Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) first emerged after methicillin was introduced to combat penicillin resistance, and its prevalence in Canada has increased since the first MRSA outbreak in the early 1980s. We reviewed the existing literature on MRSA prevalence in Canada over time and in diverse populations across the country. MRSA prevalence increased steadily in the 1990s and 2000s and remains a public health concern in Canada, especially among vulnerable populations, such as rural, remote, and Indigenous communities. Antibiotic resistance patterns and risk factors for MRSA infection were also reported. All studies reported high susceptibility (>85%) to trimethoprim-sulfamethoxazole, with no significant resistance reported for vancomycin, linezolid, or rifampin. While MRSA continues to have susceptibility to several antibiotics, the high and sometimes variable resistance rates to other drugs underscores the importance of antimicrobial stewardship. Risk factors for high MRSA infection rates related to infection control measures, low socioeconomic status, and personal demographic characteristics were also reported. Additional surveillance, infection control measures, enhanced anti-microbial stewardship, and community education programs are necessary to decrease MRSA prevalence and minimize the public health risk posed by this pathogen.
1. Introduction
Staphylococcus aureus is part of the normal skin and nasal microbiota, and around 30% of the healthy adult population is colonized mainly in the nasopharyngeal cavity [1]. While colonization is usually asymptomatic, symptomatic infection can occur if there is a breach in the mucosal barrier or skin [1]. The severity of symptomatic infection ranges from superficial skin and soft tissue infections (SSTIs) to life threatening diseases, such as bacteremia or sepsis [1]. While many strains of S. aureus are easily treatable with a variety of antimicrobial compounds, methicillin-resistant Staphylococcus aureus (MRSA) presents a more challenging problem. MRSA first emerged in the United Kingdom after methicillin was introduced in hospitals to combat penicillin-resistant S. aureus in 1961, and MRSA incidence has increased since that time [2].
The first recorded outbreak of MRSA in Canada occurred in the early 1980s within a hospital setting [2]. In the early 2000s, infections arose in otherwise healthy community members and a distinction was made between hospital-acquired MRSA (HA-MRSA) and community-acquired MRSA (CA-MRSA) in Canada [3]. Not only do HA- and CA-MRSA differ with regards to the context in which these infections are acquired, these strains have also emerged with distinct genetic and phenotypic characteristics [1]. Notably, CA-MRSA strains can cause severe infections in otherwise healthy individuals. CA-MRSA strains display enhanced virulence, spreading more rapidly and causing more severe illness than HA-MRSA strains [4]. Enhanced virulence of CA-MRSA is thought to be associated with the production of several toxins, such as phenol-soluble modulins (PSMs) [4]. HA-MRSA strains contain the larger Staphylococcal cassette chromosome mec (SCCmec) types, I, II, or III, whereas CA-MRSA strains contain the smaller SCCmec types, IV or V [3]. The epidemiological criteria that was initially used to classify the strains has now become less useful as CA-MRSA has also moved into the hospital setting. Importantly, unlike in other parts of the world, the majority of MRSA infections in Canada remain attributable to healthcare exposure [3].
Given the changing dynamics of MRSA transmission, several surveillance programs have been implemented in Canada to track and monitor MRSA infections. The Canadian Nosocomial Infection Surveillance Program (CNISP) has monitored MRSA prevalence since 1995 by collecting data on all hospitalized patients with MRSA from 47 hospitals located in 9 provinces [3]. Additionally, the Canadian Ward Surveillance Study (CANWARD) tracks the antimicrobial susceptibility of clinical isolates from the Emergency Department (ED), Intensive Care Unit (ICU), out-patient clinics, and medical surgical wards in 15 hospitals located in 8 provinces [3]. From 2013 to 2017 alone, the CNISP identified and tracked over 10,000 MRSA infections in Canada [5].
With a limited number of effective antibiotics available, MRSA is a growing public health concern in Canada [6]. MRSA is a leading cause of nosocomial infections, particularly in critically ill patients. The SARS-CoV2 pandemic has overcrowded intensive care units, and patients on ventilators are at high risk for MRSA infections. Such infections increase the morbidity and mortality rates of patients and lead to higher care costs and longer hospital stays [7,8,9,10]. Vulnerable communities, such as Indigenous populations living on reserves, those living in isolated rural communities, and persons who inject drugs are at particular risk of MRSA infection due to limited or discriminatory access to healthcare, crowding, lack of sanitation equipment, and inadequate living conditions [11,12,13]. Accordingly, the purpose of this review is to describe the epidemiology of MRSA in Canada from 1991 to 2017, as well as the associated risk factors and intervention strategies.
2. Results
A total of 387 citations were generated from the MEDLINE and EMbase searches. An additional 105 citations were obtained from the grey literature search. After removing duplicates, 396 articles remained for level-one screening. Level-one inclusion criteria consisted of a Canadian population and a mention of MRSA infection. These criteria resulted in the exclusion of 300 articles, while 96 citations proceeded to level two full-text screening. Subsequently, 56 full-text articles were excluded due to the reasons depicted in Figure 1. The principle reasons for exclusion were due to an absence of a full-text article (n = 23) or a lack of MRSA-specific prevalence rate (n = 23). In total, 40 articles were included for the qualitative analysis.
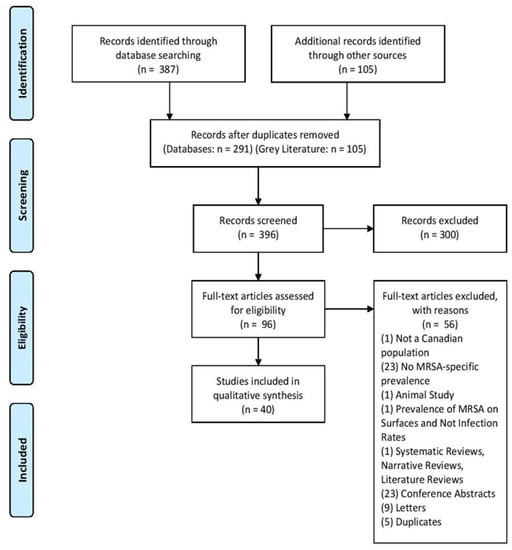
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart indicating the number of identified, screened, eligible, and included articles and the reasons for full-text exclusion.
