The Association between Weather Conditions and Admissions to the Paediatric Intensive Care Unit for Respiratory Syncytial Virus Bronchiolitis
Abstract
1. Introduction
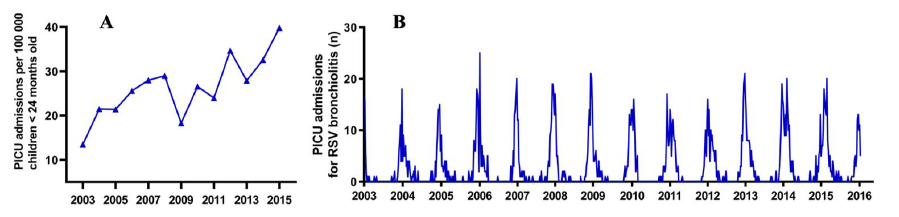
2. Results
2.1. Association between Weather Variables and PICU Admissions
2.2. Changes in Weather Variables over Time
3. Discussion
Limitations
4. Materials and Methods
4.1. Collection of Patient Data
4.2. Collection of Meteorological Data
4.3. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Component (Group) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Cloud coverage (octants) | −0.814 | ||
| Relative humidity (%) | −0.900 | ||
| % longest sunshine duration (%) | 0.890 | ||
| Sunshine duration (0.1 h) | 0.919 | ||
| Global radiation (J/cm2) | 0.812 | 0.425 | |
| Minimum temperature (°C) | 0.977 | ||
| Mean temperature (°C) | 0.974 | ||
| Maximum temperature (°C) | 0.927 | ||
| Wind speed (m/s) | 0.921 | ||
| Precipitation (mm) | 0.609 | ||
References
- Meissner, H.C. Viral bronchiolitis in Children. N. Engl. J. Med. 2016, 374, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Fujiogi, M.; Goto, T.; Yasunaga, H.; Fujishiro, J.; Mansbach, J.M.; Camargo, C.A., Jr.; Hasegawa, K. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics 2019, 144. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Straney, L.; Gelbart, B.; Alexander, J.; Franklin, D.; Beca, J.; Whitty, J.A.; Ganu, S.; Wilkins, B.; Slater, A.; et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Pham, H.; Thompson, J.; Wurzel, D.; Duke, T. Ten years of severe respiratory syncytial virus infections in a tertiary paediatric intensive care unit. J. Paediatr. Child. Health 2020, 56, 61–67. [Google Scholar] [CrossRef]
- Linssen, R.S.; Bem, R.A.; Kapitein, B.; Oude Rengerink, K.; Otten, M.H.; den Hollander, B.; Bont, L.; van Woensel, J.B.M.; the PICE Study Group. Burden of respiratory syncytial virus bronchiolitis on the Dutch pediatric intensive care units. Eur. J. Pediatr. 2021, in press. [Google Scholar] [CrossRef]
- Obando-Pacheco, P.; Justicia-Grande, A.J.; Rivero-Calle, I.; Rodriguez-Tenreiro, C.; Sly, P.; Ramilo, O.; Mejias, A.; Baraldi, E.; Papadopoulos, N.G.; Nair, H.; et al. Respiratory syncytial virus seasonality: A global overview. J. Infect. Dis. 2018, 217, 1356–1364. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Wagner, C.E.; Yang, W.; Pitzer, V.E.; Viboud, C.; Vecchi, G.A.; Metcalf, C.J.E.; Grenfell, B.T. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat. Commun 2019, 10, 5512. [Google Scholar] [CrossRef]
- Yusuf, S.; Piedimonte, G.; Auais, A.; Demmler, G.; Krishnan, S.; Van Caeseele, P.; Singleton, R.; Broor, S.; Parveen, S.; Avendano, L.; et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol. Infect. 2007, 135, 1077–1090. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Devasundaram, J.K.; Simoes, E.A. Respiratory syncytial virus epidemics: The ups and downs of a seasonal virus. Pediatr. Infect. Dis. J. 2003, 22, S21–S32. [Google Scholar] [CrossRef]
- Meerhoff, T.J.; Paget, J.W.; Kimpen, J.L.; Schellevis, F. Variation of respiratory syncytial virus and the relation with meteorological factors in different winter seasons. Pediatr. Infect. Dis. J. 2009, 28, 860–866. [Google Scholar] [CrossRef]
- Sundell, N.; Andersson, L.M.; Brittain-Long, R.; Lindh, M.; Westin, J. A four year seasonal survey of the relationship between outdoor climate and epidemiology of viral respiratory tract infections in a temperate climate. J. Clin. Virol. 2016, 84, 59–63. [Google Scholar] [CrossRef]
- Thongpan, I.; Vongpunsawad, S.; Poovorawan, Y. Respiratory syncytial virus infection trend is associated with meteorological factors. Sci. Rep. 2020, 10, 10931. [Google Scholar] [CrossRef]
