TREM1 rs2234237 (Thr25Ser) Polymorphism in Patients with Cutaneous Leishmaniasis Caused by Leishmania guyanensis: A Case-Control Study in the State of Amazonas, Brazil
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Population
2.2. Genotypes and Allele Frequencies of the TREM1 Variant rs2234237 A/T’s Polymorphism
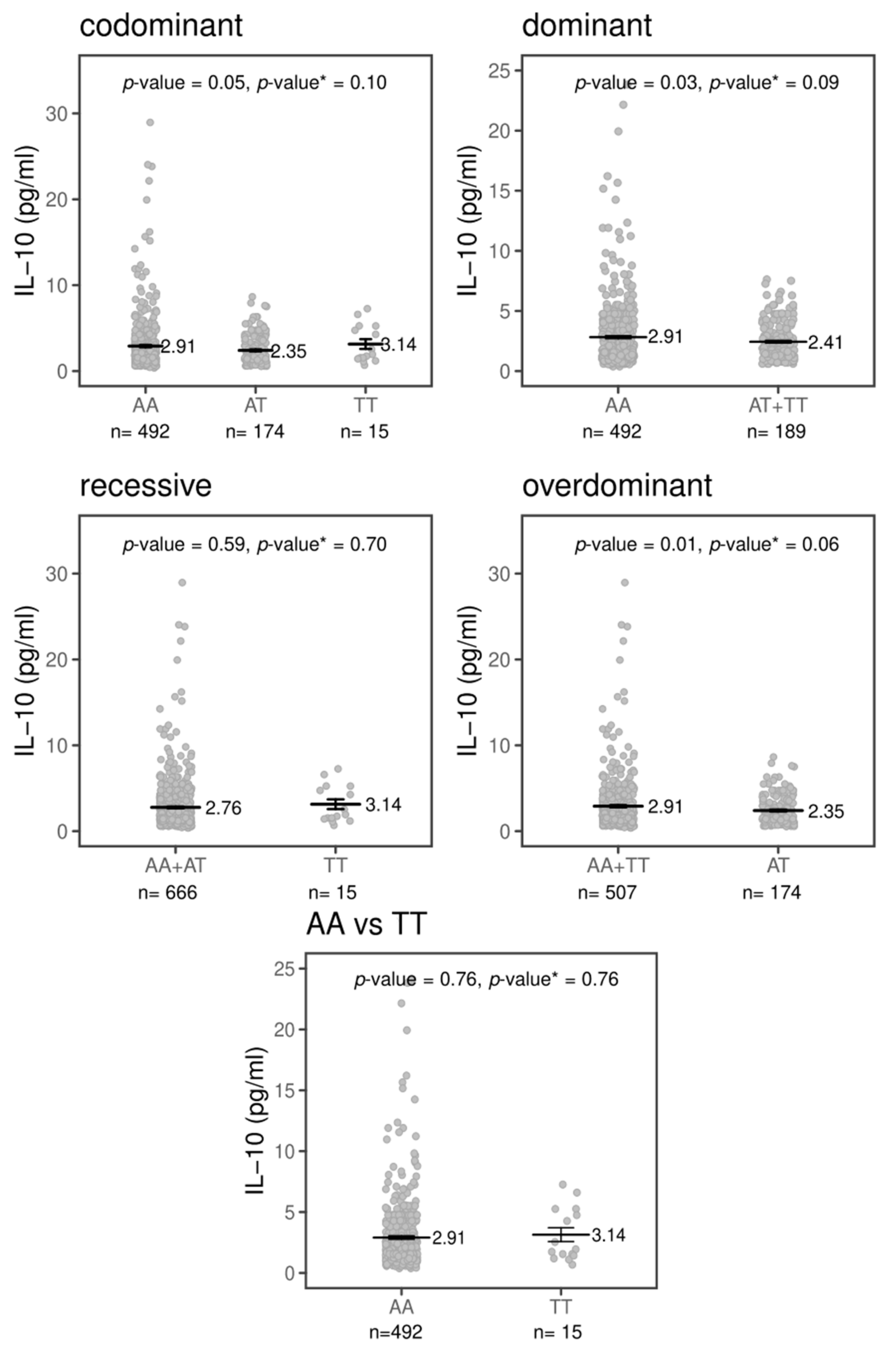
2.3. Effects of TREM1 rs2234237’s Polymorphism on Circulating Plasma Cytokine Levels
3. Discussion
4. Materials and Methods
4.1. Population Studied
4.2. Ethics Statement
4.3. Collection of Biological Samples
4.4. Leishmania Genotyping
4.5. Cytokines’ Assay
4.6. TREM1 Polymorphism Genotyping
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/publications/i/item/who-wer9525 (accessed on 17 September 2020).