All data are summarized in Table A2, Table A3, Table A4 and Table A5. After the initial MRSA outbreak in Canada, MRSA prevalence has steadily increased from levels documented in the earliest reports. Allard et al. [14] reported no MRSA cases from 1991 to 1999 in a Quebec hospital, but prevalence rates increased to 2.6 per 100,000 patients from 2000 to 2002 and again to 7.4 per 100,000 patients from 2002 to 2005. Likewise, Simor et al. [8] found similarly increasing rates in hospitals across the country from 0.25 MRSA cases per 1000 admissions in 1995 to 1.11 cases per 1000 admissions in 1999, a more than 4-fold increase in less than half a decade. While still relatively low, Warshawsky et al. [15] reported a MRSA rate of 37 cases per 100,000 patients in hospitals and clinics in London, Ontario, in 1997, and Zoutman et al. [16] reported a MRSA prevalence of 2.0 per 1000 admission in acute care hospitals across the country in 1999. Additionally, Sligl et al. [9] reported a MRSA infection rate of 3.1 per 1000 ICU admissions from 1997 to 2005 in Edmonton, documenting an overall increasing trend within this hospital setting.
Following the first incidence of CA-MRSA in Canada in the early 2000s, several studies have demonstrated a continued rise in MRSA prevalence and increased incidence rates in both clinical and non-clinical settings. Jones et. al [17] found a MRSA prevalence of 7.2% in 87 hospital sites across the country from 2000 to 2002. Golding et al. [13] found the prevalence of CA-MRSA to be 8.2 per 10,000 in Northern Saskatchewan communities in 2001, whereas Cowie et al. [18] reported a 5.39% MRSA prevalence in acute care institutions in Vancouver in 2002. Analyzing data from Calgary Laboratory Services, Gill et al. [19] found a rate of 22.2 per 100,000 people in 2004. Additionally, there was a prevalence of 3.7 cases per 10,000 patient days among patients in a Toronto hospital in 2003 [20].
Although the general trend found reports of increasing prevalence over time, a small number of studies reported lower MRSA prevalence rates. Li et al. [21] reported a MRSA prevalence of 0.32 per 100,000 Albertans in 2005, and Bracco et al. [22] reported a MRSA prevalence of only 1.1% among ICU patients in Montreal from 2002 to 2004 inclusive, suggesting that there may be some site-to-site variation in the detection or prevalence of MRSA.
At the beginning of the century, MRSA prevalence in vulnerable populations was similar to that observed in hospital patients, but this prevalence has undergone a marked increase in just a few years [23,24].
One study found a MRSA prevalence of 146–482 per 10,000 residents in three Northern communities from 2006 to 2008 [11]. Another study similarly reported a MRSA prevalence of 168.1 cases per 10,000 residents that was found in 2006 in rural and remote communities [12]. Gilbert et al. [24] reported an infection and colonization rate of 5.5% in 2005 among study participants from homeless shelters, needle exchange and detoxification programs, an inner-city medical clinic, and a corrections facility in Calgary. Similar observations were made by Al-Rawahi et al. [23], reporting a 7.4% MRSA prevalence among injection drug users in Vancouver in 2000 and a much higher MRSA prevalence of 18.6% in 2006. Expanding on these observations, one study found very high rates of MRSA SSTI infections in an inner-city emergency department in Vancouver: 54.8% of all SSTI patients were infected with MRSA in 2003 and 68% were infected in a follow-up study 21 months later [25]. Szakacs et al. [26] reported a MRSA prevalence of 4.5% among inner city shelter residents in 2006.
In addition to the vulnerable population, MRSA was also present in the general population, even in individuals who had not had any recent contact with the healthcare system. Hanselman et al. [27] found a 3.18% MRSA colonization rate among schoolteachers at a conference in Toronto in 2006. Surprisingly, in contrast, reported rates of MRSA among healthcare workers were low. Saito et al. [10] found that none of the employees at an emergency department in Toronto had MRSA in 2009, and Trépanier et al. [28] found only 1 medical resident colonized with MRSA in a Quebec City hospital.
Critically, studies that examined MRSA prevalence over a longer period of time provide further evidence of an increasing trend in prevalence. Mitchell et al. [29] found the proportion of MRSA among all healthcare-associated infections across Canada to be 3.9% in 2002 and 8.5% in 2017. Similarly, there was an increasing trend in a Vancouver emergency department (ED) from 12.0 MRSA wound isolates per 10,000 ED visits in 2003 to 34.3 per 10,000 visits in 2011 [30]. This observed growth in MRSA prevalence appears to have stabilized in the early 2000s, but rates continued to remain high from 2006 to 2010 across the country [6,31,32,33,34,35,36,37,38,39].
Moving into the last decade, Ugarte Torres et al. [40] found a MRSA colonization rate of 1.4% in Calgary sexually transmitted infection (STI) and community clinics in 2014, and Gill et al. [19] reported a MRSA prevalence of 81 cases per 100,000 people using Calgary Laboratory Services data. Despite appearing to stabilize in the early 2000s, MRSA prevalence continues to be a public health concern, especially among vulnerable populations. El Eman et al. [41] found a MRSA prevalence of 3.0 per 100 long-term care home residents in Ontario in 2011. Remote and indigenous communities continue to be disproportionately impacted: Muileboom et al. [42] reported 2482 MRSA cases per 100,000 clinic, laboratory, and hospital patients in Northwestern Ontario in 2011, mainly from remote Indigenous communities. Similarly, Jeong et al. [13] found a 14.78% MRSA prevalence in skin and soft tissue infections from 2012 to 2013 among First Nations communities across 5 provinces, and although Li et al. [21] reported a generally lower MRSA prevalence in 2012 of 1.44 per 100,000 Albertans, they noted that the majority of cases were from Indigenous communities.
