungle crows (Corvus macrorhynchos). Am. J. Trop. Med. Hyg. 2008, 78, 838–842. [Google Scholar] [CrossRef]
- Wünschmann, A.; Shivers, J.; Bender, J.; Carroll, L.; Fuller, S.; Saggese, M.; van Wettere, A.; Redig, P. Pathologic and immunohistochemical findings in goshawks (Accipiter gentilis) and great horned owls (Bubo virginianus) naturally infected with West Nile virus. Avian Dis. 2005, 49, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Banet-Noach, C.; Simanov, L.; Malkinson, M. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol. 2003, 32, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Kratz, G.E.; Bates, R.; Scherpelz, J.A.; Bowen, R.A.; Komar, N. Clinical evaluation and outcomes of naturally acquired West Nile virus infection in raptors. J. Zoo Wildl. Med. 2009, 40, 51–63. [Google Scholar] [PubMed]
- Saito, E.K.; Sileo, L.; Green, D.E.; Meteyer, C.U.; McLaughlin, G.S.; Converse, K.A.; Docherty, D.E. Raptor mortality due to West Nile virus in the United States, 2002. J. Wildl. Dis. 2009, 43, 206–213. [Google Scholar] [CrossRef]
- Busquets, N.; Bertran, K.; Costa, T.P.; Rivas, R.; de la Fuente, J.G.; Villalba, R.; Solanes, D.; Bensaid, A.; Majó, N.; Pagès, N. Experimental West Nile virus infection in Gyr-Saker hybrid falcons. Vector Borne Zoonotic Dis. 2012, 12, 482–489. [Google Scholar] [CrossRef]
- Lim, S.M.; Koraka, P.; Osterhaus, A.D.; Martina, B.E. West Nile virus: Immunity and pathogenesis. Viruses 2011, 3, 811–828. [Google Scholar] [CrossRef]
- Shrestha, B.; Gottlieb, D.; Diamond, M.S. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 2003, 77, 13203–13213. [Google Scholar] [CrossRef]
- Feyer, S.; Bartenschlager, F.; Bertram, C.A.; Ziegler, U.; Fast, C.; Klopfleisch, R.; Müller, K. Clinical, pathological and virological aspects of fatal West Nile virus infections in ten free-ranging goshawks (Accipiter gentilis) in Germany. Transbound. Emerg. Dis. 2021, 68, 907–919. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Llorente, F.; Del Amo, J.; Nowotny, N.; Jiménez-Clavero, M.Á. Susceptibility and role as competent host of the red-legged partridge after infection with lineage 1 and 2 West Nile virus isolates of Mediterranean and Central European origin. Vet. Microbiol. 2018, 222, 39–45. [Google Scholar] [CrossRef]
- Montgomery, D.L. Astrocytes: Form, functions, and roles in disease. Vet. Pathol. 1994, 31, 145–167. [Google Scholar] [CrossRef]
- Bréhin, A.C.; Mouries, J.; Frenkiel, M.P.; Dadaglio, G.; Despres, P.; Lafon, M.; Couderc, T. Dynamics of immune cell recruitment during West Nile encephalitis and identification of a new CD19+B220-BST-2+ leukocyte population. J. Immunol. 2008, 180, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Hussmann, K.L.; Samuel, M.A.; Kim, K.S.; Diamond, M.S.; Fredericksen, B.L. Differential replication of pathogenic and nonpathogenic strains of West Nile virus within astrocytes. J. Virol. 2013, 87, 2814–2822. [Google Scholar] [CrossRef]
