Exploratory Space–Time Analyses of Reported Lyme Borreliosis Cases in France, 2016–2019
Abstract
1. Introduction
2. Results
2.1. Descriptive Analyses
2.2. Space–Time K-Function Analysis
2.3. Cluster Analysis
2.3.1. Spatial Clustering Detection
2.3.2. Space–Time Clustering Detection
3. Discussion
4. Materials and Methods
4.1. Data Collation and Management
4.2. Descriptive Analyses
4.3. Space–Time K-Function Analysis
4.4. Cluster Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandekerckhove, O.; De Buck, E.; Van Wijngaerden, E. Lyme disease in Western Europe: An emerging problem? A systematic review. Acta Clin. Belg. Int. J. Clin. Lab. Med. 2019, 1–9. [Google Scholar] [CrossRef]
- Piesman, J.; Gern, L. Lyme Borreliosis in Europe and North America; Cambridge University Press: Cambridge, UK, 2008; pp. 220–252. [Google Scholar] [CrossRef]
- Sajanti, E.; Virtanen, M.; Helve, O.; Kuusi, M.; Lyytikäinen, O.; Hytönen, J.; Sane, J. Lyme borreliosis in Finland, 1995–2014. Emerg. Infect. Dis. 2017, 23, 1282–1288. [Google Scholar] [CrossRef]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence in Western Europe. J. Public Health 2016, 39, fdw017. [Google Scholar] [CrossRef]
- Hofhuis, A.; Bennema, S.; Harms, M.; Van Vliet, A.J.H.; Takken, W.; Van Den Wijngaard, C.C.; Van Pelt, W. Decrease in tick bite consultations and stabilization of early Lyme borreliosis in the Netherlands in 2014 after 15 years of continuous increase. BMC Public Health 2016, 16, 425. [Google Scholar] [CrossRef]
- Tulloch, J.S.P.; Decraene, V.; Christley, R.M.; Radford, A.D.; Warner, J.C.; Vivancos, R. Characteristics and patient pathways of Lyme disease patients: A retrospective analysis of hospital episode data in England and Wales (1998–2015). BMC Public Health 2019, 19, 931. [Google Scholar] [CrossRef]
- Fournier, L.; Roussel, V.; Couturier, E.; Jaulhac, B.; Goronflot, T.; Septfons, A.; De Martino, S.; Guerreiro, S.; Launay, T.; De Valk, H.; et al. Epidemiology of Lyme Borreliosis in general practice in France, 2009–2016. Bull. Épidémiologique Hebd. 2018, 19–20, 383–388. [Google Scholar]
- Rizzoli, A.; Hauffe, H.C.; Carpi, G.; Vourc’h, G.I.; Neteler, M.; Rosà, R. Lyme borreliosis in Europe. Eurosurveillance 2011, 16, 19906. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- van den Wijngaard, C.C.; Hofhuis, A.; Simões, M.; Rood, E.; van Pelt, W.; Zeller, H.; Van Bortel, W. Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Eurosurveillance 2017, 22, 30569. [Google Scholar] [CrossRef]
- Smith, R.; Takkinen, J.; Eurosurveillance Editorial Team. Lyme borreliosis: Europe-wide coordinated surveillance and action needed? Wkly. Releases 2006, 11, 2977. [Google Scholar] [CrossRef]
- Li, S.; Gilbert, L.; Vanwambeke, S.O.; Yu, J.; Purse, B.V.; Harrison, P.A. Lyme Disease Risks in Europe under Multiple Uncertain Drivers of Change. Environ. Health Perspect. 2019, 127, 67010. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick. Borne. Dis. 2020, 11, 101489. [Google Scholar] [CrossRef]
