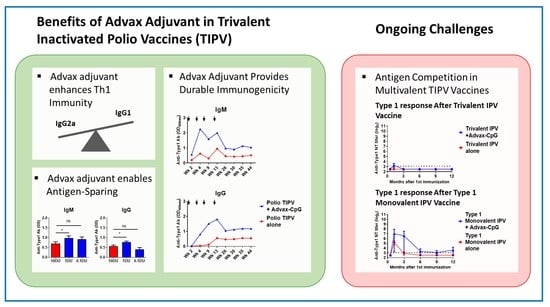
Advax-CpG Adjuvant Provides Antigen Dose-Sparing and Enhanced Immunogenicity for Inactivated Poliomyelitis Virus Vaccines
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Vaccines and Adjuvants
2.3. Immunization Schedule for Mouse and Rat Studies
2.4. ELISA for Detection of Anti-IPV Antibodies
2.5. Neutralization Assays
2.6. Statistical Analysis
3. Results
3.1. Advax-CpG Improves IPOL® TIPV Vaccine Long-Term
3.2. Advax-CpG Induces a Strong Th1 Immune Response to IPOL® TIPV Vaccine
3.3. Dose-Sparing Effects of Advax-CpG Adjuvant on SSI TIPV Immunogenicity
3.4. Effects of Advax Adjuvants on Antibody Kinetics and Longevity
3.5. TIPV Vaccine Formulated with Advax-CpG Adjuvant Induces Protective Antibody Levels in Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baicus, A. History of polio vaccination. World J. Virol. 2012, 1, 108–114. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio vaccination: Past, present and future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef]
- Jiang, B.; Patel, M.; Glass, R.I. Polio endgame: Lessons for the global rotavirus vaccination program. Vaccine 2019, 37, 3040–3049. [Google Scholar] [CrossRef]
- Alfaro-Murillo, J.A.; Ávila-Agüero, M.L.; Fitzpatrick, M.C.; Crystal, C.J.; Falleiros-Arlant, L.-H.; Galvani, A.P. The case for replacing live oral polio vaccine with inactivated vaccine in the Americas. Lancet 2020, 395, 1163–1166. [Google Scholar] [CrossRef]
- Shahzad, A.; Köhler, G. Inactivated Polio Vaccine (IPV): A strong candidate vaccine for achieving global polio eradication program. Vaccine 2009, 27, 5293–5294. [Google Scholar] [CrossRef]
- Vidor, E.; Meschievitz, C.; Plotkin, S. Fifteen years of experience with Vero-produced enhanced potency inactivated poliovirus vaccine. Pediatr. Infect. Dis. J. 1997, 16, 312–322. [Google Scholar] [CrossRef]
- Hamer, D.H.; Griffiths, J.; Maguire, J.H.; Heggenhougen, K.; Quah, S.R. Public Health and Infectious Diseases; Elsevier Science: Oxford, UK, 2010. [Google Scholar]
- Bonnet, M.-C.; Dutta, A. World wide experience with inactivated poliovirus vaccine. Vaccine 2008, 26, 4978–4983. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Fox, C.B.; Pallansch, M.A.; Coler, R.N.; Reed, S.G.; Friede, M. Increased potency of an inactivated trivalent polio vaccine with oil-in-water emulsions. Vaccine 2011, 29, 644–649. [Google Scholar] [CrossRef]
- Sureau, P.; Fabre, P.S.; N’Garo, S.B.; Butor, S.C. Simultaneous Testanus and Poliomyelitis Vaccination of Infants in a Tropical Environment. Bull. World Health Organ. 1977, 55, 739–746. [Google Scholar]
- Drescher, J.; Grutzner, L.; Godgluck, G. Further Investigations on the Immunogenic Activity of Aqueous and Aluminium Oxide Adsorbed Inactivated Poliovirus Vaccines in Macaca Mulatto. Am. J. Epidemiol. 1967, 85, 413–423. [Google Scholar] [CrossRef]
- Ivanov, A.P.; Dragunsky, E.M.; Chumakov, K.M. 1,25-Dihydroxyvitamin D3 Enhances Systemic and Mucosal Immune Responses to Inactivated Poliovirus Vaccine in Mice. J. Infect. Dis. 2006, 193, 598–600. [Google Scholar] [CrossRef]
- Yang, C.; Shi, H.; Zhou, J.; Liang, Y.; Xu, H. CpG oligodeoxynucleotides are a potent adjuvant for an inactivated polio vaccine produced from Sabin strains of poliovirus. Vaccine 2009, 27, 6558–6563. [Google Scholar] [CrossRef]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Honda-Okubo, Y.; Wilks, S.H.; Aban, M.; Barr, I.G.; Petrovsky, N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine 2016, 34, 3780–3786. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Woodman, R.J.; Honda-Okubo, Y.; Cox, M.M.; Heinzel, S.; Petrovsky, N. Randomized Clinical Trial of Immunogenicity and Safety of a Recombinant H1n1/2009 Pandemic Influenza Vaccine Containing Advax™ Polysaccharide Adjuvant. Vaccine 2012, 30, 5407–5416. [Google Scholar] [CrossRef] [PubMed]