- Eccles, R.; Wilkinson, J.E. Exposure to cold and acute upper respiratory tract infection. Rhinology 2015, 53, 99–106. [Google Scholar] [CrossRef]
- Koskela, H.; Tukiainen, H. Facial cooling, but not nasal breathing of cold air, induces bronchoconstriction: A study in asthmatic and healthy subjects. Eur. Respir. J. 1995, 8, 2088–2093. [Google Scholar] [CrossRef]
- Koskela, H.O. Cold air-provoked respiratory symptoms: The mechanisms and management. Int. J. Circumpolar Health 2007, 66, 91–100. [Google Scholar] [CrossRef]
- Kilgour, E.; Rankin, N.; Ryan, S.; Pack, R. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. 2004, 30, 1491–1494. [Google Scholar] [CrossRef]
- Asmundsson, T.; Kilburn, K.H. Mucociliary clearance rates at various levels in dog lungs. Am. Rev. Respir. Dis. 1970, 102, 388–397. [Google Scholar] [CrossRef]
- Mireku, N.; Wang, Y.; Ager, J.; Reddy, R.C.; Baptist, A.P. Changes in weather and the effects on pediatric asthma exacerbations. Ann. Allergy Asthma Immunol. 2009, 103, 220–224. [Google Scholar] [CrossRef]
- Hyrkas, H.; Ikaheimo, T.M.; Jaakkola, J.J.; Jaakkola, M.S. Asthma control and cold weather-related respiratory symptoms. Respir. Med. 2016, 113, 1–7. [Google Scholar] [CrossRef]
- Asthma and the weather. Lancet 1985, 1, 1079–1080. [CrossRef]
- Ferrari, U.; Exner, T.; Wanka, E.R.; Bergemann, C.; Meyer-Arnek, J.; Hildenbrand, B.; Tufman, A.; Heumann, C.; Huber, R.M.; Bittner, M.; et al. Influence of air pressure, humidity, solar radiation, temperature, and wind speed on ambulatory visits due to chronic obstructive pulmonary disease in Bavaria, Germany. Int. J. Biometeorol. 2012, 56, 137–143. [Google Scholar] [CrossRef]
- Estripeaut, D.; Torres, J.P.; Somers, C.S.; Tagliabue, C.; Khokhar, S.; Bhoj, V.G.; Grube, S.M.; Wozniakowski, A.; Gomez, A.M.; Ramilo, O.; et al. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J. Infect. Dis. 2008, 198, 1435–1443. [Google Scholar] [CrossRef]
- DeFord, D.M.; Nosek, J.M.; Castiglia, K.R.; Hasik, E.F.; Franke, M.E.; Nick, B.C.; Abdelnour, A.M.; Haas, C.E.; Junod, N.A.; Latsko, K.N.; et al. Evaluation of the role of respiratory syncytial virus surface glycoproteins F and G on viral stability and replication: Implications for future vaccine design. J. Gen. Virol. 2019, 100, 1112–1122. [Google Scholar] [CrossRef]
- El Saleeby, C.M.; Bush, A.J.; Harrison, L.M.; Aitken, J.A.; Devincenzo, J.P. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J. Infect. Dis. 2011, 204, 996–1002. [Google Scholar] [CrossRef]
- Colosia, A.D.; Masaquel, A.; Hall, C.B.; Barrett, A.M.; Mahadevia, P.J.; Yogev, R. Residential crowding and severe respiratory syncytial virus disease among infants and young children: A systematic literature review. Bmc Infect. Dis. 2012, 12, 95. [Google Scholar] [CrossRef]
- Chu, H.Y.; Kuypers, J.; Renaud, C.; Wald, A.; Martin, E.; Fairchok, M.; Magaret, A.; Sarancino, M.; Englund, J.A. Molecular epidemiology of respiratory syncytial virus transmission in childcare. J. Clin. Virol. 2013, 57, 343–350. [Google Scholar] [CrossRef]
- Blanken, M.O.; Korsten, K.; Achten, N.B.; Tamminga, S.; Nibbelke, E.E.; Sanders, E.A.; Smit, H.A.; Groenwold, R.H.; Bont, L. Population-attributable risk of risk factors for recurrent wheezing in moderate preterm infants during the first year of life. Paediatr. Perinat. Epidemiol. 2016, 30, 376–385. [Google Scholar] [CrossRef]
- Fisman, D. Seasonality of viral infections: Mechanisms and unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.; Rovers, M.; Bont, L. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Hambling, M.H. Survival of the respiratory syncytial virus during storage under various conditions. Br. J. Exp. Pathol. 1964, 45, 647–655. [Google Scholar]