- Kjær, L.J.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, Å.K.; Korslund, L.; Kjelland, V.; Slettan, A.; Stuen, S.; et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Eurosurveillance 2019, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bregnard, C.; Rais, O.; Voordouw, M.J. Climate and tree seed production predict the abundance of the European Lyme disease vector over a 15-year period. Parasites Vectors 2020, 13, 408. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, A.; Bertolotti, L.; Gern, L.; Gray, J. Ecology of Borrelia burgdorferi sensu lato in Europe: Transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol. Rev. 2012, 36, 837–861. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Ortega, C.; Sánchez, N.; DeSimone, L.; Sudre, B.; Suk, J.E.; Semenza, J.C. Correlation of Borrelia burgdorferi Sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the Western Palearctic. Appl. Environ. Microbiol. 2011, 77, 3838–3845. [Google Scholar] [CrossRef]
- Flahault, A.; Blanchon, T.; Dorléans, Y.; Toubiana, L.; Vibert, J.F.; Valleron, A.J. Virtual surveillance of communicable diseases: A 20-year experience in France. Stat. Methods Med. Res. 2006, 15, 413–421. [Google Scholar] [CrossRef]
- Septfons, A.; Goronflot, T.; Jaulhac, B.; Roussel, V.; De Martino, S.; Guerreiro, S.; Launay, T.; Fournier, L.; De Valk, H.; Figoni, J.; et al. Epidemiology of lyme borreliosis through two surveillance systems: The national sentinelles GP network and the national hospital discharge database, France, 2005 to 2016. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Institut Pierre Louis d’Épidémiologie et de Santé Publique (IPLESP), UMR-S 1136 (Unité Mixte de Recherche en Santé). Bilan Annuel 2018; Réseau Sentinelles: Paris, France, 2019; pp. 14, 104–110. Available online: https://www.sentiweb.fr/document/4633 (accessed on 6 April 2021).
- Andreasen, A.M.; Dehlendorff, P.B.; Knudtzen, F.C.; Bødker, R.; Kjær, L.J.; Skarphedinsson, S. Spatial and temporal patterns of Lyme Neuroborreliosis on Funen, Denmark from 1995–2014. Sci. Rep. 2020, 10, 7796. [Google Scholar] [CrossRef]
- Kulldorff, M. Spatial Scan Statistics: Models, Calculations, and Applications. In Scan Statistics and Applications; Birkhäuser Boston: Cambridge, MA, USA, 1999; pp. 303–322. [Google Scholar]
- Sanchez, J.; Stryhn, H.; Flensburg, M.; Ersbøll, A.K.; Dohoo, I. Temporal and spatial analysis of the 1999 outbreak of acute clinical infectious bursal disease in broiler flocks in Denmark. Prev. Vet. Med. 2005, 71, 209–223. [Google Scholar] [CrossRef]
- Kulldorff, M.; Athas, W.F.; Feuer, E.J.; Miller, B.A.; Key, C.R. Evaluating cluster alarms: A space-time scan statistic and brain cancer in Los Alamos, New Mexico. Am. J. Public Health 1998, 88, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kolivras, K.N.; Hong, Y.; Duan, Y.; Seukep, S.E.; Prisley, S.P.; Campbell, J.B.; Gaines, D.N. Spatial and temporal emergence pattern of lyme disease in Virginia. Am. J. Trop. Med. Hyg. 2014, 91, 1166–1172. [Google Scholar] [CrossRef]
- French, N.P.; McCarthy, H.E.; Diggle, P.J.; Proudman, C.J. Clustering of equine grass sickness cases in the United Kingdom: A study considering the effect of position-dependent reporting on the space-time K-function. Epidemiol. Infect. 2005, 133, 343–348. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 December 2019).
- SaTScan—Software for the Spatial, Temporal, and Space-Time Scan Statistics. Available online: https://www.satscan.org/ (accessed on 1 September 2020).