- Lobigs, M.; Pavy, M.; Hall, R.A.; Lobigs, P.; Cooper, P.; Komiya, T.; Toriniwa, H.; Petrovsky, N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J. Gen. Virol. 2010, 91, 1407–1417. [Google Scholar] [CrossRef]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef]
- Cosseddu, G.M.; Polci, A.; Pinoni, C.; Dondona, A.C.; Iapaolo, F.; Orsini, G.; Izzo, F.; Bortone, G.; Ronchi, F.G.; Di Ventura, M.; et al. Evaluation of Humoral Response and Protective Efficacy of an Inactivated Vaccine Against Peste des Petits Ruminants Virus in Goats. Transbound. Emerg. Dis. 2015, 63, e447–e452. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Kolpe, A.; Li, L.; Petrovsky, N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax™) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine 2014, 32, 4651–4659. [Google Scholar] [CrossRef]
- Bielefeldt-Ohmann, H.; Prow, N.A.; Wang, W.; Tan, C.S.; Coyle, M.; Douma, A.; Hobson-Peters, J.; Kidd, L.; Hall, R.A.; Petrovsky, N. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Vet. Res. 2014, 45, 130. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Ong, C.H.; Petrovsky, N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single–dose influenza vaccine protection. Vaccine 2015, 33, 4892–4900. [Google Scholar] [CrossRef]
- Chu, R.S.; Targoni, O.S.; Krieg, A.M.; Lehmann, P.V.; Harding, C.V. CpG Oligodeoxynucleotides Act as Adjuvants that Switch on T Helper 1 (Th1) Immunity. J. Exp. Med. 1997, 186, 1623–1631. [Google Scholar] [CrossRef]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4+ T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Stronsky, S.M.; Cooper, C.L.; Steffens, J.; Van Tongeren, S.; Bavari, S.; Martins, K.A.; Petrovsky, N. Adjuvant selection impacts the correlates of vaccine protection against Ebola infection. Vaccine 2020, 38, 4601–4608. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Barnard, D.L.; Ong, C.H.; Peng, B.-H.; Tseng, C.-T.K.; Petrovsky, N. Severe Acute Respiratory Syndrome-Associated Coronavirus Vaccines Formulated with Delta Inulin Adjuvants Provide Enhanced Protection while Ameliorating Lung Eosinophilic Immunopathology. J. Virol. 2014, 89, 2995–3007. [Google Scholar] [CrossRef]
- Davtyan, H.; Zagorski, K.; Rajapaksha, H.; Hovakimyan, A.; Davtyan, A.; Petrushina, I.; Kazarian, K.; Cribbs, D.H.; Petrovsky, N.; Agadjanyan, M.G.; et al. Alzheimer’s Disease Advax Cpg-Adjuvanted Multitep-Based Dual and Single Vaccines Induce High-Titer Antibodies against Various Forms of Tau and Aβ Pathological Molecules. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Simizu, B.; Abe, S.; Yamamoto, H.; Tano, Y.; Ota, Y.; Miyazawa, M.; Horie, H.; Satoh, K.; Wakabayashi, K. Development of inactivated poliovirus vaccine derived from Sabin strains. Biologicals 2006, 34, 151–154. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Romagnani, S. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 2000, 85, 9–21. [Google Scholar] [CrossRef]
- Singh, V.K.; Mehrotra, S.; Agarwal, S.S. The paradigm of Th1 and Th2 cytokines. Immunol. Res. 1999, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Andreasen, L.V.; Andersen, P.; Agger, E.M. Inducing Dose Sparing with Inactivated Polio Virus Formulated in Adjuvant CAF01. PLoS ONE 2014, 9, e100879. [Google Scholar] [CrossRef] [PubMed]
