Leishmania spp.-Infected Dogs Have Circulating Anti-Skeletal Muscle Autoantibodies Recognizing SERCA1
Abstract
1. Introduction
2. Results
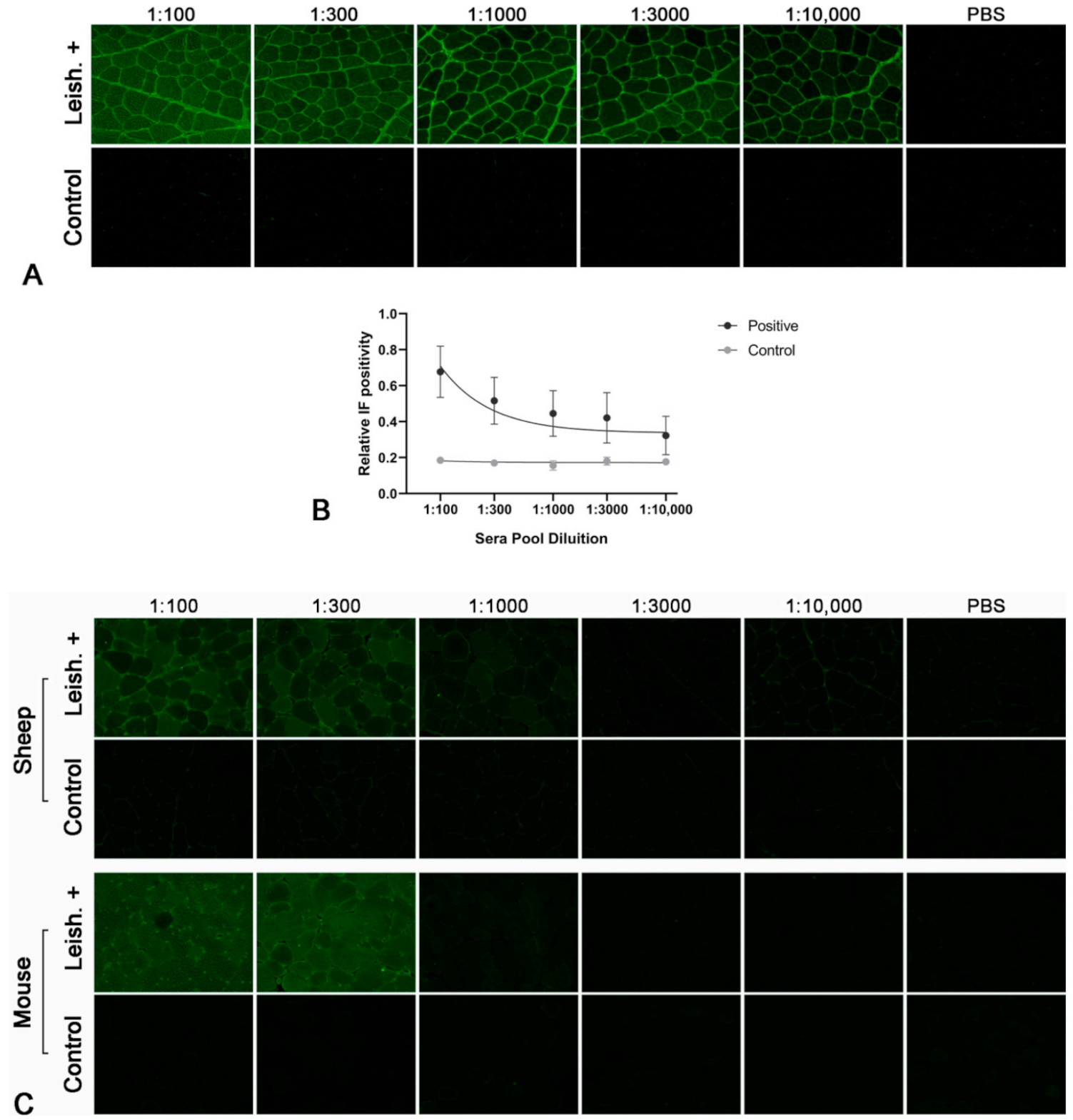
2.1. Leishmania-Infected Dogs Have Circulating Autoantibodies Recognizing Skeletal Muscle
2.2. Species Specificity of the Autoantibodies
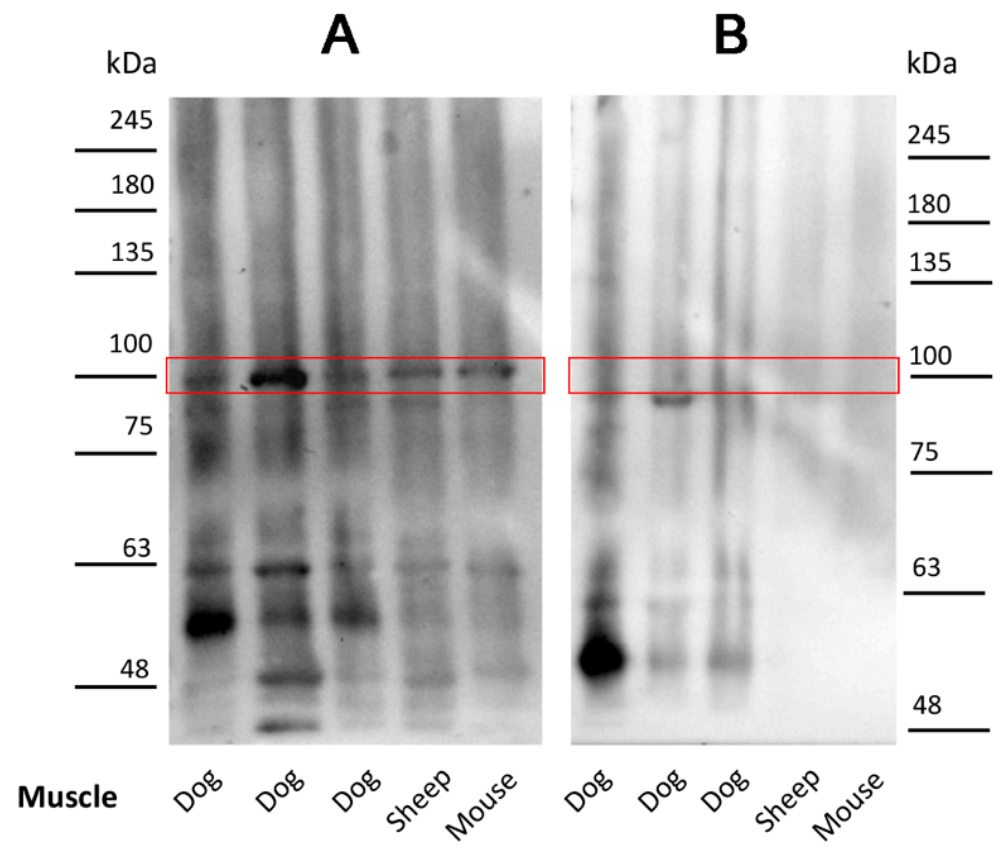
2.3. Antibodies in Leishmania-Infected Dogs Recognize a Specific Muscle Protein
2.4. Isolation and Characterization of the Canine Muscle Protein Recognized by the Leishmania-Infected Dog Sera
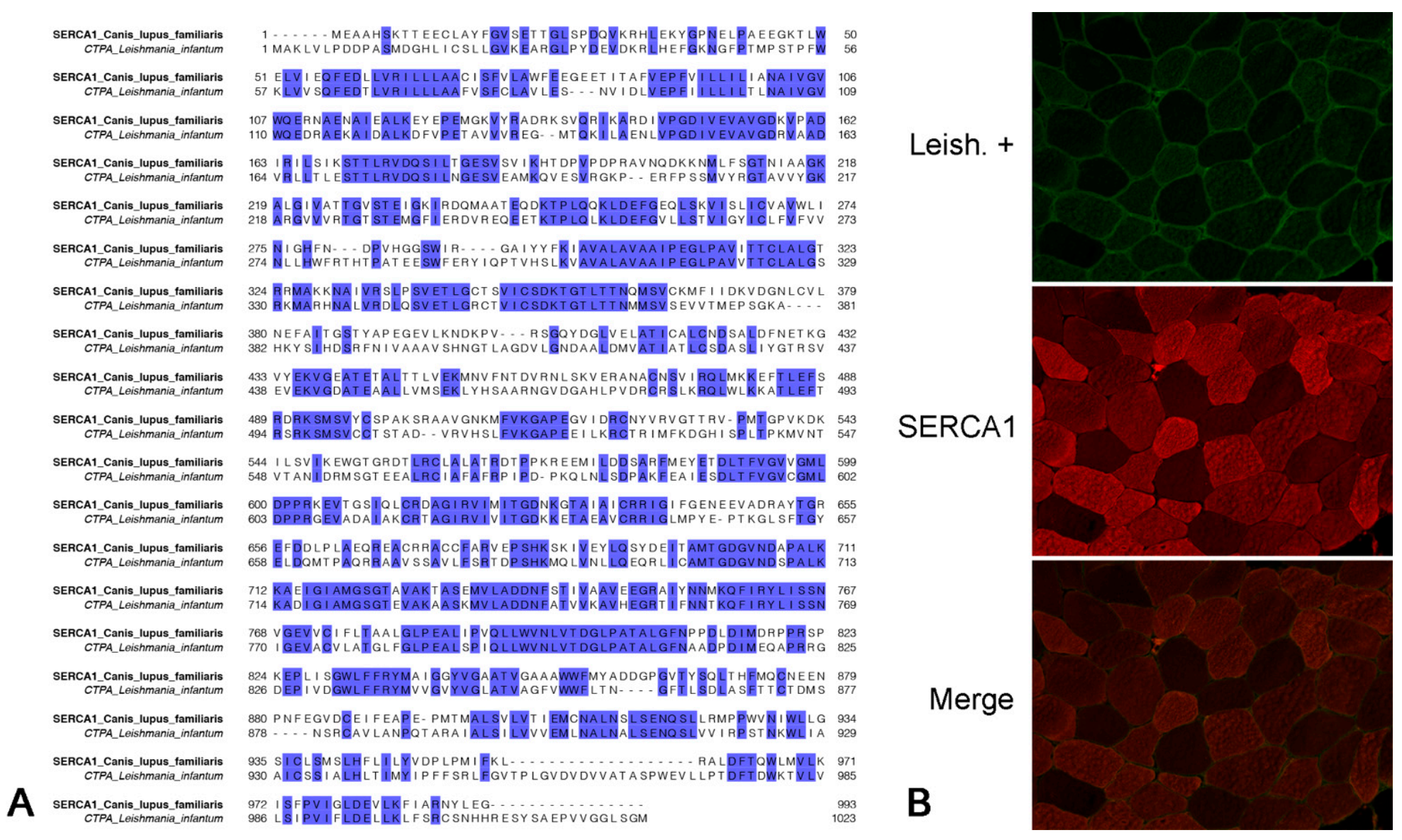
2.5. Muscle Protein Recognized by Antibodies of Leishmania-Infected Dogs Colocalizes with Anti-SERCA1 Antibodies
2.6. Antibodies of Leishmania-Infected Dogs Recognize an Immunoprecipitated SERCA1 Protein
3. Discussion
4. Materials and Methods
4.1. Sera
4.2. Tissues
4.3. Indirect Immunofluorescent Staining
4.4. Indirect Immunofluorescent with Colocalization
4.5. Western Blot Analysis and Immunoprecipitation Procedures
4.6. LC–MS/MS Analysis for Antigen Identification
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zachary, J.F. Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323357753. [Google Scholar]
- Miller, F.W.; Lamb, J.A.; Schmidt, J.; Nagaraju, K. Risk factors and disease mechanisms in myositis. Nat. Rev. Rheumatol. 2018, 14, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; de Visser, M.; Werth, V.P. Classification of myositis. Nat. Rev. Rheumatol. 2018, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Podell, M. Inflammatory myopathies. Vet. Clin. Small Anim. Pract. 2002, 32, 147–167. [Google Scholar] [CrossRef]
- Prisco, F.; de Biase, D.; Piegari, G.; Oriente, F.; Cimmino, I.; Pavone, L.M.; Ruoppolo, M.; Costanzo, M.; Pasquale, S.; Paciello, O. Pathomechanism highlights of leishmania-associated myopathy in the dog. J. Comp. Pathol. 2019, 166, 110. [Google Scholar] [CrossRef]
- Prisco, F.; Papparella, S.; Paciello, O. The correlation between cardiac and skeletal muscle pathology in animal models of idiopathic inflammatory myopathies. Acta Myol. 2020, 39, 315–321. [Google Scholar] [CrossRef]
- Shelton, G.D. From dog to man: The broad spectrum of inflammatory myopathies. Neuromuscul. Disord. 2007, 17, 663–670. [Google Scholar] [CrossRef]