- Métras, R.; Porphyre, T.; Pfeiffer, D.U.; Kemp, A.; Thompson, P.N.; Collins, L.M.; White, R.G. Exploratory Space-Time Analyses of Rift Valley Fever in South Africa in 2008–2011. PLoS Negl. Trop. Dis. 2012, 6, e1808. [Google Scholar] [CrossRef]
- Picado, A.; Speybroeck, N.; Kivaria, F.; Mosha, R.M.; Sumaye, R.D.; Casal, J.; Berkvens, D. Foot-and-mouth disease in Tanzania from 2001 to 2006. Transbound. Emerg. Dis. 2011, 58, 44–52. [Google Scholar] [CrossRef]
- Guinat, C.; Nicolas, G.; Vergne, T.; Bronner, A.; Durand, B.; Courcoul, A.; Gilbert, M.; Guérin, J.-L.; Paul, M.C. Spatio-temporal patterns of highly pathogenic avian influenza virus subtype H5N8 spread, France, 2016 to 2017. Eurosurveillance 2018, 23, 1700791. [Google Scholar] [CrossRef] [PubMed]
- Huegli, D.; Hu, C.M.; Humair, P.F.; Wilske, B.; Gern, L. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J. Clin. Microbiol. 2002, 40, 4735–4737. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Coburn, J.; Glickstein, L. The emergence of Lyme disease. J. Clin. Investig. 2004, 113, 1093–1101. [Google Scholar] [CrossRef]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Institut Pierre Louis d’Épidémiologie et de Santé Publique (IPLESP), UMR-S 1136 (Unité Mixte de Recherche en Santé). Bilan Annuel 2019; Réseau Sentinelles: Paris, France, 2020; pp. 26–27. Available online: https://www.sentiweb.fr/document/5017 (accessed on 6 April 2021).
- Bennet, L.; Halling, A.; Berglund, J. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2006, 25, 426–432. [Google Scholar] [CrossRef]
- Enkelmann, J.; Böhmer, M.; Fingerle, V.; Siffczyk, C.; Werber, D.; Littmann, M.; Merbecks, S.S.; Helmeke, C.; Schroeder, S.; Hell, S.; et al. Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci. Rep. 2018, 8, 14976. [Google Scholar] [CrossRef]
- Ceballos, L.A.; Pintore, M.D.; Tomassone, L.; Pautasso, A.; Bisanzio, D.; Mignone, W.; Casalone, C.; Mannelli, A. Habitat and occurrence of ixodid ticks in the Liguria region, northwest Italy. Exp. Appl. Acarol. 2014, 64, 121–135. [Google Scholar] [CrossRef]
- Alexander, N.S.; Morley, D.; Medlock, J.; Searle, K.; Wint, W. A First Attempt at Modelling Roe Deer (Capreolus capreolus) Distributions Over Europe. Open Health Data 2014, 2, e2. [Google Scholar] [CrossRef]
- Citique. Available online: https://www.citique.fr/distribution_signalements_2019/ (accessed on 30 March 2021).
- McCoy, K.; Boulanger, N. Tiques et Maladies à Tiques; IRD Éditions: Marseille, France, 2015; pp. 89–98. [Google Scholar] [CrossRef]
- Marchant, A.; Le Coupanec, A.; Joly, C.; Perthame, E.; Sertour, N.; Garnier, M.; Godard, V.; Ferquel, E.; Choumet, V. Infection of Ixodes ricinus by Borrelia burgdorferi sensu lato in peri-urban forests of France. PLoS ONE 2017, 12, e0183543. [Google Scholar] [CrossRef] [PubMed]
- Géoservices IGN. Available online: https://geoservices.ign.fr/documentation/diffusion/telechargement-donnees-libres.html (accessed on 6 April 2021).
- Institut National de la Statistique et des Etudes Economiques (Insee). Available online: https://www.insee.fr/fr/statistiques/4265511 (accessed on 6 April 2021).
