New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs
Abstract
1. Introduction
2. Methods of Literature Search and Review
3. Mammals (Class Mammalia)
3.1. Order Artiodactyla
3.1.1. Family Bovidae
European Cattle (Bos taurus)
Domestic Goat (Capra aegagrus Hircus)
Domestic Sheep (Ovis aries)
3.2. Order Carnivora
3.2.1. Family Canidae
Golden Jackal (Canis aureus)
Gray Wolf (Canis lupus)
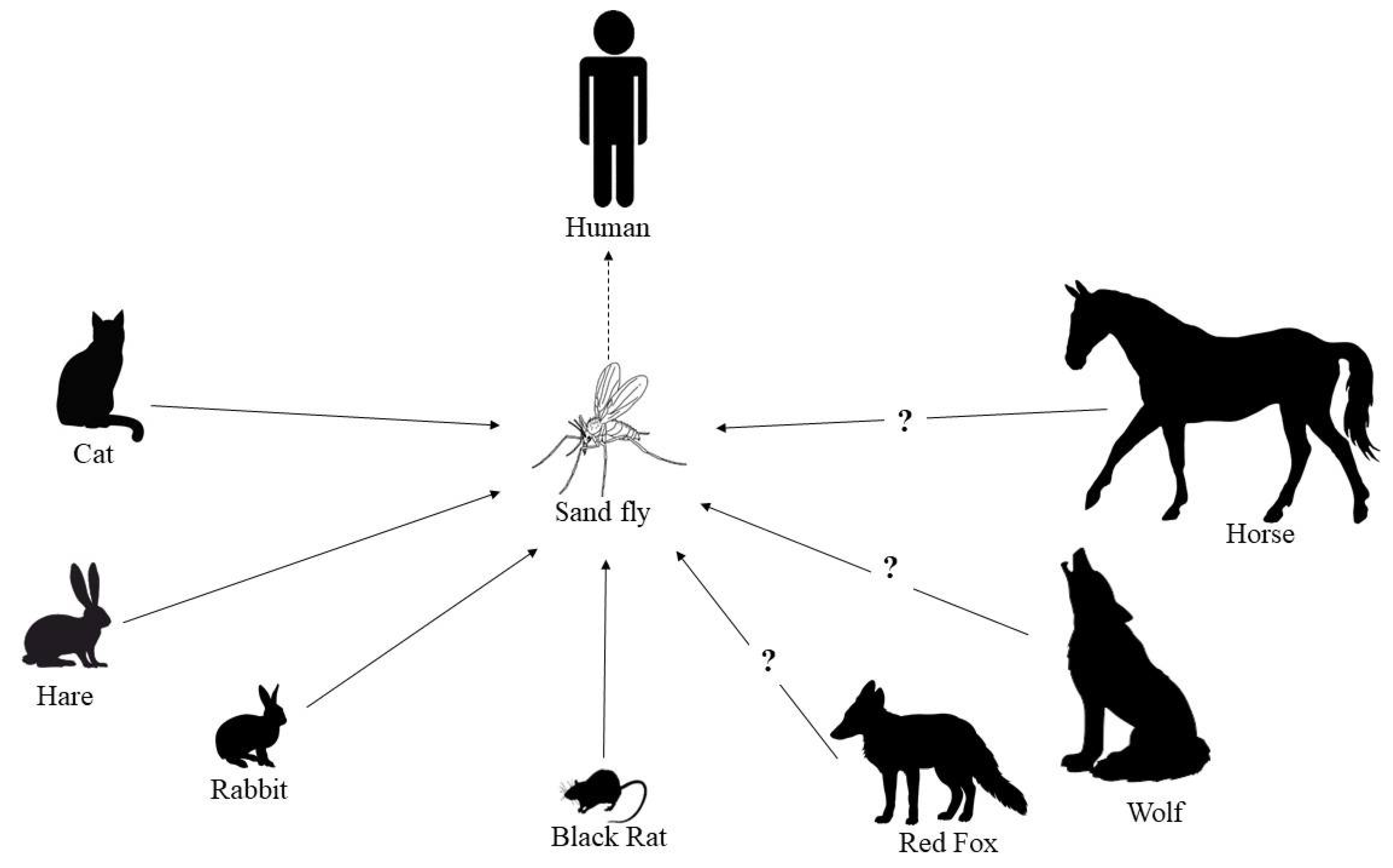
Red Fox (Vulpes vulpes)
3.2.2. Family Felidae
Domestic Cat (Felis Catus)
European Wildcat (Felis silvestris)
Iberian Lynx (Lynx pardinus)
Barbary Lion (Panthera leo)
Tiger (Panthera tigris)
3.2.3. Family Herpestidae
Egyptian Mongoose (Herpestes ichneumon)
3.2.4. Family Mustelidae
Eurasian Otter (Lutra lutra)
Beech Marten (Martes foina)
European Pine Marten (Martes martes)
European Badger (Meles meles)
European Mink (Mustela lutreola)
Polecat (Mustela putorius)
Domesticated Ferret (Mustela putorius Furo)
Stoat (Mustela erminea)
Least Weasel (Mustela nivalis)
American Mink (Neovison vison)
3.2.5. Family Phocidae
Mediterranean Monk Seal (Monachus monachus)
3.2.6. Family Ursidae
Brown Bear (Ursus arctos)
3.2.7. Family Viverridae
Common Genet (Genetta genetta)
3.2.8. Family Procyonidae
Raccoon (Procyon lotor)
3.3. Order Chiroptera
3.3.1. Family Vespertilionidae
Common Pipistrelle (Pipistrellus pipistrellus)
3.3.2. Family Miniopteridae
Common Bent-Wing Bat (Miniopterus schreibersii)
3.4. Order Diprotodontia
3.4.1. Family Macropodidae
Bennett’s Wallaby (Macropus rufogriseus)
3.5. Order Eulipotyphla
3.5.1. Family Erinaceidae
European Hedgehog (Erinaceus europaeus)
3.5.2. Family Soricidae
Greater White-Toothed Shrew (Crocidura russula)
Etruscan Shrew (Suncus etruscus)
3.6. Order Lagomorpha
3.6.1. Family Leporidae
European Hare (Lepus europaeus)
Iberian Hare (Lepus granatensis)
European Rabbit (Oryctolagyus cuniculus)
Broom Hare (Lepus castroviejoi)
3.7. Order Perissodactyla
3.7.1. Family Equidae
Horse (Equus ferus Caballus)
Donkey (Equus africanus Asinus)
3.8. Order Primates
3.8.1. Family Hominidae
North-West Bornean Orangutan (Pongo pygmaeus Pygmaeus)
3.9. Order Rodentia
3.9.1. Family Muridae
Wood Mouse (Apodemus sylvaticus)
House Mouse (Mus musculus)
Algerian Mouse (Mus spretus)
Brown Rat (Rattus norvegicus)
Black Rat (Rattus rattus)
3.9.2. Family Sciuridae
Eurasian Red Squirrel (Sciurus vulgaris)
4. Birds (Class Aves)
4.1. Order Anseriformes
4.1.1. Family Anatidae
Greylag Goose (Anser anser)
Muscovy Duck (Cairina moschata)
4.2. Order Galliformes
4.2.1. Family Phasianidae
Common Pheasant (Phasianus colchicus)
Chicken (Gallus gallus Domesticus)
4.2.2. Family Numidae
Helmeted Guineafowl (Numida meleagridis)
5. New Insights into the Future of Epidemiological Aspects of Animal Leishmaniosis in Europe
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- de Vries, H.J.; Reedijk, S.H.; Schallig, H.D. Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef]
- Courtenay, O.; Peters, N.C.; Rogers, M.E.; Bern, C. Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform leishmaniasis transmission dynamics and control. PLoS Pathog. 2017, 13, e1006571. [Google Scholar] [CrossRef]
- Kassai, T.; Cordero del Campillo, M.; Euzeby, J.; Gaafar, S.; Hiepe, T.; Himonas, C.A. Standardized nomenclature of animal parasitic diseases (SNOAPAD). Vet. Parasitol. 1988, 29, 299–326. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef]
- Miró, G.; Petersen, C.; Cardoso, L.; Bourdeau, P.; Baneth, G.; Solano-Gallego, L.; Pennisi, M.G.; Ferrer, L.; Oliva, G. Novel areas for prevention and control of canine leishmaniosis. Trends Parasitol. 2017, 33, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet Group. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Palatnik-de-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasit. Vectors 2011, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- Rugna, G.; Carra, E.; Bergamini, F.; Calzolari, M.; Salvatore, D.; Corpus, F.; Gennari, W.; Baldelli, R.; Fabbi, M.; Natalini, S.; et al. Multilocus microsatellite typing (MLMT) reveals host-related population structure in Leishmania infantum from northeastern Italy. PLoS Negl. Trop. Dis. 2018, 12, e0006595. [Google Scholar] [CrossRef]
- Millán, J.; Ferroglio, E.; Solano-Gallego, L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014, 113, 2005–2014. [Google Scholar] [CrossRef]
- Lobsiger, L.; Müller, N.; Schweizer, T.; Frey, C.F.; Wiederkehr, D.; Zumkehr, B.; Gottstein, B. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet. Parasitol. 2010, 169, 408–414. [Google Scholar] [CrossRef]
- Sukmee, T.; Siripattanapipong, S.; Mungthin, M.; Worapong, J.; Rangsin, R.; Samung, Y.; Kongkaew, W.; Bumrungsana, K.; Chanachai, K.; Apiwathanasorn, C.; et al. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int. J. Parasitol. 2008, 38, 617–622. [Google Scholar] [CrossRef]
- Müller, N.; Welle, M.; Lobsiger, L.; Stoffel, M.H.; Boghenbor, K.K.; Hilbe, M.; Gottstein, B.; Frey, C.F.; Geyer, C.; von Bomhard, W. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet. Parasitol. 2009, 166, 346–351. [Google Scholar] [CrossRef]
- Desbois, N.; Pratlong, F.; Quist, D.; Dedet, J.P. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies). Parasite 2014, 21, 12. [Google Scholar] [CrossRef]