Overall, increasing MRSA prevalence rates were observed in both hospitals and emergency departments (Figure 2), with higher prevalence seen in the emergency departments. Initially, epidemiological criteria were used to distinguish between CA-MRSA and HA-MRSA based on where the infection was acquired [3]. As previously discussed, multiple CA-MRSA strains have moved into the hospital setting, making this classification less useful [3]. Infections diagnosed in the emergency department can be used as a proxy for those acquired in the community, while infections diagnosed in the hospital are nosocomial if they have been diagnosed at least 48 h after admission. Given the higher prevalence of MRSA in the emergency department compared to hospital rates, it appears that MRSA infections occur more commonly in the community.
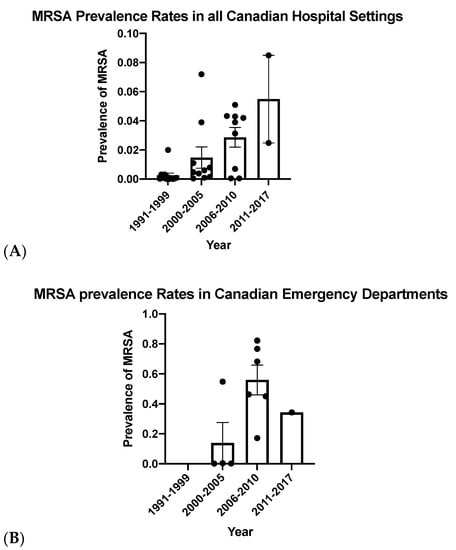
Figure 2.
Methicillin-resistant Staphylococcus aureus (MRSA) prevalence rates compared in Canadian hospitals (A) and Canadian emergency departments (B) from 1991 until 2017. Bars represent mean prevalence rates and error bars represent the standard error of the mean.
3. Discussion
After the initial MRSA outbreak in the early 1980s in Canada, MRSA prevalence rose in the 1990s and early 2000s. MRSA rates appeared to have stabilized somewhat toward the end of the 2000s and into the 2010s, but MRSA remains a public health concern in Canada, especially among vulnerable populations, such as rural and remote and Indigenous communities.
3.1. Antibiotic Resistance
A subset of the studies cited also reported antibiotic resistance patterns in the identified MRSA isolates. Although all strains were resistant to beta-lactam antibiotics, such as penicillin, cloxacillin, and cefazolin [9,42], varying susceptibilities were reported for macrolide and lincosamide antibiotics that inhibit protein synthesis and for fluoroquinolones that disrupt DNA synthesis [9,31,39,42]. Sligl et al. [9] found that all strains were resistant to erythromycin (a macrolide) but 7% remained susceptible to clindamycin (a lincosamide) in a retrospective cohort analysis of MRSA infections in the intensive care unit at the University of Alberta hospital from 1997 to 2005. Kim et al. [43] similarly found complete resistance to erythromycin in MRSA infections in Alberta from 2005 to 2008. In contrast, Achiam et al [31] found that 7.4% of MRSA isolates from adult patients with SSTIs in an Ontario urban center were susceptible to erythromycin and 75% were susceptible to clindamycin, and Muileboom et al. [42] reported nearly 100% susceptibility to clindamycin and around 60% susceptibility to erythromycin in MRSA isolates from remote first nations communities from 2008 to 2012. Zhanel et al. [39] found resistance rates over 50% to fluoroquinolones and macrolides as part of the 2008 CANWARD study. Some of these differences in susceptibility rates might be due to the differing populations or locations of the studies, but they likely also indicate MRSA’s evolving and changing resistance patterns and the non-uniform distribution of resistance strains across the broad geography of Canada (urban vs. rural vs. Northern).
All studies reported high susceptibility (>85%) to the trimethoprim-sulfamethoxazole, indicating that this is a reasonable treatment for mild to moderate MRSA infections [9,39,42,43]. Additionally, none of the studies reported significant resistance to vancomycin, linezolid, or rifampin [9,31,39,43]. While MRSA continues to have susceptibility to several antibiotics, the high resistance and sometimes variable resistance rates to other drugs underscore the importance of antimicrobial stewardship.
3.2. Risk Factors
Studies have reported various risk factors that result in higher MRSA infection rates. These risks are divided in several categories: infection control measures, factors affecting those with low socioeconomic status, and personal demographic characteristics. In the first category, multiple studies reported that large healthcare facilities have an increased risk of MRSA spread due to the high person-to-person contact. Within the ICU unit, Bracco et al. [22] suggest low compliance with hand hygiene as a risk for MRSA infection transmission. In addition, they warn that moving MRSA patients to different units before screening has been completed can cause cross-transmission in wards hyperendemic for MRSA. As a result, early identification and implementation of infection control measures must be strictly enforced within healthcare settings to prevent MRSA spread [15]. Within the community, MRSA risk factors included repeated injection drug use, having a previous MRSA infection, using antibiotics within the last 2 months and diabetes mellitus [25]. Furthermore, in two separate studies, Laupland et al. [44,45] identified that the male sex and older ages are factors at a higher risk for MRSA development.
The aforementioned MRSA prevalence rates indicated that vulnerable populations, such as remote Canadian Indigenous communities, are at a higher risk of developing MRSA infections. This is in part due to inadequate living conditions, such as crowded housing, poor access to running water, and a lack of sanitary supplies, which ultimately increase community transmission [13]. Specifically, Golding et al. [11] report that higher MRSA infection rates in remote Northern Saskatchewan communities are a result of overcrowding, poor housing conditions, inadequate hygiene, pre-existing skin conditions, and a high previous usage of antimicrobial drugs. Accordingly, Jeong et al. [13] suggests that developing a national-level surveillance system to monitor SSTI epidemiology and CA-MRSA antibiotic susceptibility test results would be essential for prevention, control, and management of disease spread, particularly in rural communities. Upstream factors of MRSA infection, including socio-environmental factors, such as poor housing and access to clean drinking water, must be addressed to prevent antibiotic resistance development and MRSA spread.