- Diggle, P.J. Second-order analysis of space-time clustering. Stat. Methods Med. Res. 1995, 4, 124–136. [Google Scholar] [CrossRef]
- Besag, J.; Diggle, P.J. Simple Monte Carlo Tests for Spatial Pattern. J. R. Stat. Soc. Ser. C Appl. Stat. 1977, 26, 327–333. [Google Scholar] [CrossRef]
- Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Linton, S.L.; Jennings, J.M.; Latkin, C.A.; Gomez, M.B.; Mehta, S.H. Application of Space-Time Scan Statistics to Describe Geographic and Temporal Clustering of Visible Drug Activity. J. Urban Health 2014, 91, 940–956. [Google Scholar] [CrossRef]
- Han, J.; Zhu, L.; Kulldorff, M.; Hostovich, S.; Stinchcomb, D.G.; Tatalovich, Z.; Lewis, D.R.; Feuer, E.J. Using Gini coefficient to determining optimal cluster reporting sizes for spatial scan statistics. Int. J. Health Geogr. 2016, 15, 27. [Google Scholar] [CrossRef] [PubMed]
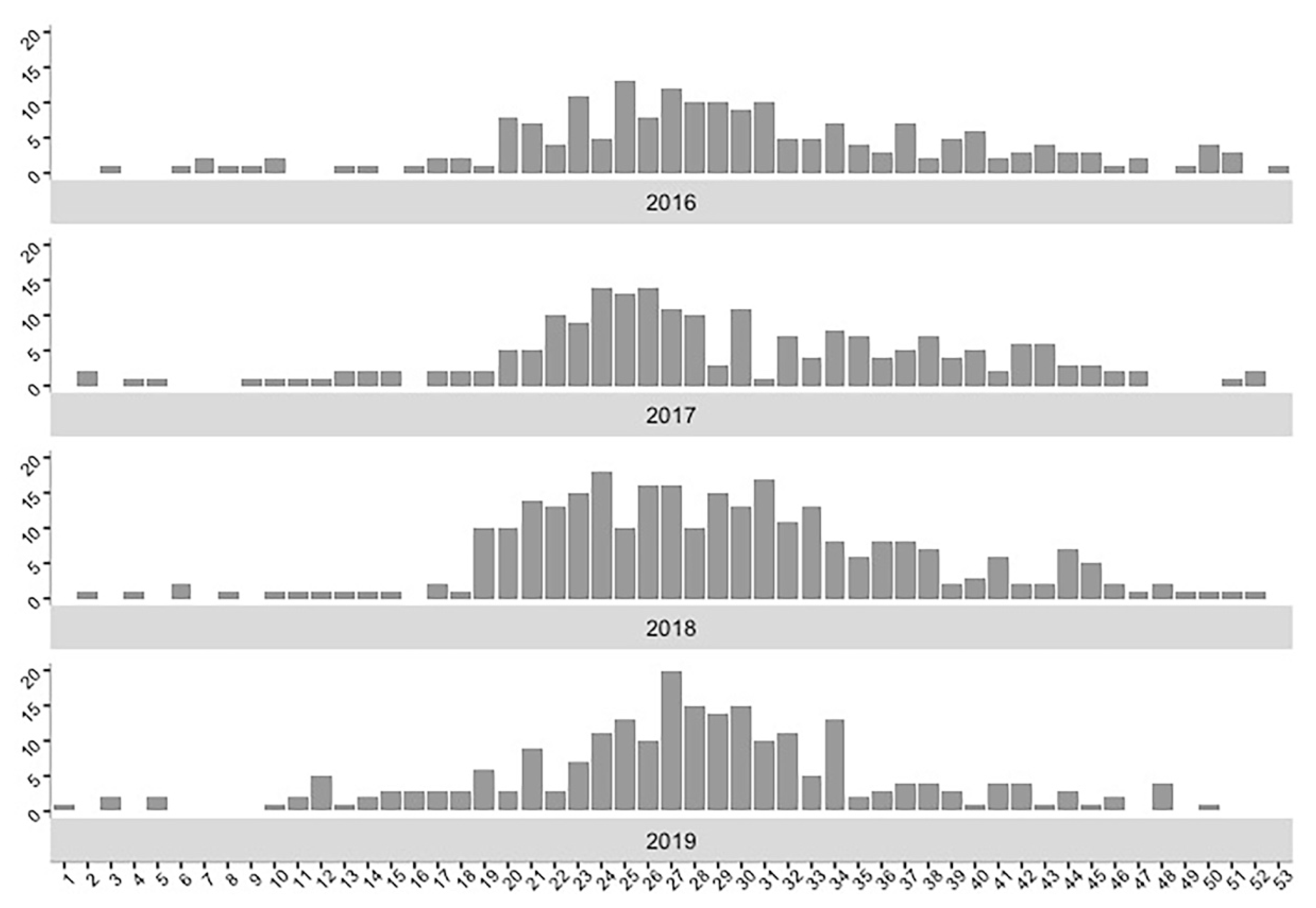
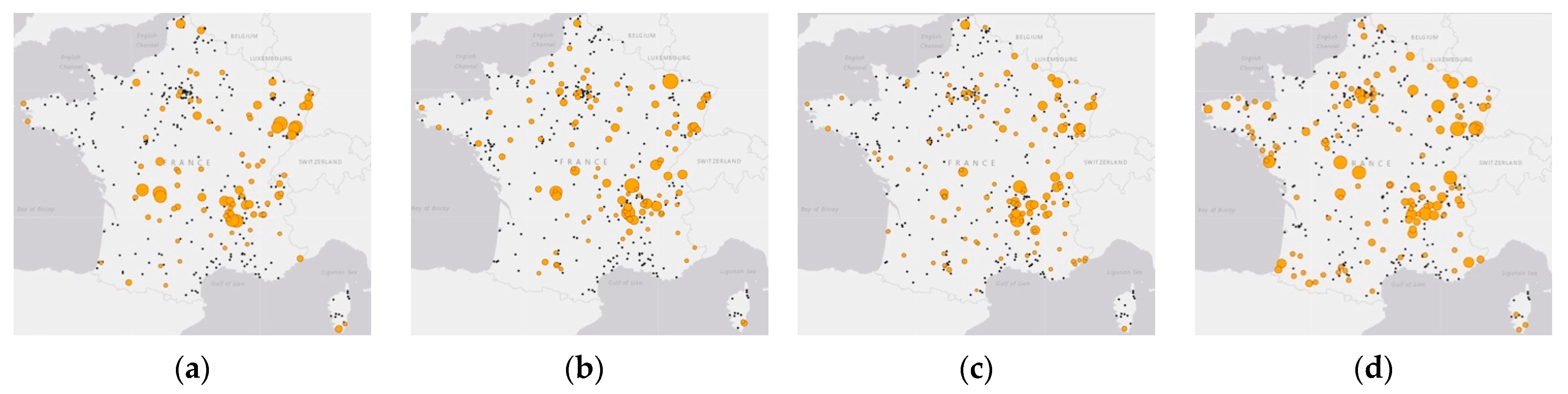

| Year | Time (90 Days) | Space (50 km) | Upper Time Window | Upper Space Window | p-Value | |
|---|---|---|---|---|---|---|
| 2016 | 7 days | 2 km | >2 | 7 days | 16 km | 0.71 |
| >1 | 7 days | 40 km | ||||
| 2017 | 7 days | 2 km | >2 | na | na | 0.18 |
| >1 | 7 days | 20 km | ||||
| 2018 | 7 days | 2 km | >2 | 7 days | 16 km | 0.02 1 |
| >1 | 7 days | 34 km | ||||
| 2019 | 7 days | 2 km | >2 | 7 days | 22 km | 0.12 |
| >1 | 7 days | 40 km |
| Year/Cluster Number (Region) | RR 1 | Radius (km) | No. of Communes | Population at Risk | Observed No. of LB Cases | Expected No. of LB Cases | p-Value |
|---|---|---|---|---|---|---|---|
| 2016 | |||||||