- Lobsiger, L.; Frey, C.; Müller, N.; Rosenberg, G.; Schweizer, T.; Gottstein, B. Breitet sich die bovine kutane Leishmaniose in der Schweiz aus [Is bovine leishmaniasis spreading in Switzerland]? Schweiz. Arch. Tierheilkd. 2012, 154, 199–207. [Google Scholar] [CrossRef]
- Kantzoura, V.; Diakou, A.; Kouam, M.K.; Feidas, H.; Theodoropoulou, H.; Theodoropoulos, G. Seroprevalence and risk factors associated with zoonotic parasitic infections in small ruminants in the Greek temperate environment. Parasitol. Int. 2013, 62, 554–560. [Google Scholar] [CrossRef]
- Cirović, D.; Chochlakis, D.; Tomanović, S.; Sukara, R.; Penezić, A.; Tselentis, Y.; Psaroulaki, A. Presence of Leishmania and Brucella species in the golden jackal Canis aureus in Serbia. Biomed. Res. Int. 2014, 2014, 728516. [Google Scholar] [CrossRef]
- Babuadze, G.; Alvar, J.; Argaw, D.; de Koning, H.P.; Iosava, M.; Kekelidze, M.; Tsertsvadze, N.; Tsereteli, D.; Chakhunashvili, G.; Mamatsashvili, T.; et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014, 8, e2725. [Google Scholar] [CrossRef] [PubMed]
- Mitková, B.; Hrazdilová, K.; D’Amico, G.; Duscher, G.G.; Suchentrunk, F.; Forejtek, P.; Gherman, C.M.; Matei, I.A.; Ionică, A.M.; Daskalaki, A.A.; et al. Eurasian golden jackal as host of canine vector-borne protists. Parasit. Vectors 2017, 10, 183. [Google Scholar] [CrossRef]
- Beck, A.; Beck, R.; Kusak, J.; Gudan, A.; Martinković, F.; Artuković, B.; Hohšteter, M.; Huber, Ð.; Marinculić, A.; Grabarević, Ž. A case of visceral leishmaniosis in a gray wolf (Canis lupus) from Croatia. J. Wildl. Dis. 2008, 44, 451–456. [Google Scholar] [CrossRef]
- Sastre, N.; Francino, O.; Ramírez, O.; Enseñat, C.; Sánchez, A.; Altet, L. Detection of Leishmania infantum in captive wolves from Southwestern Europe. Vet. Parasitol. 2008, 158, 117–120. [Google Scholar] [CrossRef]
- Muñoz-Madrid, R.; Belinchón-Lorenzo, S.; Iniesta, V.; Fernández-Cotrina, J.; Parejo, J.C.; Serrano, F.J.; Monroy, I.; Baz, V.; Gómez-Luque, A.; Gómez-Nieto, L.C. First detection of Leishmania infantum kinetoplast DNA in hair of wild mammals: Application of qPCR method to determine potential parasite reservoirs. Acta Trop. 2013, 128, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Vicente, J.; Ferroglio, E.; Pegoraro de Macedo, M.R.; Casais, R.; del Cerro, A.; Espí, A.; García, E.J.; Gortázar, C. Concomitance and interactions of pathogens in the Iberian wolf (Canis lupus). Res. Vet. Sci. 2015, 101, 22–27. [Google Scholar] [CrossRef]
- Sobrino, R.; Ferroglio, E.; Oleaga, A.; Romano, A.; Millan, J.; Revilla, M.; Arnal, M.C.; Trisciuoglio, A.; Gortázar, C. Characterization of widespread canine leishmaniasis among wild carnivores from Spain. Vet. Parasitol. 2008, 155, 198–203. [Google Scholar] [CrossRef]
- Oleaga, A.; Zanet, S.; Espí, A.; Pegoraro de Macedo, M.R.; Gortázar, C.; Ferroglio, E. Leishmania in wolves in northern Spain: A spreading zoonosis evidenced by wildlife sanitary surveillance. Vet. Parasitol. 2018, 255, 26–31. [Google Scholar] [CrossRef]
- Risueño, J.; Ortuño, M.; Pérez-Cutillas, P.; Goyena, E.; Maia, C.; Cortes, S.; Campino, L.; Bernal, L.J.; Muñoz, C.; Arcenillas, I.; et al. Epidemiological and genetic studies suggest a common Leishmania infantum transmission cycle in wildlife, dogs and humans associated to vector abundance in Southeast Spain. Vet. Parasitol. 2018, 259, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, M.; Latrofa, M.S.; Iborra, M.A.; Pérez-Cutillas, P.; Bernal, L.J.; Risueño, J.; Muñoz, C.; Bernal, A.; Sánchez-Lopez, P.F.; Segovia, M.; et al. Genetic diversity and phylogenetic relationships between Leishmania infantum from dogs, humans and wildlife in south-east Spain. Zoonoses Public Health 2019, 66, 961–973. [Google Scholar] [CrossRef]
- Battisti, E.; Zanet, S.; Khalili, S.; Trisciuoglio, A.; Hertel, B.; Ferroglio, E. Molecular survey on vector-borne pathogens in Alpine wild carnivorans. Front. Vet. Sci. 2020, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Dipineto, L.; Manna, L.; Baiano, A.; Gala, M.; Fioretti, A.; Gravino, A.E.; Menna, L.F. Presence of Leishmania infantum in red foxes (Vulpes vulpes) in southern Italy. J. Wildl. Dis. 2007, 43, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Piantedosi, D.; Veneziano, V.; Di Muccio, T.; Manzillo, V.F.; Fiorentino, E.; Scalone, A.; Neola, B.; Di Prisco, F.; D’Alessio, N.; Gradoni, L.; et al. Epidemiological survey on Leishmania infection in red foxes (Vulpes vulpes) and hunting dogs sharing the same rural area in Southern Italy. Acta Parasitol. 2016, 61, 769–775. [Google Scholar] [CrossRef]
- Verin, R.; Poli, A.; Ariti, G.; Nardoni, S.; Bertuccelli Fanucchi, M.; Mancianti, F. Detection of Leishmania infantum DNA in tissues of free-ranging red foxes (Vulpes vulpes) in Central Italy. Eur. J. Wild Res. 2010, 56, 689–692. [Google Scholar] [CrossRef]
- Abbate, J.M.; Arfuso, F.; Napoli, E.; Gaglio, G.; Giannetto, S.; Latrofa, M.S.; Otranto, D.; Brianti, E. Leishmania infantum in wild animals in endemic areas of southern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101374. [Google Scholar] [CrossRef]
- Del Río, L.; Chitimia, L.; Cubas, A.; Victoriano, I.; De la Rúa, P.; Gerrikagoitia, X.; Barral, M.; Muñoz-García, C.I.; Goyena, E.; García-Martínez, D.; et al. Evidence for widespread Leishmania infantum infection among wild carnivores in L. infantum periendemic northern Spain. Prev. Vet. Med. 2014, 113, 430–435. [Google Scholar] [CrossRef]
