S-Acylation of Proteins of Coronavirus and Influenza Virus: Conservation of Acylation Sites in Animal Viruses and DHHC Acyltransferases in Their Animal Reservoirs
Abstract
1. Introduction
1.1. Pandemics Caused by Influenza Virus and Coronaviruses
1.2. S-Acylation of Influenza Virus and Coronavirus Proteins
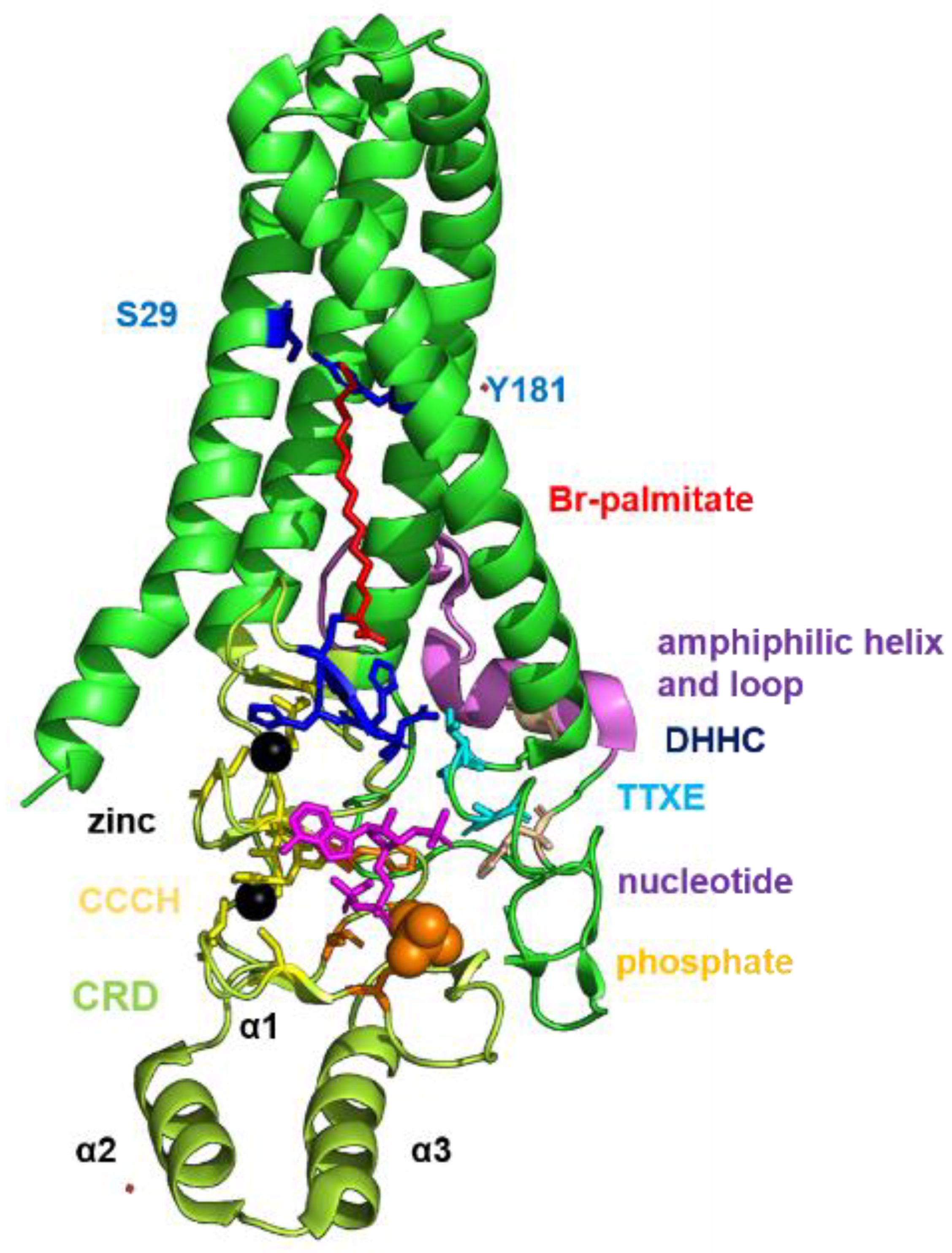
1.3. Structure and Function of DHHC Proteins
1.4. DHHCs Involved in Acylation of Influenza and Coronavirus Proteins
1.5. Rationale for This Study
2. Results
2.1. Analysis of S-Acylation Sites in HA and M2 of Human and Animal Influenza Viruses
2.2. Analysis of S-Acylation Sites in S and E of Human and Animal Coronaviruses
2.3. Number and Type of DHHC Genes in Animal Hosts of Influenza and Coronaviruses
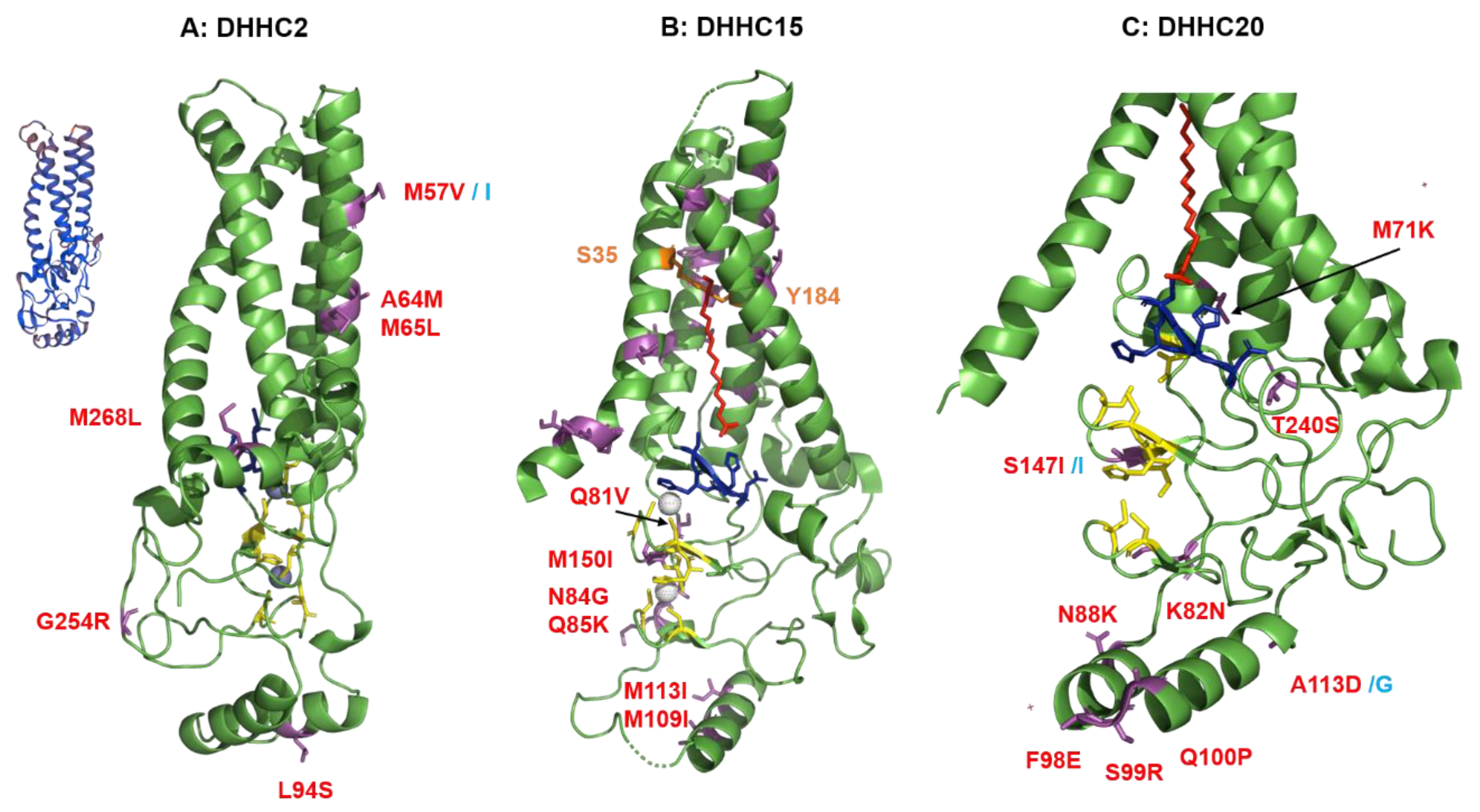
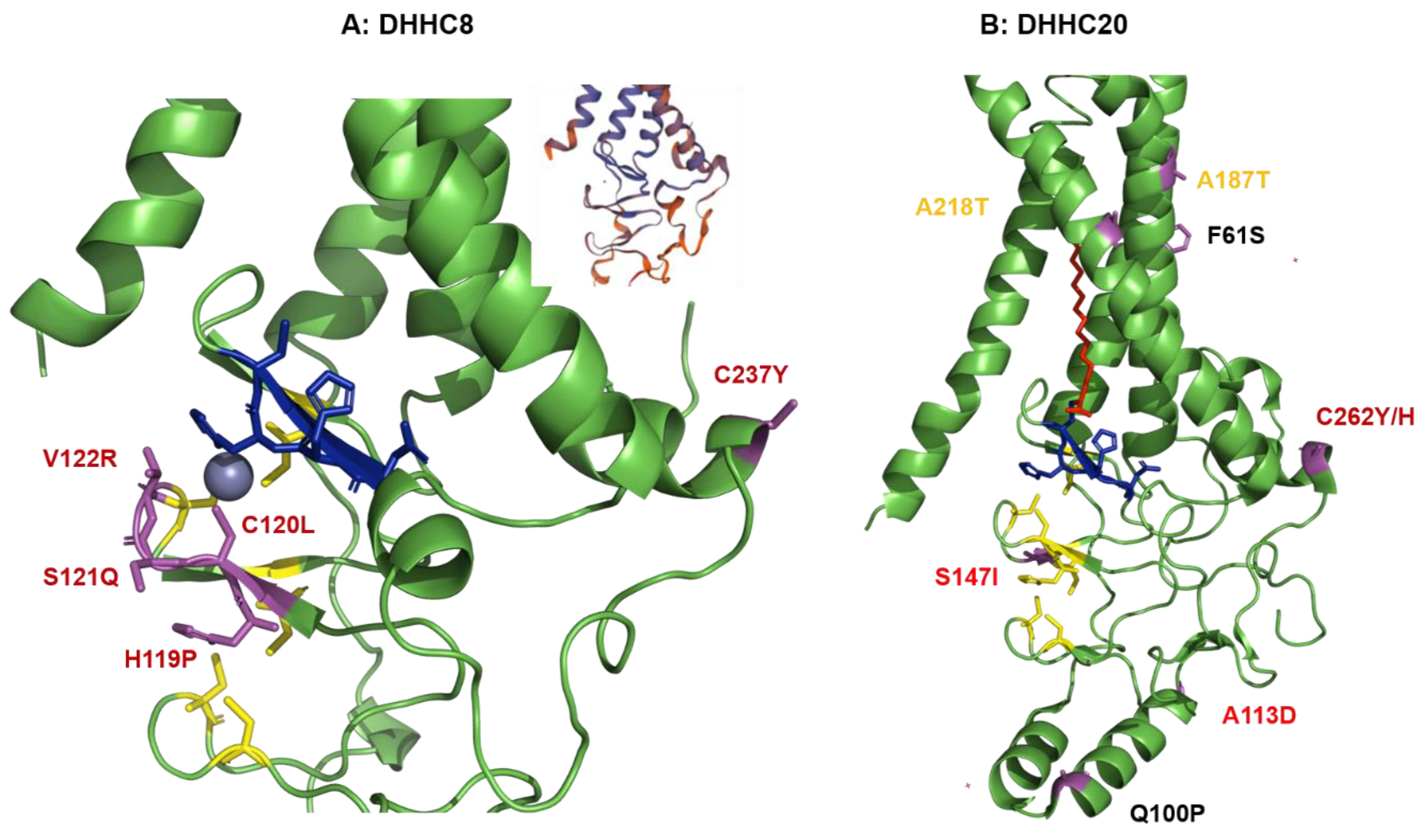
2.4. Structural Comparison of Human DHHCs with the Ortholog Proteins in Animal Hosts
2.4.1. Structural Comparison of Human DHHCs with the Ortholog Proteins in Animal Hosts of Flu A
2.4.2. Structural Comparison of Human DHHCs with the Ortholog Proteins in Animal Hosts of CoVs
3. Discussion
4. Material and Methods
4.1. Collection and Comparison of Viral Protein Sequences
4.2. Collection and Comparison of DHHC Sequences
4.3. Structural Analysis of Amino Acid Substitutions between Human and Animal DHHCs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and Pandemic Potential of Swine-Origin H1n1 Influenza Virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef]
- Palese, P. Influenza: Old and New Threats. Nat. Med. 2004, 10, S82–S87. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawaoka, Y. Pathogenesis of the 1918 Pandemic Influenza Virus. PLoS Pathog 2011, 7, e1001218. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, C. Source for Influenza Pandemics. Eur. J. Epidemiol. 1994, 10, 455–458. [Google Scholar] [CrossRef]
- Herfst, S.; Imai, M.; Kawaoka, Y.; Fouchier, R.A. Avian Influenza Virus Transmission to Mammals. Curr. Top Microbiol. Immunol. 2014, 385, 137–155. [Google Scholar] [PubMed]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and Ecology of Influenza a Viruses. Curr. Top Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar] [PubMed]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A Distinct Lineage of Influenza a Virus from Bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New World Bats Harbor Diverse Influenza a Viruses. PLoS Pathog 2013, 9, e1003657. [Google Scholar] [CrossRef]
- Karakus, U.; Thamamongood, T.; Ciminski, K.; Ran, W.; Gunther, S.C.; Pohl, M.O.; Eletto, D.; Jeney, C.; Hoffmann, D.; Reiche, S.; et al. Mhc Class Ii Proteins Mediate Cross-Species Entry of Bat Influenza Viruses. Nature 2019, 567, 109–112. [Google Scholar] [CrossRef]
- Ge, X.Y.; Li, J.L.; Yang, X.L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and Characterization of a Bat Sars-Like Coronavirus That Uses the Ace2 Receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef]