- Kelley, T.W.; Prayson, R.A.; Ruiz, A.I.; Isada, C.M.; Gordon, S.M. The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. Am. J. Clin. Pathol. 2003, 119, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Maximova, O.A.; Faucette, L.J.; Ward, J.M.; Murphy, B.R.; Pletnev, A.G. Cellular inflammatory response to flaviviruses in the central nervous system of a primate host. J. Histochem. Cytochem. 2009, 57, 973–989. [Google Scholar] [CrossRef]
- Dorries, R. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr. Top Microbiol. Immunol. 2001, 253, 219–245. [Google Scholar] [PubMed]
- Sampson, B.A.; Armbrustmacher, V. West Nile encephalitis: The neuropathology of four fatalities. Ann. N. Y. Acad. Sci. 2001, 951, 172–178. [Google Scholar] [CrossRef]
- Shrestha, B.; Diamond, M.S. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 2004, 78, 8312–8321. [Google Scholar] [CrossRef] [PubMed]
- Sitati, E.M.; Diamond, M.S. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J. Virol. 2006, 80, 12060–12069. [Google Scholar] [CrossRef]
- Wang, Y.; Lobigs, M.; Lee, E.; Mullbacher, A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 2003, 77, 13323–13334. [Google Scholar] [CrossRef] [PubMed]
- Brault, A.C.; Huang, C.Y.H.; Langevin, S.A.; Kinney, R.M.; Bowen, R.A.; Ramey, W.N.; Panella, N.A.; Holmes, E.C.; Powers, A.M.; Miller, B.R. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat. Genet. 2007, 39, 1162–1166. [Google Scholar] [CrossRef]
- Brault, A.C.; Langevin, S.A.; Ramey, W.N.; Fang, Y.; Beasley, D.W.; Barker, C.M.; Sanders, T.A.; Reisen, W.K.; Barrett, A.D.T.; Bowen, R.A. Reduced avian virulence and viremia of West Nile virus isolates from Mexico and Texas. Am. J. Trop. Med. Hyg. 2011, 85, 758–767. [Google Scholar] [CrossRef]
- Davis, C.T.; Ebel, G.D.; Lanciotti, R.S.; Brault, A.C.; Guzman, H.; Siirin, M.; Lambert, A.; Parsons, R.E.; Beasley, D.W.C.; Novak, R.J.; et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004, evidence for the emergence of a dominant genotype. Virology 2005, 342, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Fiacre, L.; Pagès, N.; Albina, E.; Richardson, J.; Lecollinet, S.; Gonzalez, G. Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts. Int. J. Mol. Sci. 2020, 21, 9117. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Diamond, M.S. Pathogenesis of flavivirus encephalitis. Adv. Virus Res. 2003, 60, 273–342. [Google Scholar] [PubMed]
- Beasley, D.W.; Whiteman, M.C.; Zhang, S.; Huang, C.Y.; Schneider, B.S.; Smith, D.R.; Gromowski, G.D.; Higgs, S.; Kinney, R.M.; Barrett, A.D.T. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 2005, 79, 8339–8347. [Google Scholar] [CrossRef]
- Brault, A.C.; Langevin, S.A.; Bowen, R.A.; Panella, N.A.; Biggerstaff, B.J.; Miller, B.R.; Komar, N. Differential virulence of West Nile strains for American crows. Emerg. Infect. Dis. 2004, 10, 2161–2168. [Google Scholar] [CrossRef]
- Gamino, V.; Gutiérrez-Guzmán, A.V.; Fernández-de-Mera, I.G.; Ortíz, J.A.; Durán-Martín, M.; de la Fuente, J.; Gortázar, C.; Hófle, C. Natural Bagaza virus infection in game birds in southern Spain. Vet. Res. 2012, 43, 65. [Google Scholar] [CrossRef]

| 3 dpi | 6 dpi | 14 dpi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SP07 | MO03 | SP07 | MO03 | SP07 | MO03 | ||||||
| Tissue | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 * | No. 10 | No. 11 |
