Tick Importin-α Is Implicated in the Interactome and Regulome of the Cofactor Subolesin
Abstract
1. Introduction
2. Results and Discussion
2.1. Importin-α Is Part of the SUB Interactome with a Putative Function in Translocation to the Cell Nucleus
2.2. The SUB-Importin-α Interaction Is Not Essential for SUB Function
2.3. SUB Interaction with Histone H4
3. Materials and Methods
3.1. SUB Cloning
3.2. Yeast Two-Hybrid Screening Procedure
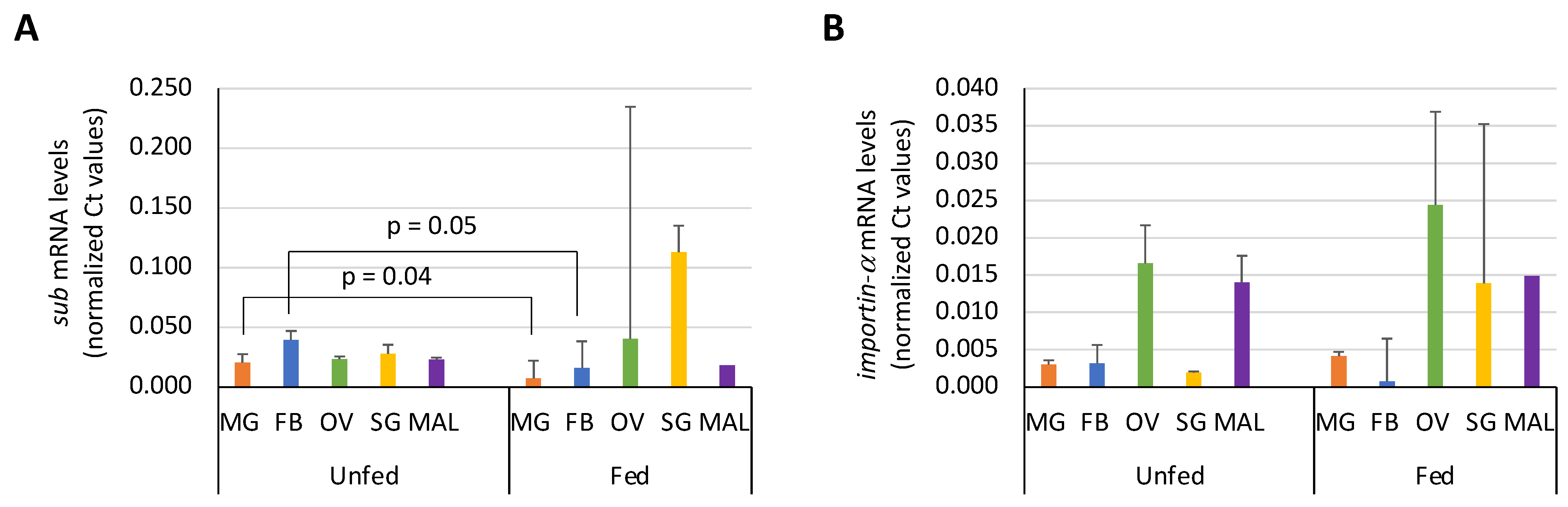
3.3. Expression Profile of Sub and Importin-α in I. Ricinus Organs
3.4. Corroboration of SUB-Importin-α Interaction by Protein Pull-Down
3.5. RNA Extraction and Synthesis of Tick cDNA for dsRNA Preparations
3.6. Gene Knockdown by RNA Interference in Ticks
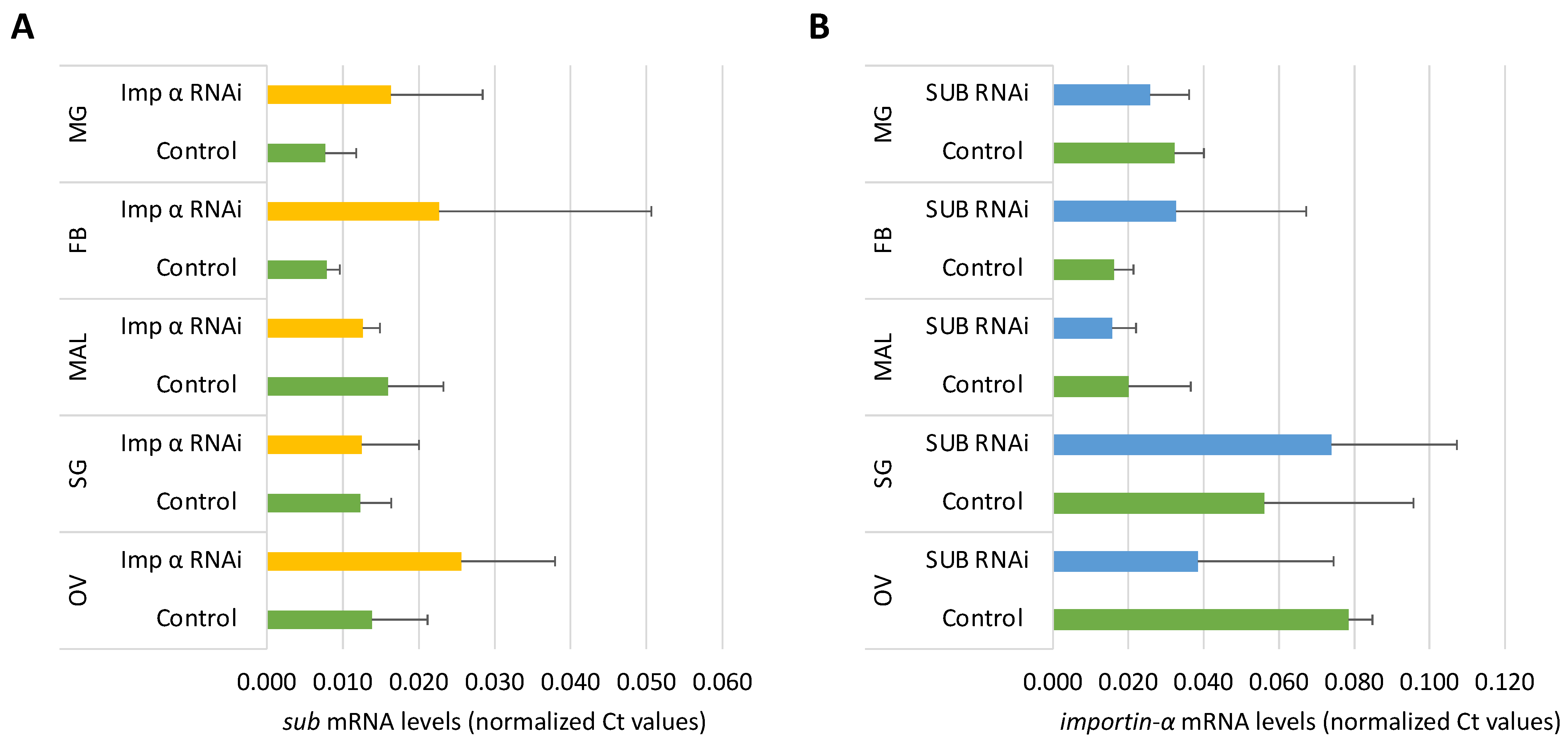
3.7. Corroboration of Sub and Importin-α Silencing by qRT-PCR in I. Ricinus Organs
3.8. Co-Regulation Assessment of Sub and Importin-α
3.9. Histone Microarray Analysis
3.10. Corroboration of SUB-Histone 4 (H4) K-Butyrylated (H4but) Peptide Interaction by Protein Pull-Down and Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de la Fuente, J.; Estrada-Peña, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- de la Fuente, J.; Almazán, C.; Blas-Machado, U.; Naranjo, V.; Mangold, A.J.; Blouin, E.F.; Gortazar, C.; Kocan, K.M. The tick protective antigen, 4D8, is a conserved protein involved in modulation of tick blood ingestion and reproduction. Vaccine 2006, 24, 4082–4095. [Google Scholar] [CrossRef]
- Schorderet-Weber, S.; Noack, S.; Selzer, P.; Kaminsky, R. Blocking transmission of vector-borne diseases. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 90–109. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Dolan, M.C. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 2016, 53, 1063–1092. [Google Scholar] [CrossRef]
- Ghosh, S.; Azhahianambi, P.; Yadav, M.P. Upcoming and future strategies of tick control: A review. J. Vector Borne Dis. 2007, 44, 79–89. [Google Scholar]
- Franzin, A.M.; Maruyama, S.R.; Garcia, G.R.; Oliveira, R.P.; Ribeiro, J.M.; Bishop, R.; Maia, A.A.; Moré, D.D.; Ferreira, B.R.; Santos, I.K. Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus. Parasit. Vectors 2017, 10, 51. [Google Scholar] [CrossRef]
- Keesing, F.; Ostfeld, R. The tick project: Testing environmental methods of preventing tick-borne diseases. Trends Parasitol. 2018, 34, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Maggi, F.; Romano, D.; Stefanini, C.; Vaseeharan, B.; Kumar, S.; Higuchi, A.; Alarfaj, A.A.; Mehlhorn, H.; Canale, A. Nanoparticles as effective acaricides against ticks-A review. Ticks Tick Borne Dis. 2017, 8, 821–826. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Contreras, M.; Estrada-Peña, A.; Cabezas-Cruz, A. Targeting a global health problem: Vaccine design and challenges for the control of tick-borne diseases. Vaccine 2017, 35, 5089–5094. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Alberdi, P.; Pérez de la Lastra, J.M.; de la Fuente, J. Tick vaccines and the control of tick-borne pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 2016, 34, 3010–3013. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.R.; Guerrero, F.D. Anti-tick vaccines in the omics era. Front. Biosci. (Elite Ed.) 2018, 10, 122–136. [Google Scholar] [CrossRef]
- Kasaija, P.D.; Contreras, M.; Kabi, F.; Mugerwa, S.; de la Fuente, J. Vaccination with Recombinant Subolesin Antigens Provides Cross-Tick Species Protection in Bos indicus and Crossbred Cattle in Uganda. Vaccines (Basel) 2020, 8, 319. [Google Scholar] [CrossRef]
- Contreras, M.; San José, C.; Estrada-Peña, A.; Talavera, V.; Rayas, E.; León, C.I.; Núñez, J.L.; García Fernández de Mera, I.; de la Fuente, J. Control of tick infestations in wild roe deer (Capreolus capreolus) vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 2020, 38, 6450–6454. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Jerónimo, S.; de la Fuente, J.; Villar, M. Interactomics and tick vaccine development: New directions for the control of tick-borne diseases. Expert Rev. Proteom. 2018, 15, 627–635. [Google Scholar] [CrossRef]
- Artigas-Jerónimo, S.; Comín, J.; Villar, M.; Contreras, M.; Alberdi, P.; Viera, I.L.; Soto, L.; Cordero, R.; Valdés, J.J.; Cabezas-Cruz, A.; et al. A Novel Combined Scientific and Artistic Approach for the Advanced Characterization of Interactomes: The Akirin/Subolesin Model. Vaccines 2020, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Kocan, K.M.; Bergman, D.K.; Garcia-Garcia, J.C.; Blouin, E.F.; de la Fuente, J. Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 2003, 21, 1492–1501. [Google Scholar] [CrossRef]
- Goto, A.; Matsushita, K.; Gesellchen, V.; El Chamy, L.; Kuttenkeuler, D.; Takeuchi, O.; Hoffmann, J.A.; Akira, S.; Boutros, M.; Reichhart, J.M. Akirins are highly conserved nuclear proteins required for NF-kappaB-dependent gene expression in Drosophila and mice. Nat. Immunol. 2008, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Jerónimo, S.; Villar, M.; Cabezas-Cruz, A.; Valdés, J.J.; Estrada-Peña, A.; Alberdi, P.; de la Fuente, J. Functional Evolution of Subolesin/Akirin. Front. Physiol. 2018, 9, 1612. [Google Scholar] [CrossRef]
- de la Fuente, J.; Maritz-Olivier, C.; Naranjo, V.; Ayoubi, P.; Nijhof, A.M.; Almazán, C.; Canales, M.; Pérez de la Lastra, J.M.; Galindo, R.C.; Blouin, E.F.; et al. Evidence of the role of tick subolesin in gene expression. BMC Genom. 2008, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Naranjo, N.; Ayllón, N.; Pérez de la Lastra, J.M.; Galindo, R.C.; Kocan, K.M.; Blouin, E.F.; Mitra, R.; Alberdi, P.; Villar, M.; de la Fuente, J. Reciprocal regulation of NF-kB (Relish) and Subolesin in the tick vector, Ixodes scapularis. PLoS ONE 2013, 8, e65915. [Google Scholar] [CrossRef]