- King, J.; Lecouteur, R.A.; Aleman, M.; Williams, D.C.; Moore, P.F.; Guo, L.T.; Mizisin, A.P.; Shelton, G.D. Vacuolar myopathy in a dog resembling human sporadic inclusion body myositis. Acta Neuropathol. 2009, 118, 711–717. [Google Scholar] [CrossRef]
- Moran, E.M.; Mastaglia, F.L. Cytokines in immune-mediated inflammatory myopathies: Cellular sources, multiple actions and therapeutic implications. Clin. Exp. Immunol. 2014, 178, 405–415. [Google Scholar] [CrossRef]
- Pagano, T.B.; Prisco, F.; de Biase, D.; Piegari, G.; Maurelli, M.P.; Rinaldi, L.; Cringoli, G.; Papparella, S.; Paciello, O. Muscular Sarcocystosis in Sheep Associated With Lymphoplasmacytic Myositis and Expression of Major Histocompatibility Complex Class I and II. Vet. Pathol. 2019, 57, 272–280. [Google Scholar] [CrossRef]
- Evans, J.; Levesque, D.; Shelton, G.D. Canine inflammatory myopathies: A clinicopathologic review of 200 cases. J. Vet. Intern. Med. 2004, 18, 679–691. [Google Scholar] [CrossRef]
- Hankel, S.; Shelton, G.D.; Engvall, E. Sarcolemma-specific autoantibodies in canine inflammatory myopathy. Vet. Immunol. Immunopathol. 2006, 113, 1–10. [Google Scholar] [CrossRef]
- Paciello, O.; Oliva, G.; Gradoni, L.; Manna, L.; Manzillo, V.F.; Wojcik, S.; Trapani, F.; Papparella, S. Canine inflammatory myopathy associated with Leishmania Infantum infection. Neuromuscul. Disord. 2009, 19, 124–130. [Google Scholar] [CrossRef]
- Podell, M.; Chen, E.; Shelton, G.D. Feline immunodeficiency virus associated myopathy in the adult cat. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1998, 21, 1680–1685. [Google Scholar] [CrossRef]
- Pasolini, M.P.; Pagano, T.B.; Costagliola, A.; de Biase, D.; Lamagna, B.; Auletta, L.; Fatone, G.; Greco, M.; Coluccia, P.; Veneziano, V.; et al. Inflammatory Myopathy in Horses With Chronic Piroplasmosis. Vet. Pathol. 2018, 55, 133–143. [Google Scholar] [CrossRef]
- Costagliola, A.; Piegari, G.; Otrocka-Domagala, I.; Ciccarelli, D.; Iovane, V.; Oliva, G.; Russo, V.; Rinaldi, L.; Papparella, S.; Paciello, O. Immunopathological features of canine myocarditis associated with leishmania infantum infection. BioMed Res. Int. 2016, 2016, 8016186. [Google Scholar] [CrossRef]
