Impact of Subclinical Haemoproteus columbae Infection on Farmed Domestic Pigeons from Central Java (Yogyakarta), Indonesia, with Special Reference to Changes in the Hemogram
Abstract
1. Introduction
2. Results
2.1. Prevalence and Morphology of Haemosporidian Species
2.2. Molecular Characterization of Cytb Sequences, and Phylogenetic Analyses
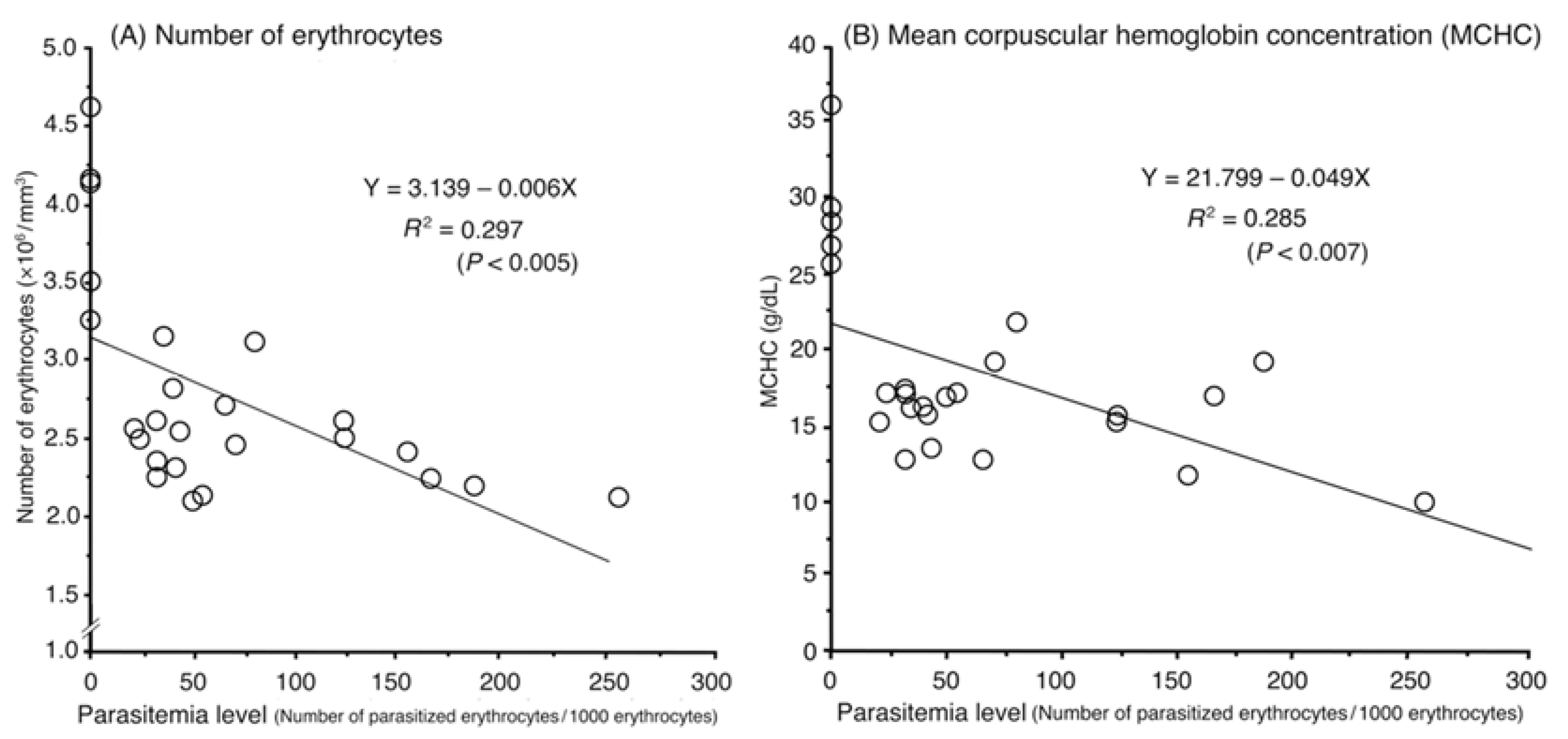
2.3. Hematological Analyses
3. Discussion
4. Materials and Methods
4.1. Blood Collection and Microscopic Examination
4.2. Hematological Analyses
4.3. DNA Extraction, Amplification and Sequencing
4.4. Phylogenetic Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bensch, S.; Pérez-Tris, J.; Waldenström, J.; Hellgren, O. Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: Multiple cases of cryptic speciation? Evolution 2004, 58, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporida; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenetics Evol. 2008, 47, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Bennett, G.F.; Peirce, M.A. The haemoproteid parasites of the pigeons and doves (family Columbidae). J. Nat. Hist. 1990, 24, 311–325. [Google Scholar] [CrossRef]
- Atkinson, C.T. Haemoproteus. In Parasitic Diseases of Wild Birds; Wiley: Hoboken, NJ, USA, 2009; pp. 11–34. [Google Scholar]
- Bennett, G.F.; Garnham, P.C.C.; Fallis, A.M. On the status of the genera Leucocytozoon Ziemann, 1893 and Haemoproteus Kruse, 1890 (Haemosporidiida: Leucocytozoidae and Haemoproteidae). Can. J. Zool. 1965, 43, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Levin, I.I.; Valkiūnas, G.; Santiago-Alarcon, D.; Cruz, L.L.; Iezhova, T.A.; O’Brien, S.L.; Hailer, F.; Dearborn, D.; Schreiber, E.; Fleischer, R.C.; et al. Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos Pelecaniform birds: Evidence from molecular and morphological studies, with a description of Haemoproteus iwa. Int. J. Parasitol. 2011, 41, 1019–1027. [Google Scholar] [CrossRef]
- Križanauskienė, A.; Iezhova, T.A.; Sehgal, R.N.M.; Carlson, J.S.; Palinauskas, V.; Bensch, S.; Valkiūnas, G. Molecular characterization of Haemoproteus sacharovi (Haemosporida, Haemoproteidae), a common parasite of columbiform birds, with remarks on classification of haemoproteids of doves and pigeons. Zootaxa 2013, 3616, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.; Zehtindjiev, P.; Bensch, S.; Ilieva, M.; Iezhova, T.; Valkiūnas, G. Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Syst. Parasitol. 2014, 87, 135–151. [Google Scholar] [CrossRef]