- Guan, Y.; Zheng, B.J.; He, Y.Q.; Liu, X.L.; Zhuang, Z.X.; Cheung, C.L.; Luo, S.W.; Li, P.H.; Zhang, L.J.; Guan, Y.J.; et al. Isolation and Characterization of Viruses Related to the Sars Coronavirus from Animals in Southern China. Science 2003, 302, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Masignani, V.; Eickmann, M.; Becker, S.; Abrignani, S.; Klenk, H.D.; Rappuoli, R. Sars–Beginning to Understand a New Virus. Nat. Rev. Microbiol. 2003, 1, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zeng, L.P.; Yang, X.L.; Ge, X.Y.; Zhang, W.; Li, B.; Xie, J.Z.; Shen, X.R.; Zhang, Y.Z.; Wang, N.; et al. Discovery of a Rich Gene Pool of Bat Sars-Related Coronaviruses Provides New Insights into the Origin of Sars Coronavirus. PLoS Pathog 2017, 13, e1006698. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Sabir, J.S.; Lam, T.T.; Ahmed, M.M.; Li, L.; Shen, Y.; Abo-Aba, S.E.; Qureshi, M.I.; Abu-Zeid, M.; Zhang, Y.; Khiyami, M.A.; et al. Co-Circulation of Three Camel Coronavirus Species and Recombination of Mers-Covs in Saudi Arabia. Science 2016, 351, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Gilardi, K.; Menachery, V.D.; Goldstein, T.; Ssebide, B.; Mbabazi, R.; Navarrete-Macias, I.; Liang, E.; Wells, H.; Hicks, A.; et al. Further Evidence for Bats as the Evolutionary Source of Middle East Respiratory Syndrome Coronavirus. mBio 2017, 8. [Google Scholar] [CrossRef]
- Anthony, S.J.; Johnson, C.K.; Greig, D.J.; Kramer, S.; Che, X.; Wells, H.; Hicks, A.L.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; et al. Global Patterns in Coronavirus Diversity. Virus Evol. 2017, 3, vex012. [Google Scholar] [CrossRef]
- Corman, V.M.; Ithete, N.L.; Richards, L.R.; Schoeman, M.C.; Preiser, W.; Drosten, C.; Drexler, J.F. Rooting the Phylogenetic Tree of Middle East Respiratory Syndrome Coronavirus by Characterization of a Conspecific Virus from an African Bat. J. Virol. 2014, 88, 11297–11303. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Weiss, S.R. Origins and Pathogenesis of Middle East Respiratory Syndrome-Associated Coronavirus: Recent Advances. F1000Research 2017, 6, 1628. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; He, W.T.; Wang, L.; Lai, A.; Ji, X.; Zhai, X.; Li, G.; Suchard, M.A.; Tian, J.; Zhou, J.; et al. Covid-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives. Trends Mol. Med. 2020, 26, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. Addendum: A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 588, E6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Lam, T.T.; Jia, N.; Zhang, Y.W.; Shum, M.H.; Jiang, J.F.; Zhu, H.C.; Tong, Y.G.; Shi, Y.X.; Ni, X.B.; Liao, Y.S.; et al. Identifying Sars-Cov-2-Related Coronaviruses in Malayan Pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef]
- Olival, K.J.; Cryan, P.M.; Amman, B.R.; Baric, R.S.; Blehert, D.S.; Brook, C.E.; Calisher, C.H.; Castle, K.T.; Coleman, J.T.H.; Daszak, P.; et al. Possibility for Reverse Zoonotic Transmission of Sars-Cov-2 to Free-Ranging Wildlife: A Case Study of Bats. PLoS Pathog 2020, 16, e1008758. [Google Scholar] [CrossRef]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188. [Google Scholar] [PubMed]
- Veit, M. Palmitoylation of Virus Proteins. Biol. Cell 2012, 104, 493–515. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Vachieri, S.G.; Xiong, X.; Zhang, J.; Martin, S.R.; Skehel, J.J. Hemagglutinin Structure and Activities. Cold Spring Harb. Perspect. Med. 2020, a038638. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-Em Structure of the 2019-Ncov Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Lamb, R.A. The Structure, Function, and Pathobiology of the Influenza a and B Virus Ion Channels. Cold Spring Harb. Perspect. Med. 2020, 10, a038505. [Google Scholar] [CrossRef]
- Ruch, T.R.; Machamer, C.E. The Coronavirus E Protein: Assembly and Beyond. Viruses 2012, 4, 363–382. [Google Scholar] [CrossRef]