| 1. Most likely cluster (NA) 2 | 9.1 | 69.0 | 7 | 7160 | 25 | 3.12 | <0.001 |
| 2. Secondary cluster (GE) | 5.7 | 79.3 | 14 | 17,649 | 37 | 7.69 | <0.001 |
| 3. Secondary cluster (ARA) | 12.8 | 14.5 | 2 | 3324 | 17 | 1.45 | <0.001 |
| 2017 | |||||||
| 4. Most likely cluster (ARA) | 3.3 | 73.9 | 39 | 49,197 | 57 | 21.43 | <0.001 |
| 5. Secondary cluster (GE) | 20.8 | 0 | 1 | 1160 | 10 | 0.51 | <0.001 |
| 6. Secondary cluster (NA-CVL) | 6.4 | 69.2 | 6 | 6585 | 17 | 2.87 | <0.001 |
| 7. Secondary cluster (BFC-GE) | 3.2 | 97.3 | 20 | 23,811 | 30 | 10.37 | <0.001 |
| 20183 | |||||||
| 8. Secondary cluster (ARA) | 3.5 | 88.3 | 48 | 60,026 | 91 | 33.48 | <0.001 |
| 9. Secondary cluster (GE) | 3.2 | 87.6 | 26 | 29,125 | 46 | 16.24 | <0.001 |
| 10. Secondary cluster (BFC) | 5.2 | 48.5 | 6 | 7275 | 20 | 4.06 | <0.001 |
| 11. Secondary cluster (NA-CVL) | 3.6 | 69.9 | 7 | 8347 | 16 | 4.66 | 0.02 |
| 2019 | |||||||
| 12. Most likely cluster (GE) | 4.0 | 92.5 | 28 | 31,388 | 41 | 11.97 | <0.001 |
| 13. Secondary cluster (ARA) | 4.2 | 62.6 | 14 | 16,137 | 24 | 6.16 | <0.001 |
| 14. Secondary cluster (NA-CVL) | 5.0 | 95.9 | 7 | 9553 | 17 | 3.64 | <0.001 |
| 15. Secondary cluster (ARA) | 10.7 | 0 | 1 | 1508 | 6 | 0.58 | 0.02 |
| 16. Secondary cluster (PACA) | 9.4 | 42.2 | 2 | 1720 | 6 | 0.66 | 0.045 |
| Year/Cluster Number (Region) | RR 2 | Radius (km) | Estimated Time Frame (Days) | Population at Risk (No. of Communes) | Observed No. of LB Cases | Expected No. of LB Cases | p-Value |
|---|---|---|---|---|---|---|---|
| 2016 | |||||||
| 17. Most likely cluster 1 (ARA-NA) | 10.1 | 96.5 | 7 July–10 August (34) | 13,566 (7) | 11 | 1.15 | 0.004 |