- Lledó, L.; Giménez-Pardo, C.; Saz, J.V.; Serrano, J.L. Wild red foxes (Vulpes vulpes) as sentinels of parasitic diseases in the province of Soria, northern Spain. Vector Borne Zoonotic Dis. 2015, 15, 743–749. [Google Scholar] [CrossRef]
- Davoust, B.; Mary, C.; Marié, J.L. Detection of Leishmania in red foxes (Vulpes vulpes) from southeastern France using real-time quantitative PCR. J. Wildl. Dis. 2014, 50, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Medkour, H.; Laidoudi, Y.; Marié, J.L.; Fenollar, F.; Davoust, B.; Mediannikov, O. Molecular investigation of vector-borne pathogens in red foxes (Vulpes vulpes) from southern France. J. Wildl. Dis. 2020, 56, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Gilad, M.; Cortes, H.C.; Nachum-Biala, Y.; Lopes, A.P.; Vila-Viçosa, M.J.; Simões, M.; Rodrigues, P.A.; Baneth, G. First report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasit. Vectors 2015, 8, 144. [Google Scholar] [CrossRef]
- Karayiannis, S.; Ntais, P.; Messaritakis, I.; Tsirigotakis, N.; Dokianakis, E.; Antoniou, M. Detection of Leishmania infantum in red foxes (Vulpes vulpes) in Central Greece. Parasitology 2015, 142, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet update and recommendations on feline leishmaniosis. Parasit. Vectors 2015, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Persichetti, M.F. Feline leishmaniosis: Is the cat a small dog? Vet. Parasitol. 2018, 251, 131–137. [Google Scholar] [CrossRef]
- Can, H.; Döşkaya, M.; Özdemir, H.G.; Şahar, E.A.; Karakavuk, M.; Pektaş, B.; Karakuş, M.; Töz, S.; Caner, A.; Döşkaya, A.D.; et al. Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of İzmir, Turkey. Exp. Parasitol. 2016, 167, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Paşa, S.; Tetik Vardarlı, A.; Erol, N.; Karakuş, M.; Töz, S.; Atasoy, A.; Balcıoğlu, İ.C.; Emek Tuna, G.; Ermiş, Ö.V.; Ertabaklar, H.; et al. Detection of Leishmania major and Leishmania tropica in domestic cats in the Ege Region of Turkey. Vet. Parasitol. 2015, 212, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Parreira, R.; Cristóvão, J.M.; Castelli, G.; Bruno, F.; Vitale, F.; Campino, L.; Maia, C. Phylogenetic insights on Leishmania detected in cats as revealed by nucleotide sequence analysis of multiple genetic markers. Infect. Genet. Evol. 2020, 77, 104069. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Zanet, S.; Gomis, M.; Trisciuoglio, A.; Negre, N.; Ferroglio, E. An investigation into alternative reservoirs of canine leishmaniasis on the endemic island of Mallorca (Spain). Transbound. Emerg. Dis. 2011, 58, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Lupo, T.; Malara, D.; Masucci, M.; Migliazzo, A.; Lombardo, G. Serological and molecular prevalence of Leishmania infantum infection in cats from Southern Italy. J. Feline. Med. Surg. 2012, 14, 656–657. [Google Scholar]
- Priolo, V.; Martínez-Orellana, P.; Pennisi, M.G.; Masucci, M.; Prandi, D.; Ippolito, D.; Bruno, F.; Castelli, G.; Solano-Gallego, L. Leishmania infantum-specific IFN-γ production in stimulated blood from cats living in areas where canine leishmaniosis is endemic. Parasit. Vectors 2019, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, M.K.; Leontides, L.; Athanasiou, L.V.; Papadopoulos, E.; Kasabalis, D.; Mylonakis, M.; Rallis, T.; Koutinas, A.F.; Andreadou, M.; Ikonomopoulos, J.; et al. Evaluation of indirect immunofluorescence antibody test and enzyme-linked immunosorbent assay for the diagnosis of infection by Leishmania infantum in clinically normal and sick cats. Exp. Parasitol. 2014, 147, 54–59. [Google Scholar] [CrossRef]
- Chatzis, M.K.; Andreadou, M.; Leontides, L.; Kasabalis, D.; Mylonakis, M.; Koutinas, A.F.; Rallis, T.; Ikonomopoulos, J.; Saridomichelakis, M.N. Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Vet. Parasitol. 2014, 202, 217–225. [Google Scholar] [CrossRef]
- Vita, S.; Santori, D.; Aguzzi, I.; Petrotta, E.; Luciani, A. Feline leishmaniasis and ehrlichiosis: Serological investigation in Abruzzo region. Vet. Res. Commun. 2005, 29 (Suppl. S2), 319–321. [Google Scholar] [CrossRef]
- Silaghi, C.; Knaus, M.; Rapti, D.; Kusi, I.; Shukullari, E.; Hamel, D.; Pfister, K.; Rehbein, S. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasit. Vectors 2014, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Attipa, C.; Papasouliotis, K.; Solano-Gallego, L.; Baneth, G.; Nachum-Biala, Y.; Sarvani, E.; Knowles, T.G.; Mengi, S.; Morris, D.; Helps, C.; et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit. Vectors 2017, 10, 130. [Google Scholar] [CrossRef]
- Diakou, A.; Papadopoulos, E.; Lazarides, K. Specific anti-Leishmania spp. antibodies in stray cats in Greece. J. Feline. Med. Surg. 2009, 11, 728–730. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Accettura, P.M.; Barros, L.; Iorio, R.; Paoletti, B.; Frangipane di Regalbono, A.; Halos, L.; Beugnet, F.; Traversa, D. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl. Trop. Dis. 2017, 11, e0005335. [Google Scholar] [CrossRef]