- Gulia-Nuss, M.; Nuss, A.B.; Meyer, J.M.; Sonenshine, D.E.; Roe, R.M.; Waterhouse, R.M.; Sattelle, D.B.; de la Fuente, J.; Ribeiro, J.M.; Megy, K.; et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016, 7, 10507. [Google Scholar] [CrossRef]
- Shaw, D.K.; Wang, X.; Brown, L.J.; Chávez, A.S.; Reif, K.E.; Smith, A.A.; Scott, A.J.; McClure, E.E.; Boradia, V.M.; Hammond, H.L.; et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun. 2017, 8, 14401. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.; Marina, A.; de la Fuente, J. Applying proteomics to tick vaccine development: Where are we? Expert Rev. Proteom. 2017, 14, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Jerónimo, S.; Villar, M.; Estrada-Peña, A.; Velázquez-Campoy, A.; Alberdi, P.; de la Fuente, J. Function of Akirin2 cofactor in the regulation of gene expression in model human Caucasian neutrophil-like HL60 cells. Sci. Rep. 2021. in revision. [Google Scholar]
- Moritsubo, M.; Miyoshi, H.; Matsuda, K.; Yoshida, N.; Nakashima, K.; Yanagida, E.; Yamada, K.; Takeuchi, M.; Suzuki, T.; Muta, H.; et al. TACC3 expression as a prognostic factor in aggressive types of adult T-cell leukemia/lymphoma patients. Int. J. Lab. Hematol. 2020, 42, 842–848. [Google Scholar] [CrossRef]
- Cheng, S.; Douglas-Jones, A.; Yang, X.; Mansel, R.E.; Jiang, W.G. Transforming acidic coiled-coil-containing protein 2 (TACC2) in human breast cancer, expression pattern and clinical/prognostic relevance. Cancer Genom. Proteom. 2010, 7, 67–73. [Google Scholar]
- Yun, M.; Rong, J.; Lin, Z.R.; He, Y.L.; Zhang, J.X.; Peng, Z.W.; Tang, L.Q.; Zeng, M.S.; Zhong, Q.; Ye, S. High expression of transforming acidic coiled coil-containing protein 3 strongly correlates with aggressive characteristics and poor prognosis of gastric cancer. Oncol. Rep. 2015, 34, 1397–1405. [Google Scholar] [CrossRef]
- Jung, C.K.; Jung, J.H.; Park, G.S.; Lee, A.; Kang, C.S.; Lee, K.Y. Expression of transforming acidic coiled-coil containing protein 3 is a novel independent prognostic marker in non-small cell lung cancer. Pathol. Int. 2006, 56, 503–509. [Google Scholar] [CrossRef]
- Takechi, R.; Galay, R.L.; Matsuo, T.; Maeda, H.; Kusakisako, K.; Talactac, M.R.; Mochizuki, M.; Fujisaki, K.; Tanaka, T. Role of the tumor necrosis factor receptor-associated factor-type zinc finger domain containing protein 1 (TRAFD1) from the hard tick Haemaphysalis longicornis in immunity against bacterial infection. Ticks Tick Borne Dis. 2016, 7, 36–45. [Google Scholar] [CrossRef]