- Paynter, S. Humidity and respiratory virus transmission in tropical and temperate settings. Epidemiol. Infect. 2015, 143, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Noyola, D.E.; Mandeville, P.B. Effect of climatological factors on respiratory syncytial virus epidemics. Epidemiol. Infect. 2008, 136, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.A.; Poynton, M.R.; Gesteland, P.H.; Maloney, C.; Staes, C.; Facelli, J.C. Predicting the start week of respiratory syncytial virus outbreaks using real time weather variables. BMC Med. Inform. Decis. Mak. 2010, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Hyrkäs-Palmu, H.; Ikäheimo, T.M.; Laatikainen, T.; Jousilahti, P.; Jaakkola, M.S.; Jaakkola, J.J.K. Cold weather increases respiratory symptoms and functional disability especially among patients with asthma and allergic rhinitis. Sci. Rep. 2018, 8, 10131. [Google Scholar] [CrossRef] [PubMed]
- Daviskas, E.; Gonda, I.; Anderson, S.D. Mathematical modeling of heat and water transport in human respiratory tract. J. Appl. Physiol. 1990, 69, 362–372. [Google Scholar] [CrossRef]
- Freed, A.N.; Davis, M.S. Hyperventilation with dry air increases airway surface fluid osmolality in canine peripheral airways. Am. J. Respir. Crit. Care Med. 1999, 159, 1101–1107. [Google Scholar] [CrossRef]
- Larsson, K.; Tornling, G.; Gavhed, D.; Müller-Suur, C.; Palmberg, L. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur. Respir. J. 1998, 12, 825–830. [Google Scholar] [CrossRef]
- Schuit, M.; Gardner, S.; Wood, S.; Bower, K.; Williams, G.; Freeburger, D.; Dabisch, P. The Influence of Simulated Sunlight on the Inactivation of Influenza Virus in Aerosols. J. Infect. Dis. 2020, 221, 372–378. [Google Scholar] [CrossRef]
- Fodha, I.; Vabret, A.; Ghedira, L.; Seboui, H.; Chouchane, S.; Dewar, J.; Gueddiche, N.; Trabelsi, A.; Boujaafar, N.; Freymuth, F. Respiratory syncytial virus infections in hospitalized infants: Association between viral load, virus subgroup, and disease severity. J. Med. Virol. 2007, 79, 1951–1958. [Google Scholar] [CrossRef]
- Sheeran, P.; Jafri, H.; Carubelli, C.; Saavedra, J.; Johnson, C.; Krisher, K.; Sánchez, P.J.; Ramilo, O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 1999, 18, 115–122. [Google Scholar] [CrossRef]
- Houben, M.L.; Coenjaerts, F.E.; Rossen, J.W.; Belderbos, M.E.; Hofland, R.W.; Kimpen, J.L.; Bont, L. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J. Med. Virol. 2010, 82, 1266–1271. [Google Scholar] [CrossRef]
- Scagnolari, C.; Midulla, F.; Selvaggi, C.; Monteleone, K.; Bonci, E.; Papoff, P.; Cangiano, G.; Di Marco, P.; Moretti, C.; Pierangeli, A.; et al. Evaluation of viral load in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. Med. Microbiol. Immunol. 2012, 201, 311–317. [Google Scholar] [CrossRef]
- Hasegawa, K.; Jartti, T.; Mansbach, J.M.; Laham, F.R.; Jewell, A.M.; Espinola, J.A.; Piedra, P.A.; Camargo, C.A., Jr. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: Multicenter cohort studies in the United States and Finland. J. Infect. Dis. 2015, 211, 1550–1559. [Google Scholar] [CrossRef]
- Monto, A.S. Epidemiology of influenza. Vaccine 2008, 26 (Suppl. 4), D45–D48. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef]
- Diagnosis and management of bronchiolitis. Pediatrics 2006, 118, 1774–1793. [CrossRef]

| Variable | Month Data (All Months) | Month Data (RSV Season) | Week Data Lag 0 (RSV Season) | Week Data Lag 7 (RSV Season) | ||||
|---|---|---|---|---|---|---|---|---|
| (β, SE) | LR test | (β, SE) | LR Test | (β, SE) | LR test | (β, SE) | LR Test | |