- González, A.D.; Lotta, I.A.; García, L.F.; Moncada, L.I.; Matta, N.E. Avian haemosporidians from Neotropical highlands: Evidence from morphological and molecular data. Parasitol. Int. 2015, 64, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Nourani, L.; Aliabadian, M.; Mirshamsi, O.; Djadid, N.D. Molecular detection and genetic diversity of avian haemosporidian parasites in Iran. PLoS ONE 2018, 13, e0206638. [Google Scholar] [CrossRef] [PubMed]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Weissenböck, H. The nuclear 18S ribosomal DNAs of avian haemosporidian parasites. Malar. J. 2019, 18, 1–19. [Google Scholar] [CrossRef]
- Aragão, H.B. Über den Entwicklungsgang und die Übertragung von Haemoproteus columbae: Vorläufige Mitteilung. Arch. Protistenkd. 1908, 12, 154–167. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Mohammed, A.H.H. Schizogony in Haemoproteus columbae Kruse. J. Protozool. 1977, 24, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Mohammed, A.-H.H. Haemoproteus columbae: Course of infection, relapse and immunity to reinfection in the pigeon. Parasitol. Res. 1978, 57, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.T.; van Riper, C. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In Bird–Parasite Interactions: Ecology, Evolution, and Behaviour; Loye, J.E., Zuk, M., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 19–48. [Google Scholar]
- Atkinson, C.T.; Forrester, D.J.; Greiner, E.C. Pathogenicity of Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. J. Parasitol. 1988, 74, 228. [Google Scholar] [CrossRef]
- Ferrell, S.T.; Snowden, K.; Marlar, A.B.; Garner, M.; Lung, N.P. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J. Zoo Wildl. Med. 2007, 38, 309–316. [Google Scholar] [CrossRef]
- Donovan, T.A.; Schrenzel, M.; Tucker, T.A.; Pessier, A.P.; Stalis, I.H. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: Eleven cases. J. Vet. Diagn. Investig. 2008, 20, 304–313. [Google Scholar] [CrossRef]