- Giannakopoulos, A.; Tsokana, C.; Papadopoulos, E.; Spyrou, V.; Chatzopoulos, D.; Valiakos, G.; Touloudi, A.; Athanasiou, L.; Billinis, C. Molecular investigation and geographical distribution of Leishmania spp. infection in stray and owned cats (Felis catus) in Thessaly, central Greece. J. Hellenic. Vet. Med. Soc. 2017, 68, 27–34. [Google Scholar] [CrossRef][Green Version]
- Morelli, S.; Colombo, M.; Dimzas, D.; Barlaam, A.; Traversa, D.; Di Cesare, A.; Russi, I.; Spoletini, R.; Paoletti, B.; Diakou, A. Leishmania infantum seroprevalence in cats from touristic areas of Italy and Greece. Front. Vet. Sci. 2020, 7, 616566. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Proverbio, D.; Migliazzo, A.; Della Pepa, A.; Perego, R.; Bagnagatti De Giorgi, G. Serological and molecular evaluation of Leishmania infantum infection in stray cats in a nonendemic area in northern Italy. ISRN Parasitol. 2013, 2013, 916376. [Google Scholar] [CrossRef][Green Version]
- Spada, E.; Canzi, I.; Baggiani, L.; Perego, R.; Vitale, F.; Migliazzo, A.; Proverbio, D. Prevalence of Leishmania infantum and co-infections in stray cats in northern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 53–58. [Google Scholar] [CrossRef]
- Spada, E.; Perego, R.; Vitale, F.; Bruno, F.; Castelli, G.; Tarantola, G.; Baggiani, L.; Magistrelli, S.; Proverbio, D. Feline Leishmania spp. infection in a non-endemic area of northern Italy. Animals 2020, 10, 817. [Google Scholar] [CrossRef]
- Morganti, G.; Veronesi, F.; Stefanetti, V.; Di Muccio, T.; Fiorentino, E.; Diaferia, M.; Santoro, A.; Passamonti, F.; Gramiccia, M. Emerging feline vector-borne pathogens in Italy. Parasit. Vectors 2019, 12, 193. [Google Scholar] [CrossRef]
- Dedola, C.; Zobba, R.; Varcasia, A.; Visco, S.; Alberti, A.; Pipia, A.P.; Scala, A.; Pinna Parpaglia, M.L. Serological and molecular detection of Leishmania infantum in cats of Northern Sardinia, Italy. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 120–123. [Google Scholar] [CrossRef]
- Persichetti, M.F.; Solano-Gallego, L.; Vullo, A.; Masucci, M.; Marty, P.; Delaunay, P.; Vitale, F.; Pennisi, M.G. Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasit. Vectors 2017, 10, 119. [Google Scholar] [CrossRef]
- Persichetti, M.F.; Pennisi, M.G.; Vullo, A.; Masucci, M.; Migliazzo, A.; Solano-Gallego, L. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasit. Vectors 2018, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Napoli, E.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Greco, G.; Lorusso, E.; Gulotta, L.; Falsone, L.; Basano, F.S.; et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet. Parasitol. 2017, 236, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Brianti, E.; Falsone, L.; Napoli, E.; Gaglio, G.; Giannetto, S.; Pennisi, M.G.; Priolo, V.; Latrofa, M.S.; Tarallo, V.D.; Solari Basano, F.; et al. Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit. Vectors 2017, 10, 334. [Google Scholar] [CrossRef]
- Iatta, R.; Furlanello, T.; Colella, V.; Tarallo, V.D.; Latrofa, M.S.; Brianti, E.; Trerotoli, P.; Decaro, N.; Lorusso, E.; Schunack, B.; et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl. Trop. Dis. 2019, 13, e0007594. [Google Scholar] [CrossRef] [PubMed]
- Urbani, L.; Tirolo, A.; Salvatore, D.; Tumbarello, M.; Segatore, S.; Battilani, M.; Balboni, A.; Dondi, F. Serological, molecular and clinicopathological findings associated with Leishmania infantum infection in cats in Northern Italy. J. Feline. Med. Surg. 2020, 22, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Guardone, L.; Marra, F.; Altomonte, I.; Nardoni, S.; Mancianti, F. Arthropod-borne pathogens in stray cats from northern Italy: A serological and molecular survey. Animals 2020, 10, 2334. [Google Scholar] [CrossRef]
- Maia, C.; Nunes, M.; Campino, L. Importance of cats in zoonotic leishmaniasis in Portugal. Vector Borne Zoonotic Dis. 2008, 8, 555–559. [Google Scholar] [CrossRef]
- Duarte, A.; Castro, I.; Pereira da Fonseca, I.M.; Almeida, V.; Madeira de Carvalho, L.M.; Meireles, J.; Fazendeiro, M.I.; Tavares, L.; Vaz, Y. Survey of infectious and parasitic diseases in stray cats at the Lisbon Metropolitan Area, Portugal. J. Feline. Med. Surg. 2010, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Gomes, J.; Cristóvão, J.; Nunes, M.; Martins, A.; Rebêlo, E.; Campino, L. Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet. Parasitol. 2010, 174, 336–340. [Google Scholar] [CrossRef]
- Cardoso, L.; Lopes, A.P.; Sherry, K.; Schallig, H.; Solano-Gallego, L. Low seroprevalence of Leishmania infantum infection in cats from northern Portugal based on DAT and ELISA. Vet. Parasitol. 2010, 174, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, H.; Martinez-Díaz, V.L.; Cardoso, L.; Vieira, L.; Altet, L.; Francino, O.; Pastor, J.; Silvestre-Ferreira, A.C. Feline vector-borne pathogens in the north and centre of Portugal. Parasit. Vectors 2013, 6, 99. [Google Scholar] [CrossRef]
- Maia, C.; Ramos, C.; Coimbra, M.; Bastos, F.; Martins, A.; Pinto, P.; Nunes, M.; Vieira, M.L.; Cardoso, L.; Campino, L. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit. Vectors 2014, 7, 115. [Google Scholar] [CrossRef]