3.3. Future Directions
There are several interventions that can address MRSA spread and antibiotic resistance rates. First, while continuing current surveillance programs, more investment should be made in local, national, and international infection surveillance and control programs in both larger hospitals and remote communities. These efforts will help characterize the continuing evolution of MRSA colonization and infection patterns and their impact on patient outcomes [46]. Additional research is needed into MRSA transmission, risk factors, and infection control strategies, such as screening and decolonization. In particular, greater screening and contact precautions, such as improved hand hygiene compliance, should be adopted in hospitals to reduce nosocomial MRSA transmission [20]. Additionally, engineering controls should be explored. For example, decreasing patient contact by increasing the number of single-patient rooms has been shown to prevent MRSA transmission, encouraging us to rethink hospital and clinic design [22]. Moreover, further genetic characterization, virulence profiling, and detection of resistant determinants should be conducted to provide a better understanding of the biology, evolution, and adaptation of this clinically important pathogen.
Lastly, among vulnerable populations, educational tools have proven to be effective in combating MRSA infection rates. Golding et al. [12] introduced a “Germs Away” curriculum among Northern communities in Saskatchewan. This program, which featured proper handwashing techniques and cough etiquette, resulted in a decrease in MRSA rates in targeted communities over a 2-year period. This study demonstrates the promise of educational programs in schools and rural communities in mitigating CA-MRSA infection rates and the rates of other communicable diseases.
4. Materials and Methods
A systematic review study protocol was created a priori and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocols, statements, and guidelines [47].
4.1. Search Strategy
MEDLINE and EMbase were searched from inception to 5 March 2020. Search clusters consisted of keywords and database-specific words. The keywords were used in both the MEDLINE and EMbase searches. The main clusters were “MRSA” terms, “Canada” terms, and “Prevalence” terms. The keywords and database-specific terms within each cluster were combined using “OR”, while each of the clusters were combined using “AND”. The search was conducted alongside an experienced librarian (Lee-Robertson, H., personal communication, 5 March 2020.) at the University of Calgary. A grey literature search was also conducted to augment the search on Google Scholar from inception to 30 March 2020, using only the keywords shown in Table A1. There were no language, age, or date restrictions for either the database searches or the grey literature search.
4.2. Eligibility Criteria and Selection of Articles
All articles were selected for eligibility and screened by two independent authors (B.W, E.M). To be included at the first stage screening process, each abstract needed to contain data on MRSA rates in a human population in Canada. All abstracts included at this first stage were eligible for screening at stage two. Screening by each author was performed separately and compared periodically to ensure that there was consistent agreeability between reviewers. Second stage screening consisted of the full-length text, and all included articles were assessed against the exclusion criteria outlined in Figure 1. Once again, each author independently screened the full texts at stage two, and each independent decision was compared. When the assessors disagreed on whether to include an abstract during the first or second stage of screening, a third-party adjudicator was brought in to help reach a decision of inclusion or exclusion. All articles that surpassed both stages were included at the data extraction stage.
4.3. Data Extraction Process and Synthesis of Evidence
Data was extracted in duplicate by two independent assessors. Data extraction criteria was formed together by both reviewers (B.W, E.M). The criteria were piloted using five randomly selected studies that were included after second-level screening. Both authors took part in extracting data from each included full-text, and the data was subsequently verified between authors.
5. Conclusions
MRSA infection remains a public health concern in Canada, especially among vulnerable populations. The risks associated with MRSA infections are multi-faceted and include personal demographic characteristics, infection control measures, and inadequate living conditions. Marginalized communities additionally face barriers to accessing timely and continuous medical care, highlighting inequities in our healthcare system.
Author Contributions
Conceptualization, E.M. and B.W.; methodology, E.M. and B.W; software, E.M. and B.W; validation, E.M., B.W. and C.N.J.; formal analysis, E.M. and B.W; investigation, E.M. and B.W; resources, C.N.J.; data curation, E.M. and B.W.; writing—original draft preparation, E.M. and B.W.; writing—review and editing, C.N.J. and B.G.J.S.; visualization, E.M. and B.W.; supervision, C.N.J.; project administration, C.N.J. and B.G.J.S.; funding acquisition, C.N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The systematic review search strategy within the MEDLINE and EMbase databases. MRSA terms, Canada terms, and prevalence terms were combined with “AND”, while keywords and database-specific terms within each search cluster were combined with “OR”.
Table A1.
The systematic review search strategy within the MEDLINE and EMbase databases. MRSA terms, Canada terms, and prevalence terms were combined with “AND”, while keywords and database-specific terms within each search cluster were combined with “OR”.
| Key Words | ||
|---|---|---|
| MRSA Keywords | Canada Keywords | Prevalence Keywords |
| Methicillin-Resistant Staphylococcus aureus | Canada | Prevalence |
| MRSA | Canadian | Incidence |
| Alberta | ||
| British Columbia | ||
| Manitoba | ||
| New Brunswick | ||
| Newfoundland | ||
| Labrador | ||
| Northwest Territories | ||
| Nova Scotia | ||
| Nunavut | ||
| Ontario | ||
| Prince Edward Island | ||
| Quebec | ||
| Saskatchewan | ||
| Yukon | ||
| Mesh Terms: | ||
| MRSA Terms | Canada Terms | Prevalence Terms |
| Methicillin-resistant Staphylococcus Aureus | Canada | Prevalence |
| Alberta | Incidence | |
| British Columbia | ||
| Manitoba | ||
| New Brunswick | ||
| “Newfoundland and Labrador” | ||
| Northwest Territories | ||
| Nova Scotia | ||
| Nunavut | ||
| Ontario | ||
| Prince Edward Island | ||
| Quebec | ||
| Saskatchewan | ||
| Yukon Territory | ||
| EMtree Terms: | ||
| MRSA Terms | Canada Terms | Prevalence Terms |
| Methicillin-resistant Staphylococcus aureus | Canada | Epidemiological data |
| Meticillin resistant Staphylococcus aureus | Alberta | Prevalence |
| British Columbia | Infection Rate | |
| Manitoba | Incidence | |
| New Brunswick | ||
| “Newfoundland and Labrador” | ||
| Northwest Territories | ||
| Nova Scotia | ||
| Nunavut | ||
| Ontario | ||
| Prince Edward Island | ||
| Quebec | ||
| Saskatchewan | ||
| Yukon | ||

Table A2.