- Silva-Almeida, M.; Carvalho, L.; Abreu-Silva, A.L.; D’Escoffier, L.; Calabrese, K. Leishsmania (Leishmania) amazonensis infection: Muscular involvement in BALB/c and C3H.HeN mice. Exp. Parasitol. 2010, 124, 315–318. [Google Scholar] [CrossRef]
- Paciello, O.; Wojcik, S.; Gradoni, L.; Oliva, G.; Trapani, F.; Iovane, V.; Politano, L.; Papparella, S. Syrian hamster infected with Leishmania infantum: A new experimental model for inflammatory myopathies. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2010, 41, 355–361. [Google Scholar] [CrossRef]
- Esteva, L.; Vargas, C.; de León, C.V. The role of asymptomatics and dogs on leishmaniasis propagation. Math. Biosci. 2017, 293, 46–55. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Miró, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine leishmaniasis control in the context of one health. Emerg. Infect. Dis. 2019, 25, E1–E4. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Suzan, M.; Michalick, M.; Eduardo, M.; Cheim, C.; Jean, F.; Frézard, G.; Magno, S. Canine leishmaniasis: An overview of the current status and strategies for control. BioMed Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Koutinas, C.K. Pathologic Mechanisms Underlying the Clinical Findings in Canine Leishmaniosis due to Leishmania infantum/chagasi. Vet. Pathol. 2014, 51, 527–538. [Google Scholar] [CrossRef]
- Baneth, G.; Yasur-Landau, D.; Gilad, M.; Nachum-Biala, Y. Canine leishmaniosis caused by Leishmania major and Leishmania tropica: Comparative findings and serology. Parasites Vectors 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Lopez, R.; Lucena, R.; Novales, M.; Ginel, P.J.P.; Martin, E.; Molleda, J.M. Circulating immune complexes and renal function in canine leishmaniasis. J. Vet. Med. Ser. B 1996, 43, 469–474. [Google Scholar] [CrossRef]
- Ginel, P.J.; Camacho, S.; Lucena, R. Anti-histone antibodies in dogs with leishmaniasis and glomerulonephritis. Res. Vet. Sci. 2008, 85, 510–514. [Google Scholar] [CrossRef]
- Cortese, L.; Sica, M.; Piantedosi, D.; Ruggiero, G.; Pero, M.E.; Terrazzano, G.; Mastellone, V.; Ciaramella, P. Secondary immune-mediated thrombocytopenia in dogs naturally infected by Leishmania infantum. Vet. Rec. 2009, 164, 778–782. [Google Scholar] [CrossRef]