- Maia, C.; Ramos, C.; Coimbra, M.; Cardoso, L.; Campino, L. Prevalence of Dirofilaria immitis antigen and antibodies to Leishmania infantum in cats from southern Portugal. Parasitol. Int. 2015, 64, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Cristóvão, J.M.; Vilhena, H.; Martins, Â.; Cachola, P.; Henriques, J.; Coimbra, M.; Catarino, A.; Lestinova, T.; Spitzova, T.; et al. Antibody response to Phlebotomus perniciosus saliva in cats naturally exposed to phlebotomine sand flies is positively associated with Leishmania infection. Parasit. Vectors 2019, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, J.; Acedo, C.; Muñoz-Pérez, M.; Pesson, B.; Marchal, O.; Morillas-Márquez, F. Infection by Leishmania infantum in cats: Epidemiological study in Spain. Vet. Parasitol. 2007, 145, 267–273. [Google Scholar] [CrossRef]
- Navarro, J.A.; Sánchez, J.; Peñafiel-Verdú, C.; Buendía, A.J.; Altimira, J.; Vilafranca, M. Histopathological lesions in 15 cats with leishmaniosis. J. Comp. Pathol. 2010, 143, 297–302. [Google Scholar] [CrossRef]
- Ayllon, T.; Tesouro, M.A.; Amusategui, I.; Villaescusa, A.; Rodriguez-Franco, F.; Sainz, A. Serologic and molecular evaluation of Leishmania infantum in cats from Central Spain. Ann. N. Y. Acad. Sci. 2008, 1149, 361–364. [Google Scholar] [CrossRef]
- Ayllón, T.; Diniz, P.P.; Breitschwerdt, E.B.; Villaescusa, A.; Rodríguez-Franco, F.; Sainz, A. Vector-borne diseases in client-owned and stray cats from Madrid, Spain. Vector Borne Zoonotic Dis. 2012, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Hernández, L.; Montoya, A.; Arranz-Solís, D.; Dado, D.; Rojo-Montejo, S.; Mendoza-Ibarra, J.A.; Ortega-Mora, L.M.; Pedraza-Díaz, S. First description of naturally acquired Tritrichomonas foetus infection in a Persian cattery in Spain. Parasitol. Res. 2011, 109, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.; Gramiccia, M.; Molina, R.; Dvorak, V.; Volf, P. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Eurosurveillance 2013, 18, 20540. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Álvarez, J.; García, N.; de la Fuente, S.; Martínez, I.; Marino, E.; Toraño, A.; Goyache, J.; Vilas, F.; Domínguez, L.; et al. Detection of anti-Leishmania infantum antibodies in sylvatic lagomorphs from an epidemic area of Madrid using the indirect immunofluorescence antibody test. Vet. Parasitol. 2014, 199, 264–267. [Google Scholar] [CrossRef]
- Miró, G.; Rupérez, C.; Checa, R.; Gálvez, R.; Hernández, L.; García, M.; Canorea, I.; Marino, V.; Montoya, A. Current status of L. infantum infection in stray cats in the Madrid region (Spain): Implications for the recent outbreak of human leishmaniosis? Parasit. Vectors 2014, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; García, M.; Gálvez, R.; Checa, R.; Marino, V.; Sarquis, J.; Barrera, J.P.; Rupérez, C.; Caballero, L.; Chicharro, C.; et al. Implications of zoonotic and vector-borne parasites to free-roaming cats in central Spain. Vet. Parasitol. 2018, 251, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Rodríguez-Cortés, A.; Iniesta, L.; Quintana, J.; Pastor, J.; Espada, Y.; Portús, M.; Alberola, J. Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the Northwestern Mediterranean. Am. J. Trop. Med. Hyg. 2007, 76, 676–680. [Google Scholar] [CrossRef]
- Tabar, M.D.; Altet, L.; Francino, O.; Sánchez, A.; Ferrer, L.; Roura, X. Vector-borne infections in cats: Molecular study in Barcelona area (Spain). Vet. Parasitol. 2008, 151, 332–336. [Google Scholar] [CrossRef]
- Sherry, K.; Miró, G.; Trotta, M.; Miranda, C.; Montoya, A.; Espinosa, C.; Ribas, F.; Furlanello, T.; Solano-Gallego, L. A serological and molecular study of Leishmania infantum infection in cats from the Island of Ibiza (Spain). Vector Borne Zoonotic Dis. 2011, 11, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Venza, M.; Reale, S.; Vitale, F.; Lo Giudice, S. Case report of leishmaniasis in four cats. Vet. Res. Commun. 2004, 28 (Suppl. S1), 363–366. [Google Scholar] [CrossRef]
- Hervás, J.; Chacón-Manrique de Lara, F.; López, J.; Gómez-Villamandos, J.C.; Guerrero, M.J.; Moreno, A. Granulomatous (pseudotumoral) iridociclitis associated with leishmaniasis in a cat. Vet. Rec. 2001, 149, 624–625. [Google Scholar] [CrossRef]
- Poli, A.; Abramo, F.; Barsotti, P.; Leva, S.; Gramiccia, M.; Ludovisi, A.; Mancianti, F. Feline leishmaniosis due to Leishmania infantum in Italy. Vet. Parasitol. 2002, 106, 181–191. [Google Scholar] [CrossRef]
- Verneuil, M. Leishmaniose oculaire féline: À propos d’un cas [Ocular leishmaniasis in a cat: Case report]. J. Fr. Ophtalmol. 2013, 36, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Grevot, A.; Jaussaud Hugues, P.; Marty, P.; Pratlong, F.; Ozon, C.; Haas, P.; Breton, C.; Bourdoiseau, G. Leishmaniosis due to Leishmania infantum in a FIV and FelV positive cat with a squamous cell carcinoma diagnosed with histological, serological and isoenzymatic methods. Parasite 2005, 12, 271–275. [Google Scholar] [CrossRef]
- Rüfenacht, S.; Sager, H.; Müller, N.; Schaerer, V.; Heier, A.; Welle, M.M.; Roosje, P.J. Two cases of feline leishmaniosis in Switzerland. Vet. Rec. 2005, 156, 542–545. [Google Scholar] [CrossRef]
- Maroli, M.; Pennisi, M.G.; Di Muccio, T.; Khoury, C.; Gradoni, L.; Gramiccia, M. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet. Parasitol. 2007, 145, 357–360. [Google Scholar] [CrossRef]