- Ministério da Saúde. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z-1/l/leishmaniose-tegumentar-lt (accessed on 17 September 2020).
- Von Stebut, E.; Udey, M.C. Requirements for Th1-dependent immunity against infection with Leishmania major. Microbes Infect. 2004, 6, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Noben-Trauth, N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002, 2, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef]
- Bouchon, A.; Facchetti, F.; Weigand, M.A.; Colonna, M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001, 410, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Tessarz, A.S.; Cerwenka, A. The TREM-1/DAP12 pathway. Immunol. Lett. 2008, 116, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, A.; Sun, M.; Deng, Q.; Hazlett, L.D.; Yuan, J.; Liu, X.; Gao, Q.; Feng, L.; He, J.; et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating Toll-like receptor signaling and Th1/Th2-type immune responses. Infect. Immun. 2011, 79, 2709–2716. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, W.; Deng, Q.; Wu, M.; Jiang, H.; Lin, X.; Sun, Y.; Huang, X.; Yuan, J. Inhibition of TREM-1 and Dectin-1 Alleviates the Severity of Fungal Keratitis by Modulating Innate Immune Responses. PLoS ONE 2016, 11, e0150114. [Google Scholar] [CrossRef] [PubMed]
- Kozik, J.H.; Trautmann, T.; Carambia, A.; Preti, M.; Lütgehetmann, M.; Krech, T.; Wiegard, C.; Heeren, J.; Herkel, J. Attenuated viral hepatitis in Trem1-/- mice is associated with reduced inflammatory activity of neutrophils. Sci. Rep. 2016, 6, 28556. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, X.; Staitieh, B.; Bedi, C.; Spearman, P.; Guidot, D.M.; Sadikot, R.T. HIV-related proteins prolong macrophage survival through induction of Triggering receptor expressed on myeloid cells-1. Sci. Rep. 2017, 7, 42028. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, Y.M.; Ding, L.; Liu, X.; Francisco, N.M.; Wen, J.; Liao, C.; Ma, Z.; Li, Z.; Li, M.; et al. Activation and Regulation of Blood Vδ2 T Cells Are Amplified by TREM-1+ during Active Pulmonary Tuberculosis. J. Immunol. 2018, 200, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Schuster, S.; Zysset, D.; Rihs, S.; Dickgreber, N.; Schürch, C.; Riether, C.; Siegrist, M.; Schneider, C.; Pawelski, H.; et al. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog. 2014, 10, e1003900. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.W.; Fukutani, K.F.; Andrade, B.B.; Curvelo, R.P.; Cristal, J.R.; Carvalho, A.M.; Barral, A.; Van Weyenbergh, J.; Barral-Netto, M.; de Oliveira, C.I. Gene Expression Profile of High IFN-γ Producers Stimulated with Leishmania braziliensis Identifies Genes Associated with Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2016, 10, e0005116. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Silva, I.B.; Ampuero, M.R.; de Noronha, A.L.L.; de Souza, L.C.L.; Correia, T.C.; Khouri, R.; Boaventura, V.S.; Barral, A.; Ramos, P.I.P.; et al. Integrated Analysis Reveals That miR-193b, miR-671, and TREM-1 Correlate with a Good Response to Treatment of Human Localized Cutaneous Leishmaniasis Caused by Leishmania braziliensis. Front Immunol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of TREM-1 activation in human neutrophils: Activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2007, 19, 41–50. [Google Scholar] [CrossRef]
- Buckland, K.F.; Ramaprakash, H.; Murray, L.A.; Carpenter, K.J.; Choi, E.S.; Kunkel, S.L.; Lukacs, N.W.; Xing, Z.; Aoki, N.; Hartl, D.; et al. Triggering receptor expressed on myeloid cells-1 (TREM-1) modulates immune responses to Aspergillus fumigatus during fungal asthma in mice. Immunol. Investig. 2011, 40, 692–722. [Google Scholar] [CrossRef]