- Makaritsis, K.P.; Gatselis, N.K.; Ioannou, M.; Petinaki, E.; Dalekos, G.N. Polyclonal hypergammaglobulinemia and high smooth-muscle autoantibody titers with specificity against filamentous actin: Consider visceral leishmaniasis, not just autoimmune hepatitis. Int. J. Infect. Dis. 2009, 13, e157–e160. [Google Scholar] [CrossRef][Green Version]
- Paciello, O.; Shelton, G.D.; Papparella, S. Expression of major histocompatibility complex class I and class II antigens in canine masticatory muscle myositis. Neuromuscul. Disord. 2007, 17, 313–320. [Google Scholar] [CrossRef]
- Prisco, F.; de Biase, D.; Piegari, G.; D’Aquino, I.; Lama, A.; Comella, F.; Mercogliano, R.; Dipineto, L.; Papparella, S.; Paciello, O. Pathologic characterization of white striping myopathy in broiler chickens. Poult. Sci. 2021, 100, 101150. [Google Scholar] [CrossRef]
- Vamvakidis, C.D.; Koutinas, A.F.E.; Saridomichelakis, M.; Kanakoudis, G.; Georgiadis, G.; Saridomichelakis, M. Masticatory and skeletal muscle myositis in canine leishmaniasis (Leishmania infantum). Vet. Rec. 2000, 146, 698–703. [Google Scholar] [CrossRef]
- Chaabouni, A.; Boubaker Elandoulsi, R.; Mhadhbi, M.; Gharbi, M.; Sassi, A. Comparative analysis of the Leishmania infantum-specific antibody repertoires and the autoantibody repertoires between asymptomatic and symptomatic dogs. Vet. Parasitol. 2018, 261, 9–17. [Google Scholar] [CrossRef]
- Lucena, R.; Ginel, P.J.; Lopez, R.; Novales, M.; Martin, E.; Molleda, J.M. Antinuclear Antibodies in Dogs with Leishmaniasis. J. Vet. Med. Ser. A 1996, 43, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Prisco, F.; de Biase, D.; Ilsami, A.; Cardillo, L.; Fusco, G.; Santoro, P.; Papparella, S.; Paciello, O. Feline Immunodeficiency Virus-Associated Myopathy in Cats: An Autoimmune Disorder? J. Comp. Pathol. 2020, 174, 148. [Google Scholar] [CrossRef]