Prevalence of MRSA incidence, year(s) of data collection, region in Canada. and population from studies initiated between the years 1991 and 1999. All prevalence and incidence rates are infection rates unless otherwise specified.
Table A2.
Prevalence of MRSA incidence, year(s) of data collection, region in Canada. and population from studies initiated between the years 1991 and 1999. All prevalence and incidence rates are infection rates unless otherwise specified.
| First Author, Year | MRSA Prevalence | Year | Region | Population |
|---|---|---|---|---|
| Allard et al., 2008 | (Bacteremia) Prevalence in 1991–1999: 0 per 100,000 2000–2002: 2.6 per 100,000 patients Prevalence in 2002–2005: 7.4/100,000 | 1991–2005 | Quebec | Hospital Patients with at least 1 blood culture positive for S. aureus |
| Simor et al., 2001 | Prevalence (per 1000 admissions): 1995: 0.25 1999:1.11 | 1995–1999 | Across Canada | Hospital Patients |
| Simor et al., 2010 | Prevalence (per 10,000 patient days): 1995: 0.36 2007: 3.43 | 1995–2007 | Across Canada | Hospital Patients |
| Warshawsky et al., 2000 | Prevalence: 37 per 100,000 patients | 1997 | London, ON | Hospital and Clinic Patients |
| Sligl et al., 2007 | Infection Rate: 3.1 per 1000 ICU admissions (overall), Prevalence (per 1000 ICU admissions): 1997: 0.8 1998: 0.8 1999: 3.1 2000: 6.0 2001: 3.8 2002: 0.9 2003: 1.5 2004: 4.7 2005: 7.9 | 1997–2005 | Edmonton, AB | ICU Patients |
| Zoutman et al., 2005 | Prevalence: 2.0 per 1000 admissions | 1999 | Across Canada | Acute care hospital patients |

Table A3.
Prevalence of MRSA incidence, year(s) of data collection, region in Canada, and population from studies initiated between the years 2000 and 2005. All prevalence and incidence rates are infection rates unless otherwise specified.
Table A3.
Prevalence of MRSA incidence, year(s) of data collection, region in Canada, and population from studies initiated between the years 2000 and 2005. All prevalence and incidence rates are infection rates unless otherwise specified.
| First Author, Year | MRSA Prevalence | Year | Region | Population |
|---|---|---|---|---|
| Jones et. al, 2004 | Prevalence: 7.20% | 2000–2002 | Across Canada | 87 hospital sites |
| Al-Rawahi et al., 2008 | Prevalence: 2000: 7.4% 2006: 18.6% | 2000, 2006 | Vancouver | Injection Drug Users |
| Golding et al., 2012 | Prevalence: CA-MRSA 8.2/10,000 | 2001 | Northern Saskatchewan | Northern Health Regions in Saskatchewan |
| Cowie et al., 2005 | Prevalence: 22/408 participants = 5.39% | 2002 | Vancouver, BC | Acute Care Institutions |
| Bracco et al., 2007 | Prevalence: 1.1% | 2002–2004 | Montreal | ICU patients |
| Mitchell et al., 2019 | Prevalence (of all healthcare-associated infections): 2002: 3.9% 2017: 8.5% | 2002, 2017 | Across Canada | Hospital Inpatients |
| Poutanen et al., 2005 | Prevalence: 3.7 per 10,000 patient days | 2003 | Toronto, ON | Hospital Patients |
| Stenstrom et al., 2009 | Prevalence: 54.8% of SSTI patients, 68% after 21 months | 2003–2004 | Vancouver, BC | SSTI patients in ED |
| Wilmer et al., 2014 | Prevalence (in wound isolates, per 10 000 ED visits): 2003: 12.0 2004: 23.0 2005: 40.1 2006: 76.7 2007: 82.2 2008: 68.2 2009: 45.0 2010: 46.3 2011: 34.3 | 2003–2011 | Vancouver, BC | ED Patients |
| Gill et al., 2019 | Prevalence: 22.2/100,000 | 2004 | Calgary | Calgary Laboratory Services Data |
| Li et al., 2014 | Prevalence: 0.32 per 100,000 people | 2005 | Alberta | Individuals living in Alberta |
| Gilbert et al., 2007 | Prevalence (Infection and Colonization): 5.5% among 271 participants | 2005 | Calgary | From five study sites: an outreach needle-exchange van, homeless shelters, detoxification centres and residential substance treatment programs, and inner city medical clinic and new admissions to a local corrections facility |

Table A4.
Prevalence or incidence of MRSA, year(s) of data collection, region in Canada, and population from studies initiated between the years 2006 and 2010. All prevalence and incidence rates are infection rates unless otherwise specified.
Table A4.
Prevalence or incidence of MRSA, year(s) of data collection, region in Canada, and population from studies initiated between the years 2006 and 2010. All prevalence and incidence rates are infection rates unless otherwise specified.