| Group 1 | ||||||||
| Cloud coverage (octants) | 98.95, 2.99 | 1338.29 * | 72.39, 3.01 | 714.30 * | 35.62, 1.91 | 407.59 * | 39.29, 1.97 | 475.74 * |
| Relative humidity (%) | 18.31, 0.51 | 1770.18 * | 13.37, 0.57 | 709.90 * | 8.54, 0.41 | 521.72 * | 9.85, 0.42 | 656.11 * |
| % longest sunshine duration (%) | −8.60, 0.23 | 1638.96 * | −6.22, 0.23 | 835.17 * | −3.00, 0.15 | 454.44 * | −3.38, 0.15 | 554.51 * |
| Sunshine duration (0.1 h) | −6.45, 0.16 | 2584.62 * | −5.45, 0.18 | 1262.54 * | −3.60, 0.13 | 954.97 * | −3.99, 0.14 | 1103.63 * |
| Global radiation (J/cm2) | −0.29, 0.01 | 2987.52 * | −0.29, 0.01 | 1542.92 * | −0.27, 0.01 | 1601.56 * | −0.30, 0.01 | 1790.74 * |
| Group 2 | ||||||||
| Minimum Temperature (°C) | −2.43, 0.06 | 2162.93 * | −1.95, 0.07 | 871.50 * | −1.15, 0.05 | 593.14 * | −1.06, 0.05 | 492.39 * |
| Mean Temperature (°C) | −2.36, 0.05 | 2600.07 * | −2.05, 0.06 | 1212.85 * | −1.37, 0.05 | 941.36 * | −1.28 0.05 | 820.03 * |
| Maximum Temperature (°C) | −2.15, 0.05 | 2826.94 * | −1.92, 0.06 | 1417.15 * | −1.39, 0.04 | 1188.86 * | −1.32, 0.04 | 1070.82 * |
| Group 3 | ||||||||
| Wind speed (m/s) | 9.66, 0.30 | 934.04 * | 6.30, 0.32 | 369.06 * | 3.06, 0.20 | 212.00 * | 2.47, 0.21 | 133.32 * |
| Precipitation (mm) | −0.62, 0.18 | 12.58 * | 0.67, 0.20 | 11.34 * | 0.29, 0.09 | 8.94 | 0.12, 0.10 | 1.39 |
| All Weeks | RSV Season (Weeks 35–18) | |
|---|---|---|
| Cloud coverage (octants) | β 0.18; SE 0.0, p < 0.01 | β 0.16; SE 0.0, p < 0.01 |
| Relative Humidity (%) | β −0.1, SE 0.0, p = 0.78 | β −0.03, SE 0.0, p = 0.52 |
| % Longest sunshine duration (%) | β −0.02, SE 0.00, p = 0.58 | β −0.02, SE 0.01, p = 0.74 |
| Sunshine duration (0.1 h) | β −0.02 SE 0.01 p = 0.55 | β −0.02 SE 0.01 p = 0.73 |
| Global radiation (J/cm2) | β −0.2, SE 0.12, p = 0.69 | β −0.01, SE 0.15, p = 0.89 |
| Minimum temperature (°C) | β 0.05 SE 0.01 p = 0.18 | β 0.06 SE 0.01 p = 0.19 |
| Mean temperature (°C) | β 0.02, SE 0.01 p = 0.55 | β 0.04, SE 0.02 p = 0.39 |
| Maximum temperature (°C) | β −0.001, SE 0.01, p = 0.98 | β −0.01, SE 0.02, p = 0.70 |
| Wind speed (m/s) | β 0.09, SE 0.00, p = 0.01 | β 0.08, SE 0.00, p = 0.09 |
| Precipitation (mm) | β 0.04, SE 0.00, p = 0.32 | β 0.03, SE 0.01, p = 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linssen, R.S.; den Hollander, B.; Bont, L.; van Woensel, J.B.M.; Bem, R.A.; on behalf of the PICE Study Group. The Association between Weather Conditions and Admissions to the Paediatric Intensive Care Unit for Respiratory Syncytial Virus Bronchiolitis. Pathogens 2021, 10, 567. https://doi.org/10.3390/pathogens10050567
Linssen RS, den Hollander B, Bont L, van Woensel JBM, Bem RA, on behalf of the PICE Study Group. The Association between Weather Conditions and Admissions to the Paediatric Intensive Care Unit for Respiratory Syncytial Virus Bronchiolitis. Pathogens. 2021; 10(5):567. https://doi.org/10.3390/pathogens10050567
Chicago/Turabian StyleLinssen, Rosalie S., Bibiche den Hollander, Louis Bont, Job B. M. van Woensel, Reinout A. Bem, and on behalf of the PICE Study Group. 2021. "The Association between Weather Conditions and Admissions to the Paediatric Intensive Care Unit for Respiratory Syncytial Virus Bronchiolitis" Pathogens 10, no. 5: 567. https://doi.org/10.3390/pathogens10050567
APA StyleLinssen, R. S., den Hollander, B., Bont, L., van Woensel, J. B. M., Bem, R. A., & on behalf of the PICE Study Group. (2021). The Association between Weather Conditions and Admissions to the Paediatric Intensive Care Unit for Respiratory Syncytial Virus Bronchiolitis. Pathogens, 10(5), 567. https://doi.org/10.3390/pathogens10050567