- Olias, P.; Wegelin, M.; Zenker, W.; Freter, S.; Gruber, A.D.; Klopfleisch, R. Avian malaria deaths in parrots, Europe. Emerg. Infect. Dis. 2011, 17, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Haemosporidian vector research: Marriage of molecular and microscopical approaches is essential. Mol. Ecol. 2011, 20, 3084–3086. [Google Scholar] [CrossRef] [PubMed]
- Cannell, B.L.; Krasnec, K.V.; Campbell, K.; Jones, H.I.; Miller, R.D.; Stephens, N. The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild little penguins (Eudyptula minor). Vet. Parasitol. 2013, 197, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ilgūnas, M.; Chagas, C.R.F.; Bukauskaitė, D.; Bernotienė, R.; Iezhova, T.; Valkiūnas, G. The life-cycle of the avian haemosporidian parasite Haemoproteus majoris, with emphasis on the exoerythrocytic and sporogonic development. Parasites Vectors 2019, 12, 516. [Google Scholar] [CrossRef]
- Nebel, C.; Harl, J.; Pajot, A.; Weissenböck, H.; Amar, A.; Sumasgutner, P. High prevalence and genetic diversity of Haemoproteus columbae (Haemosporida: Haemoproteidae) in feral pigeons Columba livia in Cape Town, South Africa. Parasitol. Res. 2019, 119, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G. Squab raising. PrimeFacts 2007, 601, 1–9. [Google Scholar] [CrossRef]
- Darwati, S.; Martojo, H.; Sumantri, C.; Sihombing, D.T.H.; Mardiastuti, A. Productivity, repeatability of productive and reproductive traits of local pigeon. J. Indones. Trop. Anim. Agric. 2010, 35, 268–274. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T. Parasitic protozoa of the blood of birds in the USSR. 6. Haemoproteidae of Columbiformes and Coraciiformes. Ekologiya 1990, 2, 86–103. [Google Scholar]
- Ihedioha, J.I.; Anyogu, D.C.; Chibuezeoke, K.J. Haematological profile of the domestic pigeon (Columba livia domestica) in Nsukka Agroecological Zone, Eungu State, Nigeria. Anim. Res. Int. 2016, 13, 2368–2377. [Google Scholar]
- Earlé, R.A.; Little, R.M. Haematozoa of feral rock doves and rock pigeons in mixed flocks. S. Afr. J. Wildl. Res. 1993, 23, 98. [Google Scholar]
- Foronda, P.; Valladares, B.; Rivera-Medina, J.; Figueruelo, E.; Abreu, N.; Casanova, J. Parasites of Columba livia (Aves: Columbiformes) in Tenerife (Canary Islands) and their role in the conservation biology of the laurel pigeons. Parasite 2004, 11, 311–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hussein, N.M.; Abdelrahim, E.A. Haemoproteus columbae infection and its histopathological effects on pigeons in Qena Governorate, Egypt. OOSR J. Pharm. Biol. Sci. 2016, 11, 79–90. [Google Scholar] [CrossRef]