- Golovkin, A.S.; Matveeva, V.G.; Kudryavtsev, I.V.; Chernova, M.N.; Bayrakova, Y.V.; Shukevich, D.L.; Grigoriev, E.V. Perioperative Dynamics of TLR2, TLR4, and TREM-1 Expression in Monocyte Subpopulations in the Setting of On-Pump Coronary Artery Bypass Surgery. ISRN Inflamm. 2013, 2013, 817901. [Google Scholar] [CrossRef]
- Van Bremen, T.; Drömann, D.; Luitjens, K.; Dodt, C.; Dalhoff, K.; Goldmann, T.; Schaaf, B. Triggering receptor expressed on myeloid cells-1 (Trem-1) on blood neutrophils is associated with cytokine inducibility in human E. coli sepsis. Diagn. Pathol. 2013, 8, 24. [Google Scholar] [CrossRef]
- Jung, E.S.; Kim, S.W.; Moon, C.M.; Shin, D.J.; Son, N.H.; Kim, E.S.; Lee, H.J.; Hong, S.P.; Kim, T.I.; Kim, W.H.; et al. Relationships between genetic polymorphisms of triggering receptor expressed on myeloid cells-1 and inflammatory bowel diseases in the Korean population. Life Sci. 2011, 89, 289–294. [Google Scholar] [CrossRef]
- Su, L.; Liu, C.; Li, C.; Jiang, Z.; Xiao, K.; Zhang, X.; Li, M.; Yan, P.; Feng, D.; Xie, L. Dynamic changes in serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) and its gene polymorphisms are associated with sepsis prognosis. Inflammation 2012, 35, 1833–1843. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.A.; Huebinger, R.M.; Burris, A.; Liu, M.M.; Minei, J.P.; Hunt, J.L.; Arnoldo, B.D.; Barber, R.C. A TREM-1 Polymorphism A/T within the Exon 2 Is Associated with Pneumonia in Burn-Injured Patients. ISRN Inflamm. 2013, 2013, 431739. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, A.S.; Ponasenko, A.V.; Khutornaya, M.V.; Kutikhin, A.G.; Salakhov, R.R.; Yuzhalin, A.E.; Zhidkova, I.I.; Barbarash, O.L.; Barbarash, L.S. Association of TLR and TREM-1 gene polymorphisms with risk of coronary artery disease in a Russian population. Gene 2014, 550, 101–109. [Google Scholar] [CrossRef]
- Peng, L.S.; Li, J.; Zhou, G.S.; Deng, L.H.; Yao, H.G. Relationships between genetic polymorphisms of triggering receptor expressed on myeloid cells-1 and septic shock in a Chinese Han population. World J. Emerg. Med. 2015, 6, 123–130. [Google Scholar] [CrossRef]
- Adukpo, S.; Gyan, B.A.; Ofori, M.F.; Dodoo, D.; Velavan, T.P.; Meyer, C.G. Triggering receptor expressed on myeloid cells 1 (TREM-1) and cytokine gene variants in complicated and uncomplicated malaria. Trop. Med. Int. Health 2016, 21, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Ponasenko, A.V.; Khutornaya, M.V.; Yuzhalin, A.E.; Zhidkova, I.I.; Salakhov, R.R.; Golovkin, A.S.; Barbarash, O.L.; Barbarash, L.S. Association of TLR and TREM-1 gene polymorphisms with atherosclerosis severity in a Russian population. Meta Gene 2016, 9, 76–89. [Google Scholar] [CrossRef]
- De Araújo Santos, F.J.; da Silva, L.S.; Júnior, J.D.E.S.; Ramos de Mesquita, T.G.; de Souza, M.L.G.; de Andrade Júnior, M.C.; Talhari, S.; Ramasawmy, R. Single nucleotide polymorphisms of the genes IL-2, IL-2RB, and JAK3 in patients with cutaneous leishmaniasis caused by Leishmania (V.) guyanensis in Manaus, Amazonas, Brazil. PLoS ONE 2019, 14, e0220572. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.A.V.; de Mesquita, T.G.R.; de Souza Encarnação, H.V.; do Espírito Santo Junior, J.; da Costa Sabino, K.; de Aguiar Neres, I.; de Almeida, S.A.; de Souza, M.L.G.; Talhari, S.; Ramasawmy, R. A polymorphism in the IL1B gene (rs16944 T/C) is associated with cutaneous leishmaniasis caused by Leishmania guyanensis and plasma cytokine interleukin receptor antagonist. Cytokine 2019, 123, 154788. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.A.V.; Mesquita, T.G.; Souza, V.C.; Junior, J.D.E.S.; Gomes de Souza, M.L.; Talhari, A.C.; Talhari, S.; Naveca, F.G.; Ramasawmy, R. A Single Haplotype of IFNG Correlating with Low Circulating Levels of Interferon-γ Is Associated With Susceptibility to Cutaneous Leishmaniasis Caused by Leishmania guyanensis. Clin. Infect. Dis. 2020, 71, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, H.; Wu, S.; Wang, H.; Lv, C.; Cheng, B.; Xie, G.; Fang, X. Lack of association between TREM-1 gene polymorphisms and severe sepsis in a Chinese Han population. Hum. Immunol. 2008, 69, 220–226. [Google Scholar] [CrossRef]
- Runzheimer, J.; Mewes, C.; Büttner, B.; Hinz, J.; Popov, A.F.; Ghadimi, M.; Kristof, K.; Beissbarth, T.; Schamroth, J.; Tzvetkov, M.; et al. Lack of an Association between the Functional Polymorphism TREM-1 rs2234237 and the Clinical Course of Sepsis among Critically Ill Caucasian Patients-A Monocentric Prospective Genetic Association Study. J. Clin. Med. 2019, 8, 301. [Google Scholar] [CrossRef]
- Chin, V.K.; Asyran, A.M.Y.; Zakaria, Z.A.; Abdullah, W.O.; Chong, P.P.; Nordin, N.; Ibraheem, Z.O.; Majid, R.A.; Basir, R. TREM-1 modulation produces positive outcome on the histopathology and cytokines release profile of Plasmodium berghei-infected mice. J. Parasit. Dis. 2019, 43, 139–153. [Google Scholar] [CrossRef]
- Ornatowska, M.; Azim, A.C.; Wang, X.; Christman, J.W.; Xiao, L.; Joo, M.; Sadikot, R.T. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, 1377–1384. [Google Scholar] [CrossRef]
- Bleharski, J.R.; Kiessler, V.; Buonsanti, C.; Sieling, P.A.; Stenger, S.; Colonna, M.; Modlin, R.L. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003, 170, 3812–3818. [Google Scholar] [CrossRef]
- Polari, L.P.; Carneiro, P.P.; Macedo, M.; Machado, P.R.L.; Scott, P.; Carvalho, E.M.; Bacellar, O. Leishmania braziliensis Infection Enhances Toll-Like Receptors 2 and 4 Expression and Triggers TNF-α and IL-10 Production in Human Cutaneous Leishmaniasis. Front Cell Infect. Microbiol. 2019, 9, 120. [Google Scholar] [CrossRef]
- Koussoulas, V.; Tzivras, M.; Giamarellos-Bourboulis, E.J.; Demonakou, M.; Vassilliou, S.; Pelekanou, A.; Papadopoulos, A.; Giamarellou, H.; Barbatzas, C. Can soluble triggering receptor expressed on myeloid cells (sTREM-1) be considered an anti-inflammatory mediator in the pathogenesis of peptic ulcer disease? Dig. Dis. Sci. 2007, 52, 2166–2169. [Google Scholar] [CrossRef]
- Ruiz-Linares, A.; Adhikari, K.; Acuña-Alonzo, V.; Quinto-Sanchez, M.; Jaramillo, C.; Arias, W.; Fuentes, M.; Pizarro, M.; Everardo, P.; de Avila, F.; et al. Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals. PLoS Genet 2014, 10, e1004572. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Kindt, A.; Bermudez, H.; Llanos-Cuentas, A.; De Doncker, S.; Arevalo, J.; Wilber Quispe Tintaya, K.; Dujardin, J.C. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J. Clin. Microbiol. 2004, 42, 2294–2297. [Google Scholar] [CrossRef]
- Marfurt, J.; Nasereddin, A.; Niederwieser, I.; Jaffe, C.L.; Beck, H.P.; Felger, I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J. Clin. Microbiol. 2003, 41, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Armengol, L.; Solé, X.; Guinó, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R package to perform whole genome association studies. Bioinformatics 2007, 23, 644–645. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Strimmer, K. fdrtool: A versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 2008, 24, 1461–1462. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009; pp. 41–64. [Google Scholar]