- Pocholle, E.; Reyes-Gomez, E.; Giacomo, A.; Delaunay, P.; Hasseine, L.; Marty, P. Un cas de leishmaniose féline disséminée dans le sud de la France [A case of feline leishmaniasis in the south of France]. Parasite 2012, 19, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Santos, M.; Malhão, F.; Pereira, R.; Fernandes, A.C.; Montenegro, L.; Roccabianca, P. Pancytopenia in a cat with visceral leishmaniasis. Vet. Clin. Pathol. 2009, 38, 201–205. [Google Scholar] [CrossRef]
- Brianti, E.; Celi, N.; Napoli, E.; Abbate, J.M.; Arfuso, F.; Gaglio, G.; Iatta, R.; Giannetto, S.; Gramiccia, M.; Otranto, D. Treatment and long-term follow-up of a cat with leishmaniosis. Parasit. Vectors 2019, 12, 121. [Google Scholar] [CrossRef]
- Attipa, C.; Neofytou, K.; Yiapanis, C.; Martínez-Orellana, P.; Baneth, G.; Nachum-Biala, Y.; Brooks-Brownlie, H.; Solano-Gallego, L.; Tasker, S. Follow-up monitoring in a cat with leishmaniosis and coinfections with Hepatozoon felis and ‘Candidatus Mycoplasma haemominutum’. JFMS Open Rep. 2017, 3, 2055116917740454. [Google Scholar] [CrossRef]
- Pereira, A.; Valente, J.; Parreira, R.; Cristovão, J.M.; Azinheira, S.; Campino, L.; Maia, C. An unusual case of feline leishmaniosis with involvement of the mammary glands. Top Companion Anim. Med. 2019, 37, 100356. [Google Scholar] [CrossRef]
- Migliazzo, A.; Vitale, F.; Calderone, S.; Puleio, R.; Binanti, D.; Abramo, F. Feline leishmaniosis: A case with a high parasitic burden. Vet. Dermatol. 2015, 26, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, P.; Alves-Pimenta, S.; Barros, J.; Barbosa, P.; Rodrigues, A.; Pereira, M.J.; Maltez, L.; Gama, A.; Cristóvão, J.M.; Campino, L.; et al. Feline leishmaniosis in Portugal: 3 cases (year 2014). Vet. Parasitol. Reg. Stud. Rep. 2015, 1–2, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.A.; Marques, C.; Santos, M.; Duarte, A.; Pissarra, H.; Carreira, L.M.; Gomes, L.; Valério-Bolas, A.; Tavares, L.; Santos-Gomes, G.; et al. Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. JFMS Open Rep. 2016, 2, 2055116916630002. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.O.; Pereira, H.; Cartaxeiro, C.; Delgado, E.; Peleteiro, M.D.C.; Pereira da Fonseca, I. Granulomatous rhinitis secondary to feline leishmaniosis: Report of an unusual presentation and therapeutic complications. JFMS Open Rep. 2018, 4, 2055116918811374. [Google Scholar] [CrossRef]
- Altuzarra, R.; Movilla, R.; Roura, X.; Espada, Y.; Majo, N.; Novellas, R. Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet. Radiol. Ultrasound 2020, 61, E64–E68. [Google Scholar] [CrossRef]
- Fernandez-Gallego, A.; Feo Bernabe, L.; Dalmau, A.; Esteban-Saltiveri, D.; Font, A.; Leiva, M.; Ortuñez-Navarro, A.; Peña, M.T.; Tabar, M.D.; Real-Sampietro, L.; et al. Feline leishmaniosis: Diagnosis, treatment and outcome in 16 cats. J. Feline. Med. Surg. 2020, 22, 993–1007. [Google Scholar] [CrossRef]
- Leiva, M.; Lloret, A.; Peña, T.; Roura, X. Therapy of ocular and visceral leishmaniasis in a cat. Vet. Ophthalmol. 2005, 8, 71–75. [Google Scholar] [CrossRef]
- Jiménez, M.; González, E.; Iriso, A.; Marco, E.; Alegret, A.; Fúster, F.; Molina, R. Detection of Leishmania infantum and identification of blood meals in Phlebotomus perniciosus from a focus of human leishmaniasis in Madrid, Spain. Parasitol. Res. 2013, 112, 2453–2459. [Google Scholar] [CrossRef]
- González, E.; Jiménez, M.; Hernández, S.; Martín-Martín, I.; Molina, R. Phlebotomine sand fly survey in the focus of leishmaniasis in Madrid, Spain (2012–2014): Seasonal dynamics, Leishmania infantum infection rates and blood meal preferences. Parasit. Vectors 2017, 10, 368. [Google Scholar] [CrossRef]
- Maia, C.; Dionísio, L.; Afonso, M.O.; Neto, L.; Cristóvão, J.M.; Campino, L. Leishmania infection and host-blood feeding preferences of phlebotomine sandflies and canine leishmaniasis in an endemic European area, the Algarve Region in Portugal. Mem. Inst. Oswaldo Cruz 2013, 108, 481–487. [Google Scholar] [CrossRef]
- Alcover, M.M.; Ribas, A.; Guillén, M.C.; Berenguer, D.; Tomás-Pérez, M.; Riera, C.; Fisa, R. Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area. Prev. Vet. Med. 2020, 175, 104874. [Google Scholar] [CrossRef]
- Libert, C.; Ravel, C.; Pratlong, F.; Lami, P.; Dereure, J.; Keck, N. Leishmania infantum infection in two captive barbary lions (Panthera leo leo). J. Zoo Wildl. Med. 2012, 43, 685–688. [Google Scholar] [CrossRef]
- Iatta, R.; Zatelli, A.; Laricchiuta, P.; Legrottaglie, M.; Modry, D.; Dantas-Torres, F.; Otranto, D. Leishmania infantum in tigers and sand flies from a leishmaniasis-endemic area, southern Italy. Emerg. Infect. Dis. 2020, 26, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, A.; Iatta, R.; Laricchiuta, P.; Passantino, G.; Abramo, F.; Mendoza-Roldan, J.A.; Otranto, D.; Zatelli, A. Clinical, haematological and biochemical findings in tigers infected by Leishmania infantum. BMC Vet. Res. 2020, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Rocha, H.; Carvalho, C.; Bandeira, V.; Fonseca, C.; Rosalino, L.M.; Cunha, M.V. Molecular detection and characterization of Leishmania infantum in free-ranging Egyptian mongoose (Herpestes ichneumon). Int. J. Parasitol. Parasites Wildl. 2020, 11, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Cantos-Barreda, A.; Navarro, R.; Pardo-Marín, L.; Martínez-Subiela, S.; Ortega, E.; Cerón, J.J.; Tecles, F.; Escribano, D. Clinical leishmaniosis in a captive Eurasian otter (Lutra lutra) in Spain: A case report. BMC Vet. Res. 2020, 16, 312. [Google Scholar] [CrossRef] [PubMed]