- Chagas, C.R.; de Oliveira Guimarães, L.; Monteiro, E.F.; Valkiūnas, G.; Katayama, M.V.; Santos, S.V.; Guida, F.J.V.; Simões, R.F.; Kirchgatter, K. Hemosporidian parasites of free-living birds in the São Paulo Zoo, Brazil. Parasitol. Res. 2016, 115, 1443–1452. [Google Scholar] [CrossRef]
- Cepeda, A.S.; Lotta-Arévalo, I.A.; Pinto-Osorio, D.F.; Macías-Zacipa, J.; Valkiūnas, G.; Barato, P.; Matta, N.E. Experimental characterization of the complete life cycle of Haemoproteus columbae, with a description of a natural host-parasite system used to study this infection. Int. J. Parasitol. 2019, 49, 975–984. [Google Scholar] [CrossRef]
- Coatney, G.R. Relapse and associated phenomena in the Haemoproteus infection of the pigeon. Am. J. Epidemiol. 1933, 18, 133–160. [Google Scholar] [CrossRef]
- Lee-Cruz, L.; Cunningham, A.A.; Martínez, P.; Cruz, M.; Goodman, S.J.; Hamer, K.C. Prevalence of Haemoproteus sp. in Galápagos blue-footed boobies: Effects on health and reproduction. Parasitol. Open 2016, 2, e1. [Google Scholar] [CrossRef]
- Sorci, G.; Møller, A.P. Comparative evidence for a positive correlation between haematozoan prevalence and mortality in waterfowl. J. Evol. Biol. 1997, 10, 731–741. [Google Scholar] [CrossRef]
- Merino, S.; Moreno, J.; Sanz, J.J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. R. Soc. B Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; de Lope, F.; Navarro, C.; Moller, A.P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Z̆ic̆kus, T.; Shapoval, A.P.; Iezhova, T.A. Effect of Haemoproteus belopolskyi (Haemosporida: Haemoproteidae) on body mass of the blackcap Sylvia atricapilla. J. Parasitol. 2006, 92, 1123–1125. [Google Scholar] [CrossRef]
- Møller, A.P.; Nielsen, J.T. Malaria and risk of predation: A comparative study of birds. Ecology 2007, 88, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.S.; Atwill, E.R.; Hunter, A. Farm and management variables linked to fecal shedding of Campylobacter and Salmonella in commercial squab production. Poult. Sci. 2001, 80, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Garvin, M.C.; Homer, B.L.; Greiner, E.C. Pathogenicity of Haemoproteus danilewskyi, Kruse, 1890, in blue jays (Cyanocitta cristata). J. Wildl. Dis. 2003, 39, 161–169. [Google Scholar] [CrossRef]