- Wolff, T.; Veit, M. Influenza B, C and D Viruses (Orthomyxoviridae). In Encyclopedia of Virology; Zuckerman, M., Bamford, D.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 561–574. [Google Scholar] [CrossRef]
- Kordyukova, L.V.; Serebryakova, M.V.; Baratova, L.A.; Veit, M. S-Acylation of the Hemagglutinin of Influenza Viruses: Mass Spectrometry Reveals Site-Specific Attachment of Stearic Acid to a Transmembrane Cysteine. J. Virol. 2008, 82, 9288–9292. [Google Scholar] [CrossRef] [PubMed]
- Veit, M.; Kretzschmar, E.; Kuroda, K.; Garten, W.; Schmidt, M.F.; Klenk, H.D.; Rott, R. Site-Specific Mutagenesis Identifies Three Cysteine Residues in the Cytoplasmic Tail as Acylation Sites of Influenza Virus Hemagglutinin. J. Virol. 1991, 65, 2491–2500. [Google Scholar] [CrossRef]
- Bos, E.C.; Heijnen, L.; Luytjes, W.; Spaan, W.J. Mutational Analysis of the Murine Coronavirus Spike Protein: Effect on Cell-to-Cell Fusion. Virology 1995, 214, 453–463. [Google Scholar] [CrossRef]
- Sugrue, R.J.; Belshe, R.B.; Hay, A.J. Palmitoylation of the Influenza a Virus M2 Protein. Virology 1990, 179, 51–56. [Google Scholar] [CrossRef]
- Veit, M.; Klenk, H.D.; Kendal, A.; Rott, R. The M2 Protein of Influenza a Virus Is Acylated. J. Gen. Virol. 1991, 72, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Takeda, M.; Lamb, R.A. Influenza Virus Hemagglutinin (H3 Subtype) Requires Palmitoylation of Its Cytoplasmic Tail for Assembly: M1 Proteins of Two Subtypes Differ in Their Ability to Support Assembly. J. Virol. 2005, 79, 13673–13684. [Google Scholar] [CrossRef]
- Siche, S.; Brett, K.; Moller, L.; Kordyukova, L.V.; Mintaev, R.R.; Alexeevski, A.V.; Veit, M. Two Cytoplasmic Acylation Sites and an Adjacent Hydrophobic Residue, but No Other Conserved Amino Acids in the Cytoplasmic Tail of Ha from Influenza a Virus Are Crucial for Virus Replication. Viruses 2015, 7, 6458–6475. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Herwig, A.; Azzouz, N.; Klenk, H.D. Acylation-Mediated Membrane Anchoring of Avian Influenza Virus Hemagglutinin Is Essential for Fusion Pore Formation and Virus Infectivity. J. Virol. 2005, 79, 6449–6458. [Google Scholar] [CrossRef]
- Zurcher, T.; Luo, G.; Palese, P. Mutations at Palmitylation Sites of the Influenza Virus Hemagglutinin Affect Virus Formation. J. Virol. 1994, 68, 5748–5754. [Google Scholar] [CrossRef]
- Thorp, E.B.; Boscarino, J.A.; Logan, H.L.; Goletz, J.T.; Gallagher, T.M. Palmitoylations on Murine Coronavirus Spike Proteins Are Essential for Virion Assembly and Infectivity. J. Virol. 2006, 80, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, J.A.; Logan, H.L.; Lacny, J.J.; Gallagher, T.M. Envelope Protein Palmitoylations Are Crucial for Murine Coronavirus Assembly. J. Virol. 2008, 82, 2989–2999. [Google Scholar] [CrossRef] [PubMed]
- Gelhaus, S.; Thaa, B.; Eschke, K.; Veit, M.; Schwegmann-Wessels, C. Palmitoylation of the Alphacoronavirus Tgev Spike Protein S Is Essential for Incorporation into Virus-Like Particles but Dispensable for S-M Interaction. Virology 2014, 464–465, 397–405. [Google Scholar] [CrossRef]