- Rockman, S.; Becher, D.; Dyson, A.; Koernig, S.; Morelli, A.B.; Barnden, M.; Camuglia, S.; Soupourmas, P.; Pearse, M.; Maraskovsky, E. Role of viral RNA and lipid in the adverse events associated with the 2010 Southern Hemisphere trivalent influenza vaccine. Vaccine 2014, 32, 3869–3876. [Google Scholar] [CrossRef]
- Lambert, S.B.; Chuk, L.R.; Nissen, M.D.; Nolan, T.M.; McVernon, J.; Booy, R.; Heron, L.; Richmond, P.C.; Walls, T.; Marshall, H.S.; et al. Safety and tolerability of a 2009 trivalent inactivated split-virion influenza vaccine in infants, children and adolescents. Influenza Other Respir. Viruses 2013, 7, 676–685. [Google Scholar] [CrossRef]
- Li, L.; Honda-Okubo, Y.; Li, C.; Sajkov, D.; Petrovsky, N. Delta Inulin Adjuvant Enhances Plasmablast Generation, Expression of Activation-Induced Cytidine Deaminase and B-Cell Affinity Maturation in Human Subjects Receiving Seasonal Influenza Vaccine. PLoS ONE 2015, 10, e0132003. [Google Scholar] [CrossRef]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef]
- Heddle, R.; Smith, A.; Woodman, R.; Hissaria, P.; Petrovsky, N. Randomized controlled trial demonstrating the benefits of delta inulin adjuvanted immunotherapy in patients with bee venom allergy. J. Allergy Clin. Immunol. 2019, 144, 504–513.e16. [Google Scholar] [CrossRef]





| Sanofi-Pasteur (IPOL) Polio Vaccine | |||||
| Study No. | Animals | Vaccine Antigen | Adjuvant | Dose Schedule | Purpose of Study |
| 1 | Female BALB/c | Trivalent inactivated polio (8 DU type 1, 1.6 DU type 2 and 6.4 DU type 3) | Alone or with Advax or Advax-CpG | 4 doses (weeks 0, 3, 7 and 12) | Long term immunogenicity/Neutralization Activity |
| Statens Serum Institut (SSI) Polio Vaccine | |||||
| Study No. | Animals | Vaccine Formulation | Adjuvant | Dose Schedule | Purpose of Study |
| 2 | Female BALB/c | Trivalent inactivated polio (10 DU type 1, 1 DU type 2 and 9 DU type 3) at 1, 1:10 and 1:100 dose | Alone or with Advax or Advax-CpG | 2-doses (3 weeks apart) | Immunogenicity/Neutralization Activity/Antigen dose sparing capacity |
| 3 | Female BALB/c | Trivalent inactivated polio and Monovalent at three different doses at 1, 1:10 and 1:100 dose | Alone or with Advax-CpG or CpG | 2-doses (3 weeks apart) | Antigen competition/Long-term Immunogencity |
| 4 | Female Wistar rats | Trivalent inactivated polio (10 DU type 1, 1 DU type 2 and 9 DU type 3) at 1, 1:10 and 1:100 dose | Alone or with Advax or Advax-CpG | 2-doses (3 weeks apart) | Species-specific differences Immunogenicity/Neutralization Activity/Weight loss |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda-Okubo, Y.; Baldwin, J.; Petrovsky, N. Advax-CpG Adjuvant Provides Antigen Dose-Sparing and Enhanced Immunogenicity for Inactivated Poliomyelitis Virus Vaccines. Pathogens 2021, 10, 500. https://doi.org/10.3390/pathogens10050500
Honda-Okubo Y, Baldwin J, Petrovsky N. Advax-CpG Adjuvant Provides Antigen Dose-Sparing and Enhanced Immunogenicity for Inactivated Poliomyelitis Virus Vaccines. Pathogens. 2021; 10(5):500. https://doi.org/10.3390/pathogens10050500
Chicago/Turabian StyleHonda-Okubo, Yoshikazu, Jeremy Baldwin, and Nikolai Petrovsky. 2021. "Advax-CpG Adjuvant Provides Antigen Dose-Sparing and Enhanced Immunogenicity for Inactivated Poliomyelitis Virus Vaccines" Pathogens 10, no. 5: 500. https://doi.org/10.3390/pathogens10050500
APA StyleHonda-Okubo, Y., Baldwin, J., & Petrovsky, N. (2021). Advax-CpG Adjuvant Provides Antigen Dose-Sparing and Enhanced Immunogenicity for Inactivated Poliomyelitis Virus Vaccines. Pathogens, 10(5), 500. https://doi.org/10.3390/pathogens10050500