- Polanowska, J.; Chen, J.X.; Soulé, J.; Omi, S.; Belougne, J.; Taffoni, C.; Pujol, N.; Selbach, M.; Zugasti, O.; Ewbank, J.J. Evolutionary plasticity in the innate immune function of Akirin. PLoS Genet. 2018, 14, e1007494. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Kasaija, P.D.; Merino, O.; de la Cruz-Hernandez, N.I.; Gortazar, C.; de la Fuente, J. Oral vaccination with a formulation combining Rhipicephalus microplus Subolesin with heat inactivated Mycobacterium bovis reduces tick infestations in cattle. Front. Cell. Infect. Microbiol. 2019, 9, 45. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Corbett, A.H.; Mason, D.A.; Harreman, M.T.; Adam, S.A. Importin alpha: A multipurpose nuclear-transport receptor. Trends Cell. Biol. 2004, 14, 505–514. [Google Scholar] [CrossRef]
- Oka, M.; Yoneda, Y. Importin α: Functions as nuclear transport factor and beyond. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 259–274. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Yamada, K.; Yoneda, Y. Importin-α: A key molecule in nuclear transport and non-transport functions. J. Biochem. 2016, 160, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kutay, U.; Bischoff, F.R.; Kostka, S.; Kraft, R.; Gorlich, D. Export of Importin-α from the nucleus is mediated by a specific nuclear transport factor. Cell 1997, 90, 1061–1071. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, T.Y.; Yan, F. Expression pattern of subA in different tissues and blood-feeding status in Haemaphysalis flava. Exp. Appl. Acarol. 2016, 70, 511–522. [Google Scholar] [CrossRef]
- Ratan, R.; Mason, D.A.; Sinnot, B.; Goldfarb, D.S.; Fleming, R.J. Drosophila importin alpha1 performs paralog-specific functions essential for gametogenesis. Genetics 2008, 178, 839–850. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Almazán, C.; Naranjo, V.; Blouin, E.F.; Kocan, K.M. Synergistic effect of silencing the expression of tick protective antigens 4D8 and Rs86 in Rhipicephalus sanguineus by RNA interference. Parasitol. Res. 2006, 99, 108–113. [Google Scholar] [CrossRef]
- Chan, C.K.; Jans, D.A. Synergy of importin a recognition and DNA binding by the yeast transcriptional activator GAL4. FEBS Lett. 1999, 462, 221–224. [Google Scholar] [CrossRef]
- Huang, S.M.; Huang, S.P.; Wang, S.L.; Liu, P.Y. Importin a1 is involved in the nuclear localization of Zac1 and the induction of p21WAF1/CIP1 by Zac1. Biochem. J. 2007, 402, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Klocko, A.D.; Rountree, M.R.; Grisafi, P.L.; Hays, S.M.; Adhvaryu, K.K.; Selker, E.U. Neurospora importin a is required for normal heterochromatic formation and DNA methylation. PLoS Genet. 2015, 11, e1005083. [Google Scholar] [CrossRef]