- Waldenström, J.; Bensch, S.; Kiboi, S.; Hasselquist, D.; Ottosson, U. Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol. Ecol. 2002, 11, 1545–1554. [Google Scholar] [CrossRef]
- Beadell, J.S.; Gering, E.; Austin, J.; Dumbacher, J.P.; Peirce, M.A.; Pratt, T.K.; Atkinson, C.T.; Fleischer, R.C. Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol. Ecol. 2004, 13, 3829–3844. [Google Scholar] [CrossRef]
- Reullier, J.; Pérez-Tris, J.; Bensch, S.; Secondi, J. Diversity, distribution and exchange of blood parasites meeting at an avian moving contact zone. Mol. Ecol. 2008, 15, 753–763. [Google Scholar] [CrossRef]
- Karamba, K.I.; Kawo, A.H.; Dabo, N.T.; Mukhtar, M.D. A survey of avian malaria parasite in Kano State, Northern Nigeria. Int. J. Biotechnol. Mol. Biol. Res. 2012, 3, 8–14. [Google Scholar] [CrossRef]
- Scaglione, F.E.; Pregel, P.; Cannizzo, F.T.; Pérez-Rodríguez, A.D.; Ferroglio, E.; Bollo, E. Prevalence of new and known species of haemoparasites in feral pigeons in northwest Italy. Malar. J. 2015, 14, 99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godfrey, R.D.; Fedynich, A.M.; Pence, D.B. Quantification of the Hematozoa in blood smears. J. Wildl. Dis. 1987, 23, 558–565. [Google Scholar] [CrossRef]
- Benjamin, M.M. Outline of Veterinary Pathology, 3rd ed.; Kalyani Publishers: New Delhi, India, 1985. [Google Scholar]
- Coles, E.H. Veterinary Clinical Pathology, 4th ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1986. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Örjan, Ö.; Hannson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. B Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A Simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef] [PubMed]
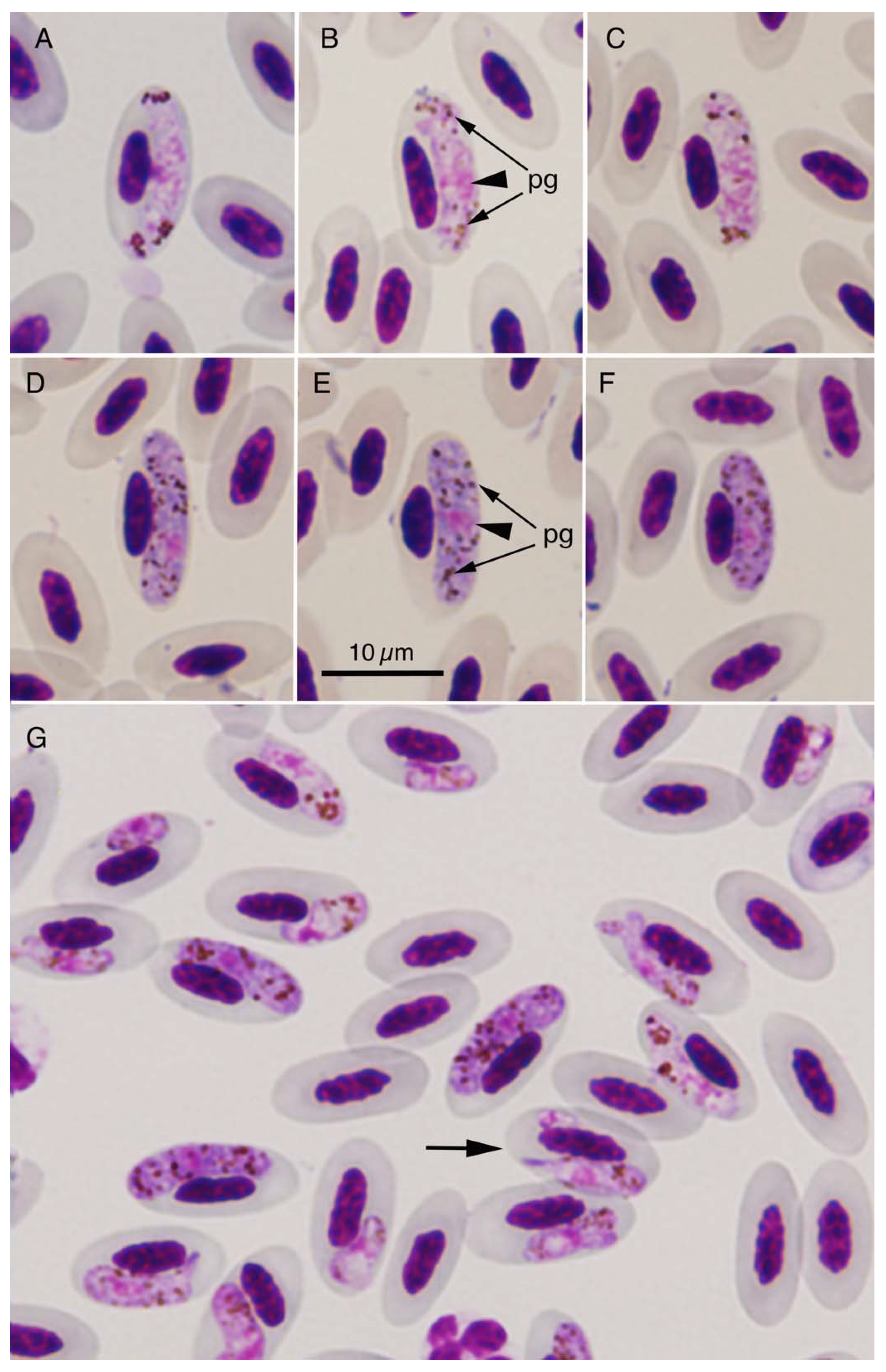
| Locality | Number of Pigeons Examined | Prevalence of Haemosporidians | Detected Haemosporidians | Parasitemia (%) 1 | Ratio of Immature Gametocytes: Mature Gametocytes |
|---|---|---|---|---|---|
| Mlati, Yogyakarta (7°43′53″ S, 110°19′52″ E) | 10 | 100% (10/10) | Haemoproteus columbae | 1.1–18.9 (3.9) | 0.67–4.39 (1.61): 1 |
| Ngemplak, Yogyakarta (7°41′57″ S, 110°26′42″ E) | 9 | 100% (9/9) | Haemoproteus columbae | 1.6–25.7 (5.6) | 0.80–15.00 (3.35): 1 |