- McBride, C.E.; Machamer, C.E. Palmitoylation of Sars-Cov S Protein Is Necessary for Partitioning into Detergent-Resistant Membranes and Cell-Cell Fusion but Not Interaction with M Protein. Virology 2010, 405, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.M.; Chouljenko, V.N.; Iyer, A.; Colgrove, R.; Farzan, M.; Knipe, D.M.; Kousoulas, K.G. Palmitoylation of the Cysteine-Rich Endodomain of the Sars-Coronavirus Spike Glycoprotein Is Important for Spike-Mediated Cell Fusion. Virology 2007, 360, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Shulla, A.; Gallagher, T. Role of Spike Protein Endodomains in Regulating Coronavirus Entry. J. Biol. Chem. 2009, 284, 32725–32734. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Zhang, S.; Wang, Q.; Anang, S.; Wang, J.; Ding, H.; Kappes, J.C.; Sodroski, J. Spike Glycoprotein and Host Cell Determinants of Sars-Cov-2 Entry and Cytopathic Effects. J. Virol. 2020. [Google Scholar] [CrossRef]
- Mesquita, F.S.; Abrami, L.; Sergeeva, O.; Turelli, P.; Kunz, B.; Raclot, C.; Montoya, J.P.; Abriata, L.A.; Peraro, M.D.; Trono, D.; et al. S-Acylation Controls Sars-Cov-2 Membrane Lipid Organization and Enhances Infectivity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lopez, L.A.; Riffle, A.J.; Pike, S.L.; Gardner, D.; Hogue, B.G. Importance of Conserved Cysteine Residues in the Coronavirus Envelope Protein. J. Virol. 2008, 82, 3000–3010. [Google Scholar] [CrossRef]
- Castrucci, M.R.; Hughes, M.; Calzoletti, L.; Donatelli, I.; Wells, K.; Takada, A.; Kawaoka, Y. The Cysteine Residues of the M2 Protein Are Not Required for Influenza a Virus Replication. Virology 1997, 238, 128–134. [Google Scholar] [CrossRef]
- Stewart, S.M.; Pekosz, A. Mutations in the Membrane-Proximal Region of the Influenza a Virus M2 Protein Cytoplasmic Tail Have Modest Effects on Virus Replication. J. Virol. 2011, 85, 12179–12187. [Google Scholar] [CrossRef] [PubMed]
- Thaa, B.; Tielesch, C.; Moller, L.; Schmitt, A.O.; Wolff, T.; Bannert, N.; Herrmann, A.; Veit, M. Growth of Influenza a Virus Is Not Impeded by Simultaneous Removal of the Cholesterol-Binding and Acylation Sites in the M2 Protein. J. Gen. Virol. 2012, 93, 282–292. [Google Scholar] [CrossRef]
- Gottlieb, C.D.; Linder, M.E. Structure and Function of Dhhc Protein S-Acyltransferases. Biochem. Soc. Trans. 2017, 45, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Lemonidis, K.; Salaun, C.; Kouskou, M.; Diez-Ardanuy, C.; Chamberlain, L.H.; Greaves, J. Substrate Selectivity in the Zdhhc Family of S-Acyltransferases. Biochem. So.c Tran.s 2017, 45, 751–758. [Google Scholar] [CrossRef]
- Lemonidis, K.; Werno, M.W.; Greaves, J.; Diez-Ardanuy, C.; Sanchez-Perez, M.C.; Salaun, C.; Thomson, D.M.; Chamberlain, L.H. The Zdhhc Family of S-Acyltransferases. Biochem. Soc. Trans. 2015, 43, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Zaballa, M.E.; van der Goot, F.G. The Molecular Era of Protein S-Acylation: Spotlight on Structure, Mechanisms, and Dynamics. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 420–451. [Google Scholar] [CrossRef]
- Rana, M.S.; Kumar, P.; Lee, C.J.; Verardi, R.; Rajashankar, K.R.; Banerjee, A. Fatty Acyl Recognition and Transfer by an Integral Membrane S-Acyltransferase. Science 2018, 359, eaa06326. [Google Scholar] [CrossRef]
- Jennings, B.C.; Linder, M.E. Dhhc Protein S-Acyltransferases Use Similar Ping-Pong Kinetic Mechanisms but Display Different Acyl-Coa Specificities. J. Biol. Chem. 2012, 287, 7236–7245. [Google Scholar] [CrossRef]
- Greaves, J.; Munro, K.R.; Davidson, S.C.; Riviere, M.; Wojno, J.; Smith, T.K.; Tomkinson, N.C.; Chamberlain, L.H. Molecular Basis of Fatty Acid Selectivity in the Zdhhc Family of S-Acyltransferases Revealed by Click Chemistry. Proc. Natl. Acad. Sci. USA 2017, 114, E1365–E1374. [Google Scholar] [CrossRef]