| First Author, Year | MRSA Prevalence or Incidence | Year | Region | Population |
|---|---|---|---|---|
| Hanselman et al., 2008 | 3.18% (7/220) | 2006 | Toronto | School Teachers in Toronto |
| Szakacs et al. 2007 | Prevalence: 4.5% | 2006 | Ottawa, ON | Inner City Shelter Residents |
| Golding et al., 2011 | Prevalence: 146–482/10,000 | 2006–2008 | North Saskatchewan | 3 Northern Saskatchewan communities |
| Roth et al., 2016 | Prevalence: 46.79 per 100,000 patient days | 2006–2009 | Ottawa, ON | Hospital Patients |
| Li et al., 2017 | Incidence Rate Ratio: 2006–2007: 1.022, April 2007–Jan 2009: 0.989, Jan 2010–Mar 2015: 0.987 | 2006–2015 | Quebec | Teaching Facility Patients |
| Antoniou et al., 2009 | Prevalence: Nasal or Rectal Carriage (CA-MRSA) = 1.6% | 2007 | Toronto | Mainly men who have sex with men |
| Adam et al., 2011 | Prevalence: 356/8228 | 2007–2009 | British Columbia, Alberta, Manitoba, Saskatchewan, Ontario, Quebec, New Brunswick, Nova Scotia | Canadian Hospitals |
| Golding et al., 2012 | Prevalence (2006): 168.1 per 10,000 Prevalence (2008): 142.6 per 10,000 | 2006–2008 | North Saskatchewan | Northern health regions in Saskatchewan |
| Achiam et al., 2011 | Prevalence: 17.1% of all samples taken (13.2% from SSTI infection sites, 10.2% in nares cultures, 5.8% in throat cultures) | 2008 | London, Ontario | ED patients with SSTI infection |
| Zhanel et al., 2010 | Prevalence: 5.1% of all bacterial isolates | 2008 | Across Canada | Hospital Patients |
| Wang et al., 2018 | Prevalence of colonization: 3.13% | 2008–2010 | Ottawa, ON | Hospital Patients |
| Simor et al., 2016 | Prevalence: 0.45 per 1000 admissions (MRSA BSI) | 2008–2012 | Across Canada | Hospital Patients |
| Saito et al., 2013 | Prevalence: 0% | 2009 | Toronto, ON | ER Healthcare Workers |
| Trépanier et al., 2013 | Prevalence (colonization): 0.4% | 2010 | Quebec City, QC | Medical Residents |
| Simor et al., 2013 | Prevalence: 4.2% colonization, 0.3% infection rate | 2010 | All 10 Provinces | Hospital Inpatients |
| William et al., 2015 | Prevalence: 2010: 4.3% 2012: 3.9% | 2010, 2012 | All 10 provinces | Hospital Patients |
| Martin et al., 2018 | Prevalence: 0.7 per 100 inpatients | 2010, 2012, 2016 | Across Canada | Hospital Inpatients |

Table A5.
Prevalence of MRSA incidence, year(s) of data collection, region in Canada, and population from studies initiated after 2010. All prevalence and incidence rates are infection rates unless otherwise specified.
Table A5.
Prevalence of MRSA incidence, year(s) of data collection, region in Canada, and population from studies initiated after 2010. All prevalence and incidence rates are infection rates unless otherwise specified.
| First Author, Year | MRSA Prevalence | Year | Region | Population |
|---|---|---|---|---|
| Eman et al., 2014 | Prevalence: 3.0 cases per 100 residents | 2011 | Ontario | Long-term Care Homes in Ontario |
| Muileboom et al., 2013 | Prevalence: 2482 per 100,000 patients | 2011 | NW Ontario | Clinic, Laboratory and Hospital Patients (mainly remote Indigenous communities) |
| Li et al., 2014 | Prevalence: 1.44 per 100,000 people | 2012 | Alberta | Alberta Residents, most infected were Indigenous |
| Jeong et al., 2020 | Prevalence: 14.78% (55/372) | 2012–2013 | AB, SK, MB, ON, QC | Individuals in First Nations Communities Across 5 Provinces with SSTIs |
| Ugarte Torres et al., 2017 | Prevalence (colonization): 1.4% | 2014 | Calgary, AB | STI and Community Clinic Patients |
| Gill et al., 2019 | Prevalence: 81/100,000 | 2014 | Calgary | Calgary Laboratory Services Patients |
References
- Loewen, K.; Schreiber, Y.; Kirlew, M.; Bocking, N.; Kelly, L. Community-associated methicillin-resistant. Can. Fam. Physician 2005, 63, 512–520. [Google Scholar]
- Cimolai, N. Methicillin-resistant Staphylococcus aureus in Canada: A historical perspective and lessons learned. Can. J. Microbiol. 2010, 56, 89–120. [Google Scholar] [CrossRef]
- Methicillin-Resistant Staphylococcus aureus: An Update. (2012, February). Toronto Invasion Bacterial Disease Network. Available online: https://eportal.mountsinai.ca/Microbiology/research/tibdn/tibdnnews/TIBDN_Newsletter_16.pdf (accessed on 21 March 2021).
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.H.; Queck, S.Y.; Li, M.; Otto, M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Canadian Nosocomial Infection Surveillance Program. (Cnisp): Summary Report of Healthcare Associated Infection (Hai), Antimicrobial Resistance (Amr) and Antimicrobial Use (Amu) Surveillance Data from 1 January 2013 to 31 December 2017. Available online: https://www.canada.ca/en/public-health/services/publications/science-research-data/summary-report-healthcare-associated-infection-antimicrobial-resistance-antimicrobial-use-surveillance-data-2013-2017.html#a4.2. (accessed on 21 March 2021).