| Brain | 30.3 | 32.8 | 31.4 | 33.9 | 25.2 | 26.5 | 25.2 | 24.8 | NA | − | 37.6 |
| Heart | 28.7 | 28.5 | 25.4 | 24.8 | 19.6 | 20.3 | 23.7 | 18.8 | NA | 38.0 | 33.8 |
| Lung | NA | 30.9 | 31.0 | 30.1 | 26.3 | 25.7 | 29.2 | 24.2 | NA | − | 38.8 |
| Liver | NA | 30.2 | 29.6 | 30.9 | 28.7 | 25.7 | 30.8 | 28.2 | NA | − | − |
| Spleen | 25.3 | 23.7 | 26.5 | 24.4 | 29.7 | 22.7 | 24.6 | 21.8 | NA | 36.1 | 34.0 |
| Kidney | 26.3 | 27.5 | 24.5 | 26.0 | 23.0 | 22.4 | 23.7 | 20.9 | NA | 37.5 | 36.1 |
| Thymus | 27.6 | 29.3 | 31.9 | 23.5 | 30.2 | 23.4 | 33.0 | NA | NA | − | 29.0 |
| Bursa of Fabricius | NA | 32.0 | 32.8 | 34.2 | 27.9 | 24.8 | 31.5 | 29.8 | NA | − | 31.3 |
| Feather pulp | − | 31.4 | 27.4 | 31.3 | 18.2 | 20.8 | 22.8 | 16.9 | NA | 34.6 | 33.7 |
| 3 dpi | 6 dpi | 14 dpi | |||||||||
| SP07 | MO03 | SP07 | MO03 | SP07 | MO03 | ||||||
| Tissue/Lesion | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 |
| Cerebrum | |||||||||||
| Neuronal necrosis | − | − | − | − | − | − | + | + | + | − | + |
| Gliosis | − | − | − | − | − | + | + | ++ | + | + | − |
| Perivascular cuffing | − | − | − | − | − | − | − | − | − | + | + |
| Endothelial cell swelling | + | + | ++ | ++ | + | + | + | ++ | ++ | + | + |
| Cerebellum | |||||||||||
| Purkinje cell necrosis | − | − | − | − | − | − | ++ | + | + | NA | + |
| Gliosis | − | − | − | − | − | − | ++ | + | − | NA | ++ |
| Perivascular cuffing | − | − | − | − | − | − | − | − | + | NA | − |
| Endothelial cell swelling | − | − | ++ | ++ | + | + | +++ | ++ | ++ | NA | ++ |
| Heart | |||||||||||
| Myofiber necrosis-degeneration | + | + | − | + | +++ | ++ | +++ | +++ | ++ | + | ++ |
| Inflammatory infiltrate | − | ++ | − | ++ | +++ | +++ | +++ | +++ | + | ++ | +++ |
| Lung | |||||||||||
| Inflammatory infiltrate | − | +++ | − | ++ | +++ | +++ | +++ | +++ | + | − | +++ |
| Liver | |||||||||||
| Hepatocyte necrosis | − | − | − | − | − | +++ | − | +++ | − | − | − |
| Inflammatory infiltrate | − | ++ | ++ | ++ | ++ | ++ | ++ | +++ | − | ++ | + |
| Hemosiderosis | − | − | − | − | + | + | ++ | ++ | ++ | + | + |
| Tissue/Lesion | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 |
| Spleen | |||||||||||
| Lymphoid cell necrosis | − | − | − | − | − | − | − | − | ++ | − | − |
| Lymphoid cell depletion | − | − | ++ | + | + | + | + | + | − | − | − |
| Granulocytic infiltrate | +++ | +++ | ++ | ++ | + | +++ | − | − | +++ | ++ | +++ |
| Eosinophilic material deposits | − | − | − | − | +++ | +++ | ++ | ++ | ++ | − | ++ |
| Hemosiderosis | − | − | − | − | +++ | +++ | + | +++ | ++ | − | + |
| Kidney | |||||||||||
| Tubular epithelial cell necrosis | − | − | − | − | − | − | − | − | +++ | +++ | +++ |
| Inflammatory infiltrate | − | ++ | − | ++ | − | − | − | + | + | − | ++ |
| Duodenum | |||||||||||
| Inflammatory infiltrate | + | + | − | − | − | + | − | − | NA | − | + |
| Large intestine | |||||||||||
| Inflammatory infiltrate | − | + | − | ++ | − | − | − | − | NA | − | ++ |
| Cecal tonsils | |||||||||||
| Lymphoid cell necrosis | NA | NA | NA | + | NA | ++ | NA | NA | NA | − | NA |
| Bursa of Fabricius | |||||||||||