- Boeuf, A.; Schnell, G.; Bernard, Q.; Kern, A.; Westermann, B.; Ehret-Sabatier, L.; Grillon, A.; Schramm, F.; Jaulhac, B.; Boulanger, N. Dissociating effect of salivary gland extract from Ixodes ricinus on human fibroblasts: Potential impact on Borrelia transmission. Ticks Tick Borne Dis. 2019, 10, 433–441. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Alberdi, P.; Ayllón, N.; Valdés, J.J.; Pierce, R.; Villar, M.; de la Fuente, J. Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 2016, 11, 303–319. [Google Scholar] [CrossRef]
- Caignard, G.; Guerbois, M.; Labernardiere, J.L.; Jacob, Y.; Jones, L.M.; Wild, F.; Tangy, F.; Vidalain, P.O.; Infectious Mapping Project I-MAP. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 2007, 368, 351–362. [Google Scholar] [CrossRef]
- Lemasson, M.; Caignard, G.; Unterfinger, Y.; Attoui, H.; Bell-Sakyi, L.; Hirchaud, E.; Moutailler, S.; Johnson, N.; Vitour, D.; Richardson, J.; et al. Exploration of binary protein–protein interactions between tick-borne flaviviruses and Ixodes ricinus. Parasit. Vectors 2021, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Ayllón, N.; Villar, M.; Busby, A.T.; Kocan, K.M.; Blouin, E.F.; Bonzón-Kulichenko, E.; Galindo, R.C.; Mangold, A.J.; Alberdi, P.; Pérez de la Lastra, J.M.; et al. Anaplasma phagocytophilum inhibits apoptosis and promotes cytoskeleton rearrangement for infection of tick cells. Infect. Immun. 2013, 81, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Moreno-Cid, J.A.; Domingos, A.; Canales, M.; Díez-Delgado, I.; Pérez de la Lastra, J.M.; Sánchez, E.; Merino, O.; Zavala, R.L.; Ayllón, N.; et al. Bacterial membranes enhance the immunogenicity and protective capacity of the surface exposed tick Subolesin-Anaplasma marginale MSP1a chimeric antigen. Ticks Tick Borne Dis. 2015, 6, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Krull, C.; Böhme, B.; Clausen, P.H.; Nijhof, A.M. Optimization of an artificial tick feeding assay for Dermacentor reticulatus. Parasit. Vectors 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; Blouin, E.; de la Fuente, J. RNA interference in ticks. J. Vis. Exp. 2011, 20, 2474. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Morel, O.; Noël, V.; Buysse, M.; Binetruy, F.; Lancelot, R.; Loire, E.; Ménard, C.; Bouchez, O.; Vavre, F.; et al. Tick-bacteria mutualism depends on B vitamin synthesis pathways. Curr. Biol. 2018, 28, 1896–1902. [Google Scholar] [CrossRef]
- Contreras, M.; Alberdi, P.; Mateos-Hernández, L.; Fernández de Mera, I.G.; García-Pérez, A.L.; Vancová, M.; Villar, M.; Ayllón, N.; Cabezas-Cruz, A.; Valdés, J.J.; et al. Anaplasma phagocytophilum MSP4 and HSP70 Proteins Are Involved in Interactions with Host Cells during Pathogen Infection. Front. Cell. Infect. Microbiol. 2017, 7, 307. [Google Scholar] [CrossRef]