- Lemonidis, K.; Sanchez-Perez, M.C.; Chamberlain, L.H. Identification of a Novel Sequence Motif Recognized by the Ankyrin Repeat Domain of Zdhhc17/13 S-Acyltransferases. J. Biol. Chem. 2015, 290, 21939–21950. [Google Scholar] [CrossRef] [PubMed]
- Verardi, R.; Kim, J.S.; Ghirlando, R.; Banerjee, A. Structural Basis for Substrate Recognition by the Ankyrin Repeat Domain of Human Dhhc17 Palmitoyltransferase. Structure 2017, 25, 1337–1347.e6. [Google Scholar] [CrossRef] [PubMed]
- Hodson, N.; Invergo, B.; Rayner, J.C.; Choudhary, J.S. Palmitoylation and Palmitoyl-Transferases in Plasmodium Parasites. Biochem. Soc. Trans. 2015, 43, 240–245. [Google Scholar] [CrossRef]
- Wittouck, S.; van Noort, V. Correlated Duplications and Losses in the Evolution of Palmitoylation Writer and Eraser Families. BMC Evol. Biol. 2017, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, M.R.; Abrami, L.; van der Goot, F.G.; Veit, M. Hemagglutinin of Influenza a, but Not of Influenza B and C Viruses Is Acylated by Zdhhc2, 8, 15 and 20. Biochem. J. 2020, 477, 285–303. [Google Scholar] [CrossRef]
- Veit, M.; Siche, S. S-Acylation of Influenza Virus Proteins: Are Enzymes for Fatty Acid Attachment Promising Drug Targets? Vaccine 2015, 33, 7002–7007. [Google Scholar] [CrossRef]
- Gadalla, M.R.; Veit, M. Toward the Identification of Zdhhc Enzymes Required for Palmitoylation of Viral Protein as Potential Drug Targets. Expert Opin. Drug Discov. 2020, 15, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Kordyukova, L.V.; Serebryakova, M.V.; Polyansky, A.A.; Kropotkina, E.A.; Alexeevski, A.V.; Veit, M.; Efremov, R.G.; Filippova, I.Y.; Baratova, L.A. Linker and/or Transmembrane Regions of Influenza a/Group-1, a/Group-2, and Type B Virus Hemagglutinins Are Packed Differently within Trimers. Biochim. Biophys. Acta. 2011, 1808, 1843–1854. [Google Scholar] [CrossRef]
- Worobey, M.; Han, G.Z.; Rambaut, A. A Synchronized Global Sweep of the Internal Genes of Modern Avian Influenza Virus. Nature 2014, 508, 254–257. [Google Scholar] [CrossRef]
- Su, S.; Fu, X.; Li, G.; Kerlin, F.; Veit, M. Novel Influenza D Virus: Epidemiology, Pathology, Evolution and Biological Characteristics. Virulence 2017, 8, 1580–1591. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.D.; Chen, X.; Tian, J.H.; Chen, L.J.; Li, K.; Wang, W.; Eden, J.S.; Shen, J.J.; Liu, L.; et al. The Evolutionary History of Vertebrate Rna Viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Khurana, S.K.; Chakraborty, S.; Malik, Y.S.; Virmani, N.; Singh, R.; et al. A Comprehensive Review on Equine Influenza Virus: Etiology, Epidemiology, Pathobiology, Advances in Developing Diagnostics, Vaccines, and Control Strategies. Front. Microbiol. 2018, 9, 1941. [Google Scholar] [CrossRef]
- Wasik, B.R.; Voorhees, I.E.H.; Parrish, C.R. Canine and Feline Influenza. Cold Spring Harb. Perspect. Med. 2021, 11, a038562. [Google Scholar] [CrossRef]
- Neumann, G.; Kawaoka, Y. The First Influenza Pandemic of the New Millennium. Influenza Other Respir. Viruses 2011, 5, 157–166. [Google Scholar] [CrossRef]
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J. Virol. 2015, 89, 8671–8676. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Woo, P.C.; Li, K.S.; Huang, Y.; Tsoi, H.W.; Wong, B.H.; Wong, S.S.; Leung, S.Y.; Chan, K.H.; Yuen, K.Y. Severe Acute Respiratory Syndrome Coronavirus-Like Virus in Chinese Horseshoe Bats. Proc. Natl. Acad. Sc.i USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats Are Natural Reservoirs of Sars-Like Coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.C.; Zhang, J.X.; Zhang, S.Y.; Wang, P.; Fan, X.H.; Li, L.F.; Li, G.; Dong, B.Q.; Liu, W.; Cheung, C.L.; et al. Prevalence and Genetic Diversity of Coronaviruses in Bats from China. J. Virol. 2006, 80, 7481–7490. [Google Scholar] [CrossRef]