| Lymphoid cell necrosis | − | − | − | − | NA | − | − | ++ | NA | NA | − |
| Lymphoid cell depletion | − | + | − | − | NA | ++ | ++ | ++ | NA | NA | − |
| Skin + feather follicle | |||||||||||
| Inflammatory infiltrate skin | − | NA | − | +++ | − | NA | NA | − | NA | ++ | − |
| Inflammatory infiltrate feather pulp | +++ | NA | +++ | NA | NA | NA | NA | +++ | NA | − | +++ |
| 3 dpi | 6 dpi | 14 dpi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SP07 | MO03 | SP07 | MO03 | SP07 | MO03 | ||||||
| Tissue | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 |
| Heart | ± | − | + | + | ++ | ++ | + | +++ | − | − | − |
| Lung | − | − | − | − | − | ± | − | + | − | − | − |
| Liver | − | − | − | − | − | − | − | + | − | − | − |
| Spleen | + | + | + | ± | − | + | + | + | − | − | − |
| Kidney | − | − | + | + | + | + | + | ++ | − | − | − |
| Duodenum | − | − | − | − | − | − | − | + | NA | − | − |
| Large intestine | − | − | − | − | + | + | + | + | NA | − | − |
| Pancreas | − | − | ± | ± | + | + | + | + | NA | − | − |
| Cecal tonsils | NA | NA | NA | + | NA | − | NA | NA | NA | − | NA |
| Primary Antibody a | Cell Population | Pretreatment b | Primary Antibody Dilution and Incubation c | Secondary Antibody d | Detection System e |
|---|---|---|---|---|---|
| Lectin RCA-1 biotinylated | Microglia-macrophages | Citrate buffer Microwave heat (22 min) | 1:600, 45 min RT | Goat anti-rabbit IgG | ABC-DAB |
| Polyclonal rabbit anti-GFAP | Astrocytes | Proteinase K (7 min RT) | 1:500, 4 °C ON | Labelled polymer-HRP anti-rabbit | AEC + substrate chromogen |
| Polyclonal rabbit anti-human CD3 | T cells | Citrate buffer Microwave heat (22 min) | 1:500, 4 °C ON | Labelled polymer-HRP anti-rabbit | AEC + substrate chromogen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamino, V.; Pérez-Ramírez, E.; Gutiérrez-Guzmán, A.V.; Sotelo, E.; Llorente, F.; Jiménez-Clavero, M.Á.; Höfle, U. Pathogenesis of Two Western Mediterranean West Nile Virus Lineage 1 Isolates in Experimentally Infected Red-Legged Partridges (Alectoris rufa). Pathogens 2021, 10, 748. https://doi.org/10.3390/pathogens10060748
Gamino V, Pérez-Ramírez E, Gutiérrez-Guzmán AV, Sotelo E, Llorente F, Jiménez-Clavero MÁ, Höfle U. Pathogenesis of Two Western Mediterranean West Nile Virus Lineage 1 Isolates in Experimentally Infected Red-Legged Partridges (Alectoris rufa). Pathogens. 2021; 10(6):748. https://doi.org/10.3390/pathogens10060748
Chicago/Turabian StyleGamino, Virginia, Elisa Pérez-Ramírez, Ana Valeria Gutiérrez-Guzmán, Elena Sotelo, Francisco Llorente, Miguel Ángel Jiménez-Clavero, and Ursula Höfle. 2021. "Pathogenesis of Two Western Mediterranean West Nile Virus Lineage 1 Isolates in Experimentally Infected Red-Legged Partridges (Alectoris rufa)" Pathogens 10, no. 6: 748. https://doi.org/10.3390/pathogens10060748
APA StyleGamino, V., Pérez-Ramírez, E., Gutiérrez-Guzmán, A. V., Sotelo, E., Llorente, F., Jiménez-Clavero, M. Á., & Höfle, U. (2021). Pathogenesis of Two Western Mediterranean West Nile Virus Lineage 1 Isolates in Experimentally Infected Red-Legged Partridges (Alectoris rufa). Pathogens, 10(6), 748. https://doi.org/10.3390/pathogens10060748