- Stammers, A.N.; Susser, S.E.; Hamm, N.C.; Hlynsky, M.W.; Kimber, D.E.; Kehler, D.S.; Duhamel, T.A. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA). Can. J. Physiol. Pharmacol. 2015, 93, 834–854. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.; Massilamany, C.; Basavalingappa, R.H.; Gangaplara, A.; Rajasekaran, R.A.; Afzal, M.Z.; Khalilzad-Sharghi, V.; Zhou, Y.; Riethoven, J.-J.; Nandi, S.S.; et al. Epitope Mapping of SERCA2a Identifies an Antigenic Determinant that Induces Mainly Atrial Myocarditis in A/J Mice. J. Immunol. 2018, 200, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Brooks, R.; Komives, E.A.; Torpey, J.W.; Engvall, E.; Gonias, S.L.; Shelton, G.D. Autoantibodies in Canine Masticatory Muscle Myositis Recognize a Novel Myosin Binding Protein-C Family Member. J. Immunol. 2007, 179, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
- McHugh, N.J.; Tansley, S.L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 2018, 14, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Dubowitz, V.; Sewry, C.A.; Oldfors, A.; Lane, R.J.M. Muscle Biopsy: A Practical Approach, 4th ed.; Saunders: Philadelphia, PA, USA, 2013; ISBN 9780702050305. [Google Scholar]
- Sharaf, A.E.R.; Narula, J.; Nicol, P.D.; Southern, J.F.; Khaw, B.A. Cardiac sarcoplasmic reticulum calcium ATPase, an autoimmune antigen in experimental cardiomyopathy. Circulation 1994, 89, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Khaw, B.A.; Narula, J.; Sharaf, A.R.; Nicol, P.D.; Southern, J.F.; Carles, M. SR-Ca2+ATPase as an autoimmunogen in experimental myocarditis. Eur. Heart J. 1995, 16, 92–96. [Google Scholar] [CrossRef]
- Summerfield, N.; Peters, M.E.; Hercock, C.A.; Mobasheri, A.; Young, I.S. Immunohistochemical evidence for expression of fast-twitch type sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA1) in German shepherd dogs with dilated cardiomyopathy myocardium. J. Vet. Cardiol. 2010, 12, 17–23. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Bobe, R.; Adnot, S.; Hajjar, R.J.; Lipskaia, L. Sarco (Endo) Plasmic Reticulum Calcium Atpases (SERCA) Isoforms in the Normal and Diseased Cardiac, Vascular and Skeletal Muscle. J. Cardiovasc. Dis. Diagn. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Ferrari, I.; Levin, M.J.; Wallukat, G.; Elies, R.; Lebesgue, D.; Chiale, P.; Elizari, M.; Rosenbaum, M.; Hoebeke, J. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J. Exp. Med. 1995, 182, 59–65. [Google Scholar] [CrossRef]