| 2017 | |||||||
| 18. Most likely cluster (ARA-BFC) | 7.9 | 96.8 | 30 May–30 Jun (32) | 22,643 (7) | 12 | 1.60 | 0.01 |
| 19. Secondary cluster (GE) | 17.1 | 77.8 | 28 June–10 July (14) | 14,742 (6) | 7 | 0.42 | 0.02 |
| 2018 | |||||||
| 20. Most likely cluster (ARA) | 3.6 | 96.0 | 28 May–25 August (89) | 106,123 (25) | 69 | 21.57 | <0.001 |
| 21. Secondary cluster (GE) | 3.6 | 92.6 | 21 May–14 August (85) | 43,935 (15) | 31 | 9.30 | 0.001 |
| 2019 | |||||||
| 22. Most likely cluster (GE) | 3.8 | 92.5 | 24 May–09 August (77) | 47,063 (15) | 29 | 8.46 | 0.002 |
| 23. Secondary cluster (ARA) | 3.4 | 97.7 | 5 June–29 August (84) | 47,350 (22) | 29 | 9.38 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Bonnet, C.; Figoni, J.; Septfons, A.; Métras, R. Exploratory Space–Time Analyses of Reported Lyme Borreliosis Cases in France, 2016–2019. Pathogens 2021, 10, 444. https://doi.org/10.3390/pathogens10040444
Fu W, Bonnet C, Figoni J, Septfons A, Métras R. Exploratory Space–Time Analyses of Reported Lyme Borreliosis Cases in France, 2016–2019. Pathogens. 2021; 10(4):444. https://doi.org/10.3390/pathogens10040444
Chicago/Turabian StyleFu, Wen, Camille Bonnet, Julie Figoni, Alexandra Septfons, and Raphaëlle Métras. 2021. "Exploratory Space–Time Analyses of Reported Lyme Borreliosis Cases in France, 2016–2019" Pathogens 10, no. 4: 444. https://doi.org/10.3390/pathogens10040444
APA StyleFu, W., Bonnet, C., Figoni, J., Septfons, A., & Métras, R. (2021). Exploratory Space–Time Analyses of Reported Lyme Borreliosis Cases in France, 2016–2019. Pathogens, 10(4), 444. https://doi.org/10.3390/pathogens10040444