- Simor, A.E.; Williams, V.; McGeer, A.; Raboud, J.; Larios, O.; Weiss, K.; Hirji, Z.; Laing, F.; Moore, C.; Gravel, D. Prevalence of colonization and infection with methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus and of Clostridium difficile infection in Canadian hospitals. Infect. Control. Hosp. Epidemiol. 2013, 34, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fortin, E.; Tremblay, C.; Ngenda-Muadi, M.; Garenc, C.; Moisan, D.; Villeneuve, J.; Quach, C. Hospital-Acquired Methicillin-Resistant Staphylococcus aureus Bloodstream Infections in Québec: Impact of Guidelines. Infect. Control. Hosp. Epidemiol. 2017, 38, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Simor, A.E.; Ofner-Agostini, M.; Bryce, E.; Green, K.; McGeer, A.; Mulvey, M.; The Canadian Nosocomial Infection Surveillance Program, Health Canada. The evolution of methicillin-resistant Staphylococcus aureus in Canadian hospitals: 5 years of national surveillance. CMAJ 2001, 165, 21–26. [Google Scholar] [PubMed]
- Sligl, W.; Taylor, G.; Gibney, R.N.; Rennie, R.; Chui, L. Methicillin-resistant Staphylococcus aureus in a Canadian intensive care unit: Delays in initiating effective therapy due to the low prevalence of infection. Can. J. Infect. Dis. Med. Microbiol 2007, 18, 139–143. [Google Scholar] [CrossRef]
- Saito, G.; Thom, J.; Wei, Y.; Gnanasuntharam, P.; Kreiswirth, N.; Willey, B.; Borgundvaag, B. Methicillin-resistant Staphylococcus aureus colonization among health care workers in a downtown emergency department in Toronto, Ontario. Can. J. Infect. Dis. Med. Microbiol 2013, 24, 57–60. [Google Scholar] [CrossRef]
- Golding, G.R.; Levett, P.N.; McDonald, R.R.; Irvine, J.; Quinn, B.; Nsungu, M.; Partnership, N.A.R. High rates of Staphylococcus aureus USA400 infection, Northern Canada. Emerg. Infect. Dis. 2011, 17, 722–725. [Google Scholar] [CrossRef]
- Golding, G.R.; Quinn, B.; Bergstrom, K.; Stockdale, D.; Woods, S.; Nsungu, M.; Partnership, N.A.R. Community-based educational intervention to limit the dissemination of community-associated methicillin-resistant Staphylococcus aureus in Northern Saskatchewan, Canada. BMC Public Health 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Nguyen, H.N.T.; Tyndall, M.; Schreiber, Y.S. Antibiotic use among twelve Canadian First Nations communities: A retrospective chart review of skin and soft tissue infections. BMC Infect. Dis. 2020, 20, 118. [Google Scholar] [CrossRef]
- Allard, C.; Carignan, A.; Bergevin, M.; Boulais, I.; Tremblay, V.; Robichaud, P.; Pepin, J. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin. Microbiol. Infect. 2008, 14, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Warshawsky, B.; Hussain, Z.; Gregson, D.B.; Alder, R.; Austin, M.; Bruckschwaiger, D.; Schiedel, L. Hospital- and community-based surveillance of methicillin-resistant Staphylococcus aureus: Previous hospitalization is the major risk factor. Infect. Control. Hosp. Epidemiol. 2000, 21, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Zoutman, D.E.; Ford, B.D. The relationship between hospital infection surveillance and control activities and antibiotic-resistant pathogen rates. Am. J. Infect. Control. 2005, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Karlowsky, J.A.; Draghi, D.C.; Thornsberry, C.; Sahm, D.F.; Bradley, J.S. Rates of antimicrobial resistance among common bacterial pathogens causing respiratory, blood, urine, and skin and soft tissue infections in pediatric patients. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Cowie, S.E.; Ma, I.; Lee, S.K.; Smith, R.M.; Hsiang, Y.N. Nosocomial MRSA infection in vascular surgery patients: Impact on patient outcome. Vasc. Endovasc. Surg. 2005, 39, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.C.; Ma, I.; Guo, M.; Gregson, D.B.; Naugler, C.; Church, D.L. Sociodemographic and geospatial associations with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections in a large Canadian city: An 11 year retrospective study. BMC Public Health 2019, 19, 914. [Google Scholar] [CrossRef]
- Poutanen, S.M.; Vearncombe, M.; McGeer, A.J.; Gardam, M.; Large, G.; Simor, A.E. Nosocomial acquisition of methicillin-resistant Staphylococcus aureus during an outbreak of severe acute respiratory syndrome. Infect. Control. Hosp. Epidemiol. 2005, 26, 134–137. [Google Scholar] [CrossRef]
- Li, V.; Chui, L.; Simmonds, K.; Nguyen, T.; Golding, G.R.; Yacoub, W.; Louie, M. Emergence of new CMRSA7/USA400 methicillin-resistant Staphylococcus aureus spa types in Alberta, Canada, from 2005 to 2012. J. Clin. Microbiol. 2014, 52, 2439–2446. [Google Scholar] [CrossRef]
- Bracco, D.; Dubois, M.J.; Bouali, R.; Eggimann, P. Single rooms may help to prevent nosocomial bloodstream infection and cross-transmission of methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2007, 33, 836–840. [Google Scholar] [CrossRef]
- Al-Rawahi, G.N.; Schreader, A.G.; Porter, S.D.; Roscoe, D.L.; Gustafson, R.; Bryce, E.A. Methicillin-resistant Staphylococcus aureus nasal carriage among injection drug users: Six years later. J. Clin. Microbiol. 2008, 46, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Macdonald, J.; Louie, M.; Gregson, D.; Zhang, K.; Elsayed, S.; Conly, J. Prevalence of USA300 colonization or infection and associated variables during an outbreak of community-associated methicillin-resistant Staphylococcus aureus in a marginalized urban population. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Stenstrom, R.; Grafstein, E.; Romney, M.; Fahimi, J.; Harris, D.; Hunte, G.; Christenson, J. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus skin and soft tissue infection in a Canadian emergency department. CJEM 2009, 11, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, T.A.; Toye, B.; Turnbull, J.M.; Muckle, W.; Roth, V.R. Prevalence of methicillin-resistant Staphylococcus aureus in a Canadian inner-city shelter. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Hanselman, B.A.; Kruth, S.A.; Rousseau, J.; Weese, J.S. Methicillin-resistant Staphylococcus aureus colonization in schoolteachers in Ontario. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 405–408. [Google Scholar] [CrossRef]