- Woo, P.C.; Wang, M.; Lau, S.K.; Xu, H.; Poon, R.W.; Guo, R.; Wong, B.H.; Gao, K.; Tsoi, H.W.; Huang, Y.; et al. Comparative Analysis of Twelve Genomes of Three Novel Group 2c and Group 2d Coronaviruses Reveals Unique Group and Subgroup Features. J. Virol. 2007, 81, 1574–1585. [Google Scholar] [CrossRef]
- Wang, L.; Su, S.; Bi, Y.; Wong, G.; Gao, G.F. Bat-Origin Coronaviruses Expand Their Host Range to Pigs. Trends Microbiol. 2018, 26, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Wille, M.; Holmes, E.C. Wild Birds as Reservoirs for Diverse and Abundant Gamma- and Deltacoronaviruses. FEMS Microbiol. Rev. 2020, 44, 631–644. [Google Scholar] [CrossRef]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and Re-Emerging Coronaviruses in Pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [PubMed]
- Grange, Z.L.; Goldstein, T.; Johnson, C.K.; Anthony, S.; Gilardi, K.; Daszak, P.; Olival, K.J.; O’Rourke, T.; Murray, S.; Olson, S.H.; et al. Ranking the Risk of Animal-to-Human Spillover for Newly Discovered Viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2002324118. [Google Scholar] [CrossRef]
- Corse, E.; Machamer, C.E. The Cytoplasmic Tail of Infectious Bronchitis Virus E Protein Directs Golgi Targeting. J. Virol. 2002, 76, 1273–1284. [Google Scholar] [CrossRef]
- Gadalla, M.R.; Morrison, E.; Serebryakova, M.V.; Han, X.; Wolff, T.; Freund, C.; Kordyukova, L.; Veit, M. Ns1-Mediated Upregulation of Zdhhc22 Acyltransferase in Influenza a Virus Infected Cells. Cell Microbiol. 2021, 23, e13322. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Zhong, L.; Lin, H.; Hu, M.M.; Zhou, Y.; Shu, H.B.; Li, S. Zdhhc11 Modulates Innate Immune Response to DNA Virus by Mediating Mita-Irf3 Association. Cell Mol. Immunol. 2018, 15, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, H.; Zhang, L. Fatty Acid Synthase Promotes the Palmitoylation of Chikungunya Virus Nsp1. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Munster, V.J.; Baas, C.; Lexmond, P.; Waldenstrom, J.; Wallensten, A.; Fransson, T.; Rimmelzwaan, G.F.; Beyer, W.E.; Schutten, M.; Olsen, B.; et al. Spatial, Temporal, and Species Variation in Prevalence of Influenza a Viruses in Wild Migratory Birds. PLoS Pathog 2007, 3, e61. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenstrom, J.; Osterhaus, A.D.; Fouchier, R.A. Global Patterns of Influenza a Virus in Wild Birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.O.; Woodley, K.T.; Choudhary, J.S. Global, Site-Specific Analysis of Neuronal Protein S-Acylation. Sci. Rep. 2017, 7, 4683. [Google Scholar] [CrossRef]
- Yang, W.; di Vizio, D.; Kirchner, M.; Steen, H.; Freeman, M.R. Proteome Scale Characterization of Human S-Acylated Proteins in Lipid Raft-Enriched and Non-Raft Membranes. Mol. Cell Proteom. 2010, 9, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, C.D.; Zhang, S.; Linder, M.E. The Cysteine-Rich Domain of the Dhhc3 Palmitoyltransferase Is Palmitoylated and Contains Tightly Bound Zinc. J. Biol. Chem. 2015, 290, 29259–29269. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.F.; Feng, Y.; Chen, L.; Davis, N.G. The Yeast Dhhc Cysteine-Rich Domain Protein Akr1p Is a Palmitoyl Transferase. J. Cell Biol. 2002, 159, 23–28. [Google Scholar] [CrossRef]