- Giner, J.; Villanueva-Saz, S.; Alcover, M.M.; Riera, C.; Fisa, R.; Basurco, A.; Yzuel, A.; Trotta, M.; Fani, C.; Verde, M.T.; et al. Treatment and follow-up of a domestic ferret (Mustela putorius furo) with clinical leishmaniosis caused by Leishmania infantum. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100423. [Google Scholar] [CrossRef]
- Giner, J.; Basurco, A.; Alcover, M.M.; Riera, C.; Fisa, R.; López, R.A.; Juan-Sallés, C.; Verde, M.T.; Fernández, A.; Yzuel, A.; et al. First report on natural infection with Leishmania infantum in a domestic ferret (Mustela putorius furo) in Spain. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100369. [Google Scholar] [CrossRef]
- Filioussis, G.; Petridou, E.; Papadopoulos, D.; Karavanis, E.; Morgan, E.; Billinis, C.; Papadopoulos, E. Hemorrhagic pneumonia in neonatal minks in Greece concomitant with Leismania infantum detection. Pol. J. Vet. Sci. 2018, 21, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakidis, Ι.; Pavlou, C.; Tamvakis, A.; Papadopoulos, T.; Christodoulou, V.; Angelopoulou, K.; Dovas, C.I.; Antoniou, Μ.; Anastasakis, C.; Diakou, A. Leishmania infection in lagomorphs and minks in Greece. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100279. [Google Scholar] [CrossRef] [PubMed]
- Toplu, N.; Aydoğan, A.; Oguzoglu, T.C. Visceral leishmaniosis and parapoxvirus infection in a Mediterranean monk seal (Monachus monachus). J. Comp. Pathol. 2007, 136, 283–287. [Google Scholar] [CrossRef]
- Millán, J.; Chirife, A.D.; Altet, L. Serum chemistry reference values for the common genet (Genetta genetta): Variations associated with Leishmania infantum infection. Vet. Q. 2015, 35, 43–47. [Google Scholar] [CrossRef]
- Azami-Conesa, I.; Martínez-Díaz, R.A.; González, F.; Gómez-Muñoz, M.T. First detection of Leishmania infantum in common urban bats Pipistrellus pipistrellus in Europe. Res. Vet. Sci. 2020, 132, 172–176. [Google Scholar] [CrossRef]
- Millán, J.; López-Roig, M.; Cabezón, O.; Serra-Cobo, J. Absence of Leishmania infantum in cave bats in an endemic area in Spain. Parasitol. Res. 2014, 113, 1993–1995. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, G.A.; Peñafiel-Verdú, C.; Altimira, J.; García-González, B.; Vilafranca, M. Naturally acquired visceral leishmaniosis in a captive Bennett’s wallaby (Macropus rufogriseus rufogriseus). Vet. Pathol. 2013, 50, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; de Quadros, L.P.; Mateo, M.; Hernández, L.; Gálvez, R.; Alcántara, G.; Checa, R.; Jiménez, M.Á.; Chicharro, C.; Cruz, I.; et al. Leishmania infantum infection in Bennett’s wallabies (Macropus rufogriseus rufogriseus) in a Spanish wildlife park. J. Zoo Wildl. Med. 2016, 47, 586–593. [Google Scholar] [CrossRef]
- Millán, J. Molecular investigation of vector-borne parasites in wild micromammals, Barcelona (Spain). Parasitol. Res. 2018, 117, 3015–3018. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; Ferroglio, E.; Gortázar, C. Leishmania infantum in free-ranging hares, Spain, 2004–2010. Euro Surveill. 2013, 18, 20541. [Google Scholar] [CrossRef]
- Tsokana, C.N.; Sokos, C.; Giannakopoulos, A.; Mamuris, Z.; Birtsas, P.; Papaspyropoulos, K.; Valiakos, G.; Spyrou, V.; Lefkaditis, M.; Chatzopoulos, D.C.; et al. First evidence of Leishmania infection in European brown hare (Lepus europaeus) in Greece: GIS analysis and phylogenetic position within the Leishmania spp. Parasitol. Res. 2016, 115, 313–321. [Google Scholar] [CrossRef]
- Tsokana, C.N.; Sokos, C.; Giannakopoulos, A.; Birtsas, P.; Athanasiou, L.V.; Valiakos, G.; Sofia, M.; Chatzopoulos, D.C.; Kantere, M.; Spyrou, V.; et al. Serological and molecular investigation of selected parasitic pathogens in European brown hare (Lepus europaeus) in Greece: Inferring the ecological niche of Toxoplasma gondii and Leishmania infantum in hares. Parasitol. Res. 2019, 118, 2715–2721. [Google Scholar] [CrossRef]
- Ebani, V.V.; Poli, A.; Rocchigiani, G.; Bertelloni, F.; Nardoni, S.; Papini, R.A.; Mancianti, F. Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy. Asian Pac. J. Trop. Med. 2016, 9, 465–469. [Google Scholar] [CrossRef]
- Rocchigiani, G.; Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Bascherini, A.; Leoni, A.; Mancianti, F.; Poli, A. Molecular survey on the occurrence of arthropod-borne pathogens in wild brown hares (Lepus europaeus) from Central Italy. Infect. Genet. Evol. 2018, 59, 142–147. [Google Scholar] [CrossRef]
- Arce, A.; Estirado, A.; Ordobas, M.; Sevilla, S.; García, N.; Moratilla, L.; de la Fuente, S.; Martínez, A.M.; Pérez, A.M.; Aránguez, E.; et al. Re-emergence of leishmaniasis in Spain: Community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013, 18, 20546. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Jiménez, M.I.; Cruz, I.; Iriso, A.; Martín-Martín, I.; Sevillano, O.; Melero, S.; Bernal, J. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet. Parasitol. 2012, 190, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Chicharro, C.; Llanes-Acevedo, I.P.; García, E.; Nieto, J.; Moreno, J.; Cruz, I. Molecular typing of Leishmania infantum isolates from a leishmaniasis outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013, 18, 20545. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, M.L.; Pérez, A.; Domínguez, M.; Moreno, I.; García, N.; Martínez, I.; Navarro, A.; Domínguez, L.; Álvarez, J. Assessment of the sensitivity and specificity of serological (IFAT) and molecular (direct-PCR) techniques for diagnosis of leishmaniasis in lagomorphs using a Bayesian approach. Vet. Med. Sci. 2016, 2, 211–220. [Google Scholar] [CrossRef]