- Trépanier, P.; Tremblay, C.; Ruest, A. Methicillin-resistant Staphylococcus aureus colonization among medical residents. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, 148937. [Google Scholar] [CrossRef]
- Mitchell, R.; Taylor, G.; Rudnick, W.; Alexandre, S.; Bush, K.; Forrester, L.; Program, C.N.I.S. Trends in health care-associated infections in acute care hospitals in Canada: An analysis of repeated point-prevalence surveys. CMAJ 2019, 191, E981–E988. [Google Scholar] [CrossRef]
- Wilmer, A.; Lloyd-Smith, E.; Romney, M.G.; Champagne, S.; Wong, T.; Zhang, W.; Hull, M.W. Reduction in community-onset methicillin-resistant Staphylococcus aureus rates in an urban Canadian hospital setting. Epidemiol. Infect. 2014, 142, 463–467. [Google Scholar] [CrossRef]
- Achiam, C.C.; Fernandes, C.M.; McLeod, S.L.; Salvadori, M.I.; John, M.; Seabrook, J.A.; Hussain, Z. Methicillin-resistant Staphylococcus aureus in skin and soft tissue infections presenting to the Emergency Department of a Canadian Academic Health Care Center. Eur. J. Emerg. Med. 2011, 18, 2–8. [Google Scholar] [CrossRef]
- Adam, H.J.; DeCorby, M.; Rennie, R.; Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G.; The Canadian Antimicrobial Resistance Alliance (CARA). Prevalence of antimicrobial resistant pathogens from blood cultures from Canadian hospitals: Results of the CANWARD 2007–2009 study. Diagn. Microbiol. Infect. Dis. 2011, 69, 307–313. [Google Scholar] [CrossRef]
- Antoniou, T.; Devlin, R.; Gough, K.; Mulvey, M.; Katz, K.C.; Zehtabchi, M.; Loutfy, M.R. Prevalence of community-associated methicillin-resistant Staphylococcus aureus colonization in men who have sex with men. Int. J. STD AIDS 2009, 20, 180–183. [Google Scholar] [CrossRef]
- Martin, P.; Chakra, C.N.A.; Williams, V.; Bush, K.; Dyck, M.; Hirji, Z.; Kiss, A.; Larios, O.E.; McGeer, A.; Moore, C.; et al. Prevalence of antibiotic-resistant organisms in Canadian Hospitals. Comparison of point-prevalence survey results from 2010, 2012, and 2016. Infect. Control. Hosp. Epidemiol. 2019, 40, 53–59. [Google Scholar] [CrossRef]
- Roth, V.R.; Longpre, T.; Taljaard, M.; Coyle, D.; Suh, K.N.; Muldoon, K.A.; Forster, A. Universal vs risk factor screening for methicillin-resistant staphylococcus aureus in a large multicenter tertiary care facility in Canada. Infect. Control. Hosp. Epidemiol. 2016, 37, 41–48. [Google Scholar] [CrossRef]
- Simor, A.E.; Pelude, L.; Golding, G.; Fernandes, R.; Bryce, E.; Frenette, C.; Program, C.N.I.S. Determinants of outcome in hospitalized patients with methicillin-resistant staphylococcus aureus bloodstream infection: Results from national surveillance in Canada, 2008–2012. Infect. Control. Hosp. Epidemiol. 2016, 37, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Suh, K.N.; Muldoon, K.A.; Oake, N.; Forster, A.; Ramotar, K.; Roth, V.R. Risk factors for methicillin-resistant staphylococcus aureus (mrsa) colonization among patients admitted to obstetrical units: A nested case-control study. J. Obstet. Gynaecol. Can. 2018, 40, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.; Simor, A.E.; Kiss, A.; McGeer, A.; Hirji, Z.; Larios, O.E.; Moore, C.; Weiss, K. Is the prevalence of antibiotic-resistant organisms changing in Canadian hospitals? Comparison of point-prevalence survey results in 2010 and 2012. Clin. Microbiol. Infect. 2015, 21, 553–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhanel, G.G.; DeCorby, M.; Adam, H.; Mulvey, M.R.; McCracken, M.; Lagacé-Wiens, P.; Alliance, C.A.R. Prevalence of antimicrobial-resistant pathogens in Canadian hospitals: Results of the Canadian Ward Surveillance Study (CANWARD 2008). Antimicrob. Agents Chemother. 2010, 54, 4684–4693. [Google Scholar] [CrossRef] [PubMed]
- Ugarte Torres, A.; Chu, A.; Read, R.; MacDonald, J.; Gregson, D.; Louie, T.; Conly, J. The epidemiology of Staphylococcus aureus carriage in patients attending inner city sexually transmitted infections and community clinics in Calgary, Canada. PLoS ONE 2017, 12, e0178557. [Google Scholar] [CrossRef]
- El Emam, K.; Arbuckle, L.; Essex, A.; Samet, S.; Eze, B.; Middleton, G.; Earle, C. Secure surveillance of antimicrobial resistant organism colonization or infection in Ontario long term care homes. PLoS ONE 2014, 9, e93285. [Google Scholar] [CrossRef]
- Muileboom, J.; Hamilton, M.; Parent, K.; Makahnouk, D.; Kirlew, M.; Saginur, R.; Kelly, L. Community-associated methicillin-resistant Staphylococcus aureus in northwest Ontario: A five-year report of incidence and antibiotic resistance. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, e42–e44. [Google Scholar] [CrossRef]
- Kim, J.; Ferrato, C.; Golding, G.R.; Mulvey, M.R.; Simmonds, K.A.; Svenson, L.W.; Louie, M. Changing epidemiology of methicillin-resistant Staphylococcus aureus in Alberta, Canada: Population-based surveillance, 2005–2008. Epidemiol. Infect. 2011, 139, 1009–1018. [Google Scholar] [CrossRef]
- Laupland, K.B.; Ross, T.; Gregson, D.B. Staphylococcus aureus bloodstream infections: Risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J. Infect. Dis. 2008, 198, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Lyytikäinen, O.; Søgaard, M.; Kennedy, K.J.; Knudsen, J.D.; Ostergaard, C.; Collaborative, I.B.S. The changing epidemiology of Staphylococcus aureus bloodstream infection: A multinational population-based surveillance study. Clin. Microbiol. Infect. 2013, 19, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Simor, A.E.; Gilbert, N.L.; Gravel, D.; Mulvey, M.R.; Bryce, E.; Loeb, M.; Program, C.N.I.S. Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: National surveillance and changing epidemiology, 1995–2007. Infect. Control. Hosp. Epidemiol. 2010, 31, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shammer, L.; Clarke, M. Preferred reporting items for systematic reviews and meta-analyses protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).