- Hou, H.; Peter, A.T.J.; Meiringer, C.; Subramanian, K.; Ungermann, C. Analysis of Dhhc Acyltransferases Implies Overlapping Substrate Specificity and a Two-Step Reaction Mechanism. Traffic 2009, 10, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Mitchell, G.; Ling, Y.; Budde, C.; Deschenes, R.J. Mutational Analysis of Saccharomyces Cerevisiae Erf2 Reveals a Two-Step Reaction Mechanism for Protein Palmitoylation by Dhhc Enzymes. J. Biol. Chem. 2010, 285, 38104–38114. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Schad, E.; Tantos, A.; Kalmar, L. Intrinsically Disordered Proteins: Emerging Interaction Specialists. Curr. Opin. Struct. Biol. 2015, 35, 49–59. [Google Scholar] [CrossRef]
- Zhang, M.; Han, X.; Osterrieder, K.; Veit, M. Palmitoylation of the Envelope Membrane Proteins Gp5 and M of Porcine Reproductive and Respiratory Syndrome Virus Is Essential for Virus Growth. PLoS Pathog 2021, 17, e1009554. [Google Scholar] [CrossRef]
- Whitt, M.A.; Rose, J.K. Fatty Acid Acylation Is Not Required for Membrane Fusion Activity or Glycoprotein Assembly into Vsv Virions. Virology 1991, 185, 875–878. [Google Scholar] [CrossRef]
- Chavda, B.; Arnott, J.A.; Planey, S.L. Targeting Protein Palmitoylation: Selective Inhibitors and Implications in Disease. Expert Opin. Drug Discov. 2014, 9, 1005–1019. [Google Scholar] [CrossRef]
- Ducker, C.E.; Griffel, L.K.; Smith, R.A.; Keller, S.N.; Zhuang, Y.; Xia, Z.; Diller, J.D.; Smith, C.D. Discovery and Characterization of Inhibitors of Human Palmitoyl Acyltransferases. Mol. Cancer Ther. 2006, 5, 1647–1659. [Google Scholar] [CrossRef]
- Jennings, B.C.; Nadolski, M.J.; Ling, Y.; Baker, M.B.; Harrison, M.L.; Deschenes, R.J.; Linder, M.E. 2-Bromopalmitate and 2-(2-Hydroxy-5-Nitro-Benzylidene)-Benzo[B]Thiophen-3-One Inhibit Dhhc-Mediated Palmitoylation in Vitro. J. Lipid Res. 2009, 50, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.P.; Chari, A.; Ciferri, C.; Liu, W.T.; Remigy, H.W.; Stark, H.; Wiesmann, C. Cryo-Em in Drug Discovery: Achievements, Limitations and Prospects. Nat. Rev. Drug Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef] [PubMed]
- De, I.; Sadhukhan, S. Emerging Roles of Dhhc-Mediated Protein S-Palmitoylation in Physiological and Pathophysiological Context. Eur. J. Cell Biol. 2018, 97, 319–338. [Google Scholar] [CrossRef] [PubMed]
- El-Husseini Ael, D.; Bredt, D.S. Protein Palmitoylation: A Regulator of Neuronal Development and Function. Nat. Rev. Neurosci. 2002, 3, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.J.; Dixon, S.J. Protein Palmitoylation and Cancer. EMBO Rep. 2018, 19, e46666. [Google Scholar] [CrossRef]
- Yeste-Velasco, M.; Linder, M.E.; Lu, Y.J. Protein S-Palmitoylation and Cancer. Biochim. Biophys. Acta. 2015, 1856, 107–120. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic. Acids. Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]

| S-Acylation Sites in Spike Proteins of Influenza and Coronaviruses | ||
| Flu A | HA/1 | LVSLGAISFWMC SNGSLQCRICI |
| Flu A | HA/7 | LAIAVGLVFICV KNGNMRCTICI |
| Flu B | HA | LMIAIFIVYMVS RDNVSCSICL |
| Flu C | HEF | LAALVISGIAIC RTK |
| MHV | S | AGVAVCVLLFFI CCCTGCGSCCFKKCGNCCDEYGGHQDSIVIHNISSHED |
| MERS-CoV | S | LVALALCVFFIL CCTGCGTNCMGKLKCNRCCDRYEEYDLEPHKVHVH |
| SARS-CoV-1 | S | GLIAIVMVTILL CCMTSCCSCLKGACSCGSCCKFDEDDSEPVLKGVKLHYT |
| SARS-CoV-2 | S | GLIAIVMVTIML CCMTSCCSCLKGCCSCGSCCKFDEDDSEPVLKGVKLHYT |