| Patients with CL | HCs 1 | ||||
|---|---|---|---|---|---|
| N = 838 | N = 818 | ||||
| Males | Females | Males | Females | p-Value 2 | |
| Sex | 629 (75%) | 210 (25%) | 567 (68.2%) | 265 (31.8%) | 0.002 |
| Age (mean ± SEM 3) | 34.04 ± 0.54 | 37.12 ± 1.09 | 42.02 ± 0.72 | 40.05 ± 1.14 | <0.001 |
| Genotype and Allele Frequencies | ||||
|---|---|---|---|---|
| Patients with CL | HCs 1 | |||
| rs2234237 | Total = 838 | Total = 818 | ||
| Genotypes | ||||
| AA | 618 (74%) | 580 (71%) | ||
| AT | 202 (24%) | 220 (27%) | ||
| TT | 18 (2%) | 18 (2%) | ||
| Alleles | ||||
| A | 1.438 (86%) | 1.380 (84%) | ||
| T | 238 (14%) | 256 (16%) | ||
| Statistical comparisons between patients with CL and HCs 1 | ||||
| Inheritance models | OR 2 [CI 3 95%] | p-value 4 | Corrected p-value 5 | q-value 6 |
| Codominant | ||||
| AA | ||||
| AT | 1.19 [0.95–1.50] | 0.31 | 0.39 | 0.12 |
| TT | ||||
| Dominant | ||||
| AA vs. AT+TT | 1.18 [0.95–1.47] | 0.14 | 0.32 | 0.09 |
| Recessive | ||||
| AA+AT vs. TT | 0.99 [0.50–1.94] | 0.97 | 0.97 | 0.29 |
| Over-dominant | ||||
| AA+TT vs. AT | 1.19 [0.95–1.50] | 0.13 | 0.32 | 0.09 |
| Log-additive | 1.14 [0.93–1.39] | 0.19 | 0.32 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Espírito Santo Júnior, J.; Gabrielle Ramos de Mesquita, T.; Diego Oliveira da Silva, L.; Jules de Araújo, F.; Lacerda de Souza, J.; Carneiro de Lacerda, T.; Santos da Silva, L.; Marcello da Silveira Júnior, C.; Layane Guimarães Duarte Queiroz, K.; dos Santos, D.M.; et al. TREM1 rs2234237 (Thr25Ser) Polymorphism in Patients with Cutaneous Leishmaniasis Caused by Leishmania guyanensis: A Case-Control Study in the State of Amazonas, Brazil. Pathogens 2021, 10, 498. https://doi.org/10.3390/pathogens10040498
do Espírito Santo Júnior J, Gabrielle Ramos de Mesquita T, Diego Oliveira da Silva L, Jules de Araújo F, Lacerda de Souza J, Carneiro de Lacerda T, Santos da Silva L, Marcello da Silveira Júnior C, Layane Guimarães Duarte Queiroz K, dos Santos DM, et al. TREM1 rs2234237 (Thr25Ser) Polymorphism in Patients with Cutaneous Leishmaniasis Caused by Leishmania guyanensis: A Case-Control Study in the State of Amazonas, Brazil. Pathogens. 2021; 10(4):498. https://doi.org/10.3390/pathogens10040498
Chicago/Turabian Styledo Espírito Santo Júnior, José, Tirza Gabrielle Ramos de Mesquita, Luan Diego Oliveira da Silva, Felipe Jules de Araújo, Josué Lacerda de Souza, Thaís Carneiro de Lacerda, Lener Santos da Silva, Cláudio Marcello da Silveira Júnior, Krys Layane Guimarães Duarte Queiroz, Diogo Matos dos Santos, and et al. 2021. "TREM1 rs2234237 (Thr25Ser) Polymorphism in Patients with Cutaneous Leishmaniasis Caused by Leishmania guyanensis: A Case-Control Study in the State of Amazonas, Brazil" Pathogens 10, no. 4: 498. https://doi.org/10.3390/pathogens10040498
APA Styledo Espírito Santo Júnior, J., Gabrielle Ramos de Mesquita, T., Diego Oliveira da Silva, L., Jules de Araújo, F., Lacerda de Souza, J., Carneiro de Lacerda, T., Santos da Silva, L., Marcello da Silveira Júnior, C., Layane Guimarães Duarte Queiroz, K., dos Santos, D. M., Chagas da Silva, C., David Graterol Sequera, H., Tamayo Hermida, M., Lúcia Gomes de Souza, M., Vinitius de Farias Guerra, M., & Ramasawmy, R. (2021). TREM1 rs2234237 (Thr25Ser) Polymorphism in Patients with Cutaneous Leishmaniasis Caused by Leishmania guyanensis: A Case-Control Study in the State of Amazonas, Brazil. Pathogens, 10(4), 498. https://doi.org/10.3390/pathogens10040498