- Zaghini, A.; Sarli, G.; Barboni, C.; Sanapo, M.; Pellegrino, V.; Diana, A.; Linta, N.; Rambaldi, J.; D’Apice, M.R.; Murdocca, M.; et al. Long term breeding of the Lmna G609G progeric mouse: Characterization of homozygous and heterozygous models. Exp. Gerontol. 2020, 130, 110784. [Google Scholar] [CrossRef]
- Zhanmu, O.; Zhao, P.; Yang, Y.; Yang, X.; Gong, H.; Li, X. Maintenance of Fluorescence During Paraffin Embedding of Fluorescent Protein-Labeled Specimens. Front. Neurosci. 2019, 13, 752. [Google Scholar] [CrossRef]
- Rapa, S.F.; Prisco, F.; Popolo, A.; Iovane, V.; Autore, G.; Di Iorio, B.R.; dal Piaz, F.; Paciello, O.; Nishijima, F.; Marzocco, S. Pro-Inflammatory Effects of Indoxyl Sulfate in Mice: Impairment of Intestinal Homeostasis and Immune Response. Int. J. Mol. Sci. 2021, 22, 1135. [Google Scholar] [CrossRef]
- Cimmino, I.; Margheri, F.; Prisco, F.; Perruolo, G.; D’Esposito, V.; Laurenzana, A.; Fibbi, G.; Paciello, O.; Doti, N.; Ruvo, M.; et al. Prep1 regulates angiogenesis through a PGC-1α-mediated mechanism. FASEB J. 2019, 33, 13893–13904. [Google Scholar] [CrossRef]
- Cimmino, I.; Lorenzo, V.; Fiory, F.; Doti, N.; Ricci, S.; Cabaro, S.; Liotti, A.; Vitagliano, L.; Longo, M.; Miele, C.; et al. A peptide antagonist of Prep1-p160 interaction improves ceramide-induced insulin resistance in skeletal muscle cells. Oncotarget 2017, 8, 71845–71858. [Google Scholar] [CrossRef]
- Caterino, M.; Zacchia, M.; Costanzo, M.; Bruno, G.; Arcaniolo, D.; Trepiccione, F.; Siciliano, R.A.; Mazzeo, M.F.; Ruoppolo, M.; Capasso, G. Urine Proteomics Revealed a Significant Correlation Between Urine-Fibronectin Abundance and Estimated-GFR Decline in Patients with Bardet-Biedl Syndrome. Kidney Blood Press. Res. 2018, 43, 389–405. [Google Scholar] [CrossRef]
- Costanzo, M.; Cevenini, A.; Marchese, E.; Imperlini, E.; Raia, M.; del Vecchio, L.; Caterino, M.; Ruoppolo, M. Label-Free Quantitative Proteomics in a Methylmalonyl-CoA Mutase-Silenced Neuroblastoma Cell Line. Int. J. Mol. Sci. 2018, 19, 3580. [Google Scholar] [CrossRef] [PubMed]