| Kalasan, Yogyakarta (7°45′18″ S, 110°29′06″) | 8 | 75.0% (6/8) | Haemoproteus columbae | 2.5–6.7 (3.9) | 2.15–11.75 (4.10): 1 |
| Sedayu, Yogyakarta (7°48′49″ S, 110°16′17″) | 8 | 62.5% (5/8) | Haemoproteus columbae | 2.1–7.1 (3.0) | 0.47–15.00 (1.47): 1 |
| Feature | The Present Study (n = 30) | Valkiūnas (2005) 1 (n = 31) | |
|---|---|---|---|
| Uninfected erythrocyte 2 | |||
| Length | 12.3–14.9 (13.4) | 12.8–14.7 (13.7) | |
| Width | 6.6–7.7 (7.0) | 6.4–7.7 (7.0) | |
| Length of nucleus | 6.1–7.7 (6.7) | 6.2–7.7 (6.7) | |
| Width of nucleus | 2.3–3.4 (2.8) | 2.1–2.9 (2.4) | |
| Infected erythrocyte with a mature microgametocyte | |||
| Length | 12.2–16.6 (14.1) | 12.9–15.9 (14.4) | |
| Width | 6.1–7.8 (7.0) | 5.3–7.8 (6.9) | |
| Length of nucleus | 5.5–7.4 (6.4) | 5.5–7.4 (6.5) | |
| Width of nucleus | 2.3–3.5 (2.7) | 2.1–2.6 (2.3) | |
| Infected erythrocyte with a mature macrogametocyte | |||
| Length | 13.0–15.9 (14.5) | 13.8–16.0 (15.0) | |
| Width | 6.2–7.9 (7.2) | 6.0–7.9 (7.1) | |
| Length of nucleus | 4.9–7.5 (6.3) | 6.0–7.4 (6.5) | |
| Width of nucleus | 2.2–2.9 (2.6) | 1.7–2.7 (2.3) | |
| Mature microgametocyte | |||
| Length | 10.5–16.6 (13.7) | 11.6–15.5 (13.3) | |
| Width | 2.7–5.0 (3.6) | 2.6–4.3 (3.6) | |
| Length of nucleus | — 3 | — | |
| Width of nucleus | 2.6–4.5 (3.1) | 2.6–4.3 (3.6) | |
| Mature macrogametocyte | |||
| Length | 12.4–16.4 (14.6) | 13.4–16.7 (14.8) | |
| Width | 2.5–4.2 (3.5) | 3.0–4.2 (3.4) | |
| Length of nucleus | 2.1–3.2 (2.6) | 2.1–3.6 (2.9) | |
| Width of nucleus | 1.5–3.2 (2.3) | 1.5–3.4 (2.3) | |
| Locality | Number of Pigeons Examined Molecular–Genetically | Detected cytb Lineages of H. columbae (Number of Pigeons) | DDBJ/EMBL/GenBank Accession No. |
|---|---|---|---|
| Mlati, Yogyakarta | 4 | HAECOL1 (3) | LC605998–LC606001 |
| COLIV03 (1) | |||
| Ngemplak, Yogyakarta | 5 | HAECOL1 (5) | LC606002–LC606008 |
| CXNEA02 (2) 1 | |||
| Kalasan, Yogyakarta | 3 | HAECOL1 (2) | LC606009–LC606011 |
| COLIV03 (1) | |||
| Sedayu, Yogyakarta | 2 | HAECOL1 (1) | LC606012–LC606013 |
| COQUI05 (1) |
| Parameter | Unit | Infected Pigeons (n = 20) | Uninfected Pigeons (n = 5) | Normal Range Reported by Ihedioha et al. [29] 1 | Change 2 | Statistical Significance |
|---|---|---|---|---|---|---|
| Number of erythrocytes | 106/mm3 | 2.10–3.15 | 3.25–4.60 | 2.12–3.95 | ↓ | p < 0.001 |