- Antunes, S.; Merino, O.; Mosqueda, J.; Moreno-Cid, J.A.; Bell-Sakyi, L.; Fragkoudis, R.; Weisheit, S.; Pérez de la Lastra, J.M.; Alberdi, P.; Domingos, A.; et al. Tick capillary feeding for the study of proteins involved in tick-pathogen interactions as potential antigens for the control of tick infestation and pathogen infection. Parasit. Vectors 2014, 7, 42. [Google Scholar] [CrossRef]
- Bao, H.; Chen, H.; Zhu, X.; Zhang, M.; Yao, G.; Yu, Y.; Qin, W.; Zeng, C.; Zen, K.; Liu, Z. MiR-223 downregulation promotes glomerular endothelial cell activation by upregulating importin a4 and a5 in IgA nephropathy. Kidney Int. 2014, 85, 624–635. [Google Scholar] [CrossRef]
- de la Fuente, J.; Villar, M.; Cabezas-Cruz, A.; Estrada-Peña, A.; Ayllón, N.; Alberdi, P. Tick–host–pathogen interactions: Conflict and cooperation. PLoS Pathog. 2016, 12, e1005488. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Jerónimo, S.; Alberdi, P.; Villar, M.; Cabezas-Cruz, A.; Prados, P.J.E.; Mateos-Hernández, L.; de la Fuente, J. Anaplasma phagocytophilum modifies tick cell microRNA expression and upregulates isc-mir-79 to facilitate infection by targeting the Roundabout protein 2 pathway. Sci. Rep. 2019, 9, 9073. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artigas-Jerónimo, S.; Villar, M.; Cabezas-Cruz, A.; Caignard, G.; Vitour, D.; Richardson, J.; Lacour, S.; Attoui, H.; Bell-Sakyi, L.; Allain, E.; et al. Tick Importin-α Is Implicated in the Interactome and Regulome of the Cofactor Subolesin. Pathogens 2021, 10, 457. https://doi.org/10.3390/pathogens10040457
Artigas-Jerónimo S, Villar M, Cabezas-Cruz A, Caignard G, Vitour D, Richardson J, Lacour S, Attoui H, Bell-Sakyi L, Allain E, et al. Tick Importin-α Is Implicated in the Interactome and Regulome of the Cofactor Subolesin. Pathogens. 2021; 10(4):457. https://doi.org/10.3390/pathogens10040457
Chicago/Turabian StyleArtigas-Jerónimo, Sara, Margarita Villar, Alejandro Cabezas-Cruz, Grégory Caignard, Damien Vitour, Jennifer Richardson, Sandrine Lacour, Houssam Attoui, Lesley Bell-Sakyi, Eleonore Allain, and et al. 2021. "Tick Importin-α Is Implicated in the Interactome and Regulome of the Cofactor Subolesin" Pathogens 10, no. 4: 457. https://doi.org/10.3390/pathogens10040457
APA StyleArtigas-Jerónimo, S., Villar, M., Cabezas-Cruz, A., Caignard, G., Vitour, D., Richardson, J., Lacour, S., Attoui, H., Bell-Sakyi, L., Allain, E., Nijhof, A. M., Militzer, N., Pinecki Socias, S., & de la Fuente, J. (2021). Tick Importin-α Is Implicated in the Interactome and Regulome of the Cofactor Subolesin. Pathogens, 10(4), 457. https://doi.org/10.3390/pathogens10040457