- Caterino, M.; Ruoppolo, M.; Fulcoli, G.; Huynth, T.; Orrù, S.; Baldini, A.; Salvatore, F. Transcription Factor TBX1 Overexpression Induces Downregulation of Proteins Involved in Retinoic Acid Metabolism: A Comparative Proteomic Analysis. J. Proteome Res. 2009, 8, 1515–1526. [Google Scholar] [CrossRef]

| Serum # | IFAT |
|---|---|
| 1, 2 | 1/80 |
| 3–8 | 1/160 |
| 9–12 | 1/320 |
| 13–15 | 1/640 |
| 16–35 | 1/1280 |
| 36–45 | Absent |
| Muscle # | Species | Breed/Strain | Sex | Age |
|---|---|---|---|---|
| 1 | Dog | Siberian husky | M | 5 years |
| 2 | Dog | Mix | M | 12 years |
| 3 | Dog | Jack Russell terrier | M | 9 years |
| 4 | Dog | Whippet | F | 6 years |
| 5 | Dog | Mix | M | 3.5 months |
| 6 | Mouse | C57 | F | 1 year |
| 7 | Mouse | C57 | F | 1 year |
| 8 | Mouse | C57 | F | 1 year |
| 9 | Sheep | Mix | F | 4 years |
| 10 | Sheep | Mix | F | 5 years |
| 11 | Sheep | Mix | F | 7 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prisco, F.; De Biase, D.; Piegari, G.; Oriente, F.; Cimmino, I.; De Pasquale, V.; Costanzo, M.; Santoro, P.; Gizzarelli, M.; Papparella, S.; et al. Leishmania spp.-Infected Dogs Have Circulating Anti-Skeletal Muscle Autoantibodies Recognizing SERCA1. Pathogens 2021, 10, 463. https://doi.org/10.3390/pathogens10040463
Prisco F, De Biase D, Piegari G, Oriente F, Cimmino I, De Pasquale V, Costanzo M, Santoro P, Gizzarelli M, Papparella S, et al. Leishmania spp.-Infected Dogs Have Circulating Anti-Skeletal Muscle Autoantibodies Recognizing SERCA1. Pathogens. 2021; 10(4):463. https://doi.org/10.3390/pathogens10040463
Chicago/Turabian StylePrisco, Francesco, Davide De Biase, Giuseppe Piegari, Francesco Oriente, Ilaria Cimmino, Valeria De Pasquale, Michele Costanzo, Pasquale Santoro, Manuela Gizzarelli, Serenella Papparella, and et al. 2021. "Leishmania spp.-Infected Dogs Have Circulating Anti-Skeletal Muscle Autoantibodies Recognizing SERCA1" Pathogens 10, no. 4: 463. https://doi.org/10.3390/pathogens10040463
APA StylePrisco, F., De Biase, D., Piegari, G., Oriente, F., Cimmino, I., De Pasquale, V., Costanzo, M., Santoro, P., Gizzarelli, M., Papparella, S., & Paciello, O. (2021). Leishmania spp.-Infected Dogs Have Circulating Anti-Skeletal Muscle Autoantibodies Recognizing SERCA1. Pathogens, 10(4), 463. https://doi.org/10.3390/pathogens10040463