| S-Acylation Sites in Ion Channels of Influenza and Coronaviruses | ||
| Flu A | M2 | IIGILHLILWIL DRLFFKCIYRRFKYGLK |
| MHV | E | VTIIVVAFLASI KLCIQLCGLCNTLVLSPSIYLYD |
| MERS-CoV | E | TLLVCMAFLTAT RLCVQCMTGFNTLLVQPALYLYN |
| SARS-CoV | E | FLLVTLAILTAL RLCAYCCNIVNVSLVKPTVY |
| SARS-CoV-2 | E | FLLVTLAILTAL RLCAYCCNIVNVSLVKPSFYVYS |
| Order or Family | Species | # DHHCs | DHHC Missing | Host of |
|---|---|---|---|---|
| Primate | Humans (Homo sapiens) | 23 | X | Flu A |
| Suidae | Pigs (Sus scrofa) | 21 | 4, 11 | Flu A |
| Anseriformes | Mallard (Anas platyrhynchos) | 20 | 11, 19, 24 | Flu A |
| Anseriformes | Duck (Aythya fuligula) | 20 | 11, 19, 24 | Flu A |
| Anseriformes | Duck (Oxyura jamaicensis) | 19 | 5, 11, 19, 24 | Flu A |
| Anseriformes | Gesse (Anser) | 19 | 5, 11, 19, 24 | Flu A |
| Anseriformes | Swans (Cygnus) | 20 | 11, 19, 24 | Flu A |
| Charadriiformes | Sandpiper (Calidris pugnax) | 20 | 11, 19, 24 | Flu A |
| Galliformes | Chicken (Gallus gallus) | 20 | 11, 19, 24 | Flu A |
| Galliformes | Turkey (Meleagris gallopavo) | 18 | 4, 11, 19,21, 24 | Flu A |
| Galliformes | Quail (Coturnix japonica) | 20 | 11, 19, 24 | Flu A |
| Falconiformes | Falcon (Falco peregrinus) | 20 | 11, 19, 24 | Flu A |
| Columbiformes | Pigeon (Columba livia) | 20 | 11, 19, 24 | Flu A |
| Mastacembelidae | Zig-zag eel (M. armatus) | 18 | 1, 7, 11, 18, 19 | Flu |
| Bufonidae | Toad (B. bufo) | 20 | 11, 18, 19 | Flu |
| Manidae | Pangolin (Manis javanica) | 23 | X | SARS-2 |
| Camelidae | Camel (Camelus dromedarius) | 23 | X | MERS |
| Rhinolophidae | Horseshoe bat (Rhinolophus s.) | 23 | X | SARS-1 +2 |
| Vespertilionidae | Pipistrelle bat (P. kuhlii) | 22 | 11 | MERS |
| Pigs (Sus scrofa) | Chicken (Gallus gallus) | Sandpiper (Calidris pugnax) | Mallard Duck (Anas platyrhynchos) | |
|---|---|---|---|---|
| DHHC2 | 96.7% | 84.2% | 84.5% | 84.5% |
| DHHC8 | 93.3% | 65.1% | 62.8% | 66.2% |
| DHHC15 | 98.2% | 75.3% | 75.9% | 75.3% |
| DHHC20 | 91.3% | 77.7% | 76.6% | 73.1% |
| Horseshoe Bat (Rhinolophus sinicus) | Pangolin (Manis javanica) | Camel (C. dromedarius) | Pipistrelle Bat (P. kuhlii) | |
|---|---|---|---|---|
| DHHC8 | 89.31% | 90.00% | 92.38% | 88.82% |
| DHHC9 | 96.98% | 98.63% | 98.08% | 95.99% |
| DHHC20 | 87.27% | 88.54% | 91.39% | 86.17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahman, D.A.; Meng, X.; Veit, M. S-Acylation of Proteins of Coronavirus and Influenza Virus: Conservation of Acylation Sites in Animal Viruses and DHHC Acyltransferases in Their Animal Reservoirs. Pathogens 2021, 10, 669. https://doi.org/10.3390/pathogens10060669
Abdulrahman DA, Meng X, Veit M. S-Acylation of Proteins of Coronavirus and Influenza Virus: Conservation of Acylation Sites in Animal Viruses and DHHC Acyltransferases in Their Animal Reservoirs. Pathogens. 2021; 10(6):669. https://doi.org/10.3390/pathogens10060669
Chicago/Turabian StyleAbdulrahman, Dina A., Xiaorong Meng, and Michael Veit. 2021. "S-Acylation of Proteins of Coronavirus and Influenza Virus: Conservation of Acylation Sites in Animal Viruses and DHHC Acyltransferases in Their Animal Reservoirs" Pathogens 10, no. 6: 669. https://doi.org/10.3390/pathogens10060669
APA StyleAbdulrahman, D. A., Meng, X., & Veit, M. (2021). S-Acylation of Proteins of Coronavirus and Influenza Virus: Conservation of Acylation Sites in Animal Viruses and DHHC Acyltransferases in Their Animal Reservoirs. Pathogens, 10(6), 669. https://doi.org/10.3390/pathogens10060669