| (2.49 ± 0.29) | (3.93 ± 0.55) | (3.34 ± 0.38) | ||||
| Number of leukocytes | 103/mm3 | 11.95–12.85 | 8.15–11.20 | 12.50–35.50 | ↑ | p < 0.007 |
| (12.44 ± 0.34) | (9.61 ± 1.22) | (23.36 ± 7.06) | ||||
| PCV | % | 34–50 | 45–48 | 32–55 | ↓ | p < 0.001 |
| (39.8 ± 4.6) | (46.2 ± 1.6) | (44.5 ± 4.7) | ||||
| MCV | fl | 134.10–218.60 | 97.83–138.46 | 109.82–169.09 | ↑ | p < 0.001 |
| (161.04 ± 18.44) | (119.24 ± 15.34) | (133.86 ± 19.37) | ||||
| Hemoglobin | g/dL | 4.5–10.9 | 11.5–17.2 | 7.76–16.00 | ↓ | p < 0.001 |
| (6.30 ± 1.28) | (13.54 ± 2.26) | (12.89 ± 1.55) | ||||
| MCH | pg | 18.60–35.16 | 26.30–41.45 | n.d. 3 | ↓ | p < 0.001 |
| (25.39 ± 4.11) | (34.79 ± 5.95) | |||||
| MCHC | g/dL | 10.00–21.80 | 25.56–35.83 | 23.57–33.75 | ↓ | p < 0.001 |
| (15.90 ± 2.72) | (29.22 ± 3.97) | (28.97 ± 2.59) | ||||
| Plasma protein | g/dL | 1.0–4.0 | 4.1–5.2 | n.d. 3 | ↓ | p < 0.001 |
| (2.4 ± 1.0) | (4.7 ± 0.5) | |||||
| Fibrinogen | g/dL | 0.2–1.0 | 0.1–0.4 | n.d. 3 | ↑ | p < 0.001 |
| (0.8 ± 0.3) | (0.2 ± 0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosyadi, I.; Salasia, S.I.O.; Argamjav, B.; Sato, H. Impact of Subclinical Haemoproteus columbae Infection on Farmed Domestic Pigeons from Central Java (Yogyakarta), Indonesia, with Special Reference to Changes in the Hemogram. Pathogens 2021, 10, 440. https://doi.org/10.3390/pathogens10040440
Rosyadi I, Salasia SIO, Argamjav B, Sato H. Impact of Subclinical Haemoproteus columbae Infection on Farmed Domestic Pigeons from Central Java (Yogyakarta), Indonesia, with Special Reference to Changes in the Hemogram. Pathogens. 2021; 10(4):440. https://doi.org/10.3390/pathogens10040440
Chicago/Turabian StyleRosyadi, Imron, Siti Isrina Oktavia Salasia, Bayanzul Argamjav, and Hiroshi Sato. 2021. "Impact of Subclinical Haemoproteus columbae Infection on Farmed Domestic Pigeons from Central Java (Yogyakarta), Indonesia, with Special Reference to Changes in the Hemogram" Pathogens 10, no. 4: 440. https://doi.org/10.3390/pathogens10040440
APA StyleRosyadi, I., Salasia, S. I. O., Argamjav, B., & Sato, H. (2021). Impact of Subclinical Haemoproteus columbae Infection on Farmed Domestic Pigeons from Central Java (Yogyakarta), Indonesia, with Special Reference to Changes in the Hemogram. Pathogens, 10(4), 440. https://doi.org/10.3390/pathogens10040440