- Ortega, M.V.; Moreno, I.; Domínguez, M.; de la Cruz, M.L.; Martín, A.B.; Rodríguez-Bertos, A.; López, R.; Navarro, A.; González, S.; Mazariegos, M.; et al. Application of a specific quantitative real-time PCR (qPCR) to identify Leishmania infantum DNA in spleen, skin and hair samples of wild Leporidae. Vet. Parasitol. 2017, 243, 92–99. [Google Scholar] [CrossRef]
- Ortega-García, M.V.; Salguero, F.J.; Rodríguez-Bertos, A.; Moreno, I.; García, N.; García-Seco, T.; Luz Torre, G.; Domínguez, L.; Domínguez, M. A pathological study of Leishmania infantum natural infection in European rabbits (Oryctolagus cuniculus) and Iberian hares (Lepus granatensis). Transbound. Emerg. Dis. 2019, 66, 2474–2481. [Google Scholar] [CrossRef]
- Díaz-Sáez, V.; Merino-Espinosa, G.; Morales-Yuste, M.; Corpas-López, V.; Pratlong, F.; Morillas-Márquez, F.; Martín-Sánchez, J. High rates of Leishmania infantum and Trypanosoma nabiasi infection in wild rabbits (Oryctolagus cuniculus) in sympatric and syntrophic conditions in an endemic canine leishmaniasis area: Epidemiological consequences. Vet. Parasitol. 2014, 202, 119–127. [Google Scholar] [CrossRef]
- Chitimia, L.; Muñoz-García, C.I.; Sánchez-Velasco, D.; Lizana, V.; Del Río, L.; Murcia, L.; Fisa, R.; Riera, C.; Giménez-Font, P.; Jiménez-Montalbán, P.; et al. Cryptic leishmaniosis by Leishmania infantum, a feature of canines only? A study of natural infection in wild rabbits, humans and dogs in southeastern Spain. Vet. Parasitol. 2011, 181, 12–16. [Google Scholar] [CrossRef]
- Jiménez, M.; González, E.; Martín-Martín, I.; Hernández, S.; Molina, R. Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania infantum in the focus of Madrid, Spain? Vet. Parasitol. 2014, 202, 296–300. [Google Scholar] [CrossRef] [PubMed]
- García, N.; Moreno, I.; Alvarez, J.; de la Cruz, M.L.; Navarro, A.; Pérez-Sancho, M.; García-Seco, T.; Rodríguez-Bertos, A.; Conty, M.L.; Toraño, A.; et al. Evidence of Leishmania infantum infection in rabbits (Oryctolagus cuniculus) in a natural area in Madrid, Spain. Biomed. Res. Int. 2014, 2014, 318254. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Stechele, M.; Hetzel, U.; Domingo, M.; Schönian, G.; Zahner, H.; Burkhardt, E. Cutaneous leishmaniosis in a horse in southern Germany caused by Leishmania infantum. Vet. Parasitol. 2002, 109, 9–17. [Google Scholar] [CrossRef]
- Rolão, N.; Martins, M.J.; João, A.; Campino, L. Equine infection with Leishmania in Portugal. Parasite 2005, 12, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.; Elias, J.; Ribeiro, A.J.; Alegria, N.; Schallig, H.D.; Silva, F.; Santarém, N.; Cardoso, L.; Cotovio, M. Cutaneous leishmaniosis in a horse from northern Portugal. Vet. Parasitol. 2014, 200, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bellon, H.; Solano-Gallego, L.; Bardagí, M.; Alberola, J.; Ramis, A.; Ferrer, L. Immune response to Leishmania infantum in healthy horses in Spain. Vet. Parasitol. 2006, 135, 181–185. [Google Scholar] [CrossRef]
- Kouam, M.K.; Diakou, A.; Kanzoura, V.; Papadopoulos, E.; Gajadhar, A.A.; Theodoropoulos, G. A seroepidemiological study of exposure to Toxoplasma, Leishmania, Echinococcus and Trichinella in equids in Greece and analysis of risk factors. Vet. Parasitol. 2010, 170, 170–175. [Google Scholar] [CrossRef]
- Lopes, A.P.; Sousa, S.; Dubey, J.P.; Ribeiro, A.J.; Silvestre, R.; Cotovio, M.; Schallig, H.D.; Cardoso, L.; Cordeiro-da-Silva, A. Prevalence of antibodies to Leishmania infantum and Toxoplasma gondii in horses from the north of Portugal. Parasit. Vectors 2013, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Sgorbini, M.; Bonelli, F.; Pizzolli, I.; Tognetti, R.; Corazza, M. Seroprevalence of Leishmania sp. infection in healthy horses housed in endemic areas in Tuscany. J. Equine Vet. Sci. 2014, 34, 572–574. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Bertero, F.; Moretta, I.; Morganti, G.; Mortarino, M.; Villa, L.; Zanzani, S.A.; Morandi, B.; Rinnovati, R.; Vitale, F.; et al. Detecting antibodies to Leishmania infantum in horses from areas with different epizooticity levels of canine leishmaniosis and a retrospective revision of Italian data. Parasit. Vectors 2020, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.T.; Pereira, C.; Dubey, J.P.; Nóvoa, M.; Quaresma, M.; Schallig, H.; Cardoso, L.; Lopes, A.P. Seroprevalence of Toxoplasma gondii and Leishmania spp. in domestic donkeys from Portugal. Rev. Bras. Parasitol. Vet. 2019, 28, 172–176. [Google Scholar] [CrossRef]
- Nardoni, S.; Altomonte, I.; Salari, F.; Martini, M.; Mancianti, F. Serological and molecular findings of Leishmania infection in healthy donkeys (Equus asinus) from a canine leishmaniosis endemic focus in Tuscany, Italy: A preliminary report. Pathogens 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Troyano, A.; Montoya, A.; Fariñas, F.; Fermín, M.L.; Flores, L.; Rojo, C.; Checa, R.; Gálvez, R.; Marino, V.; et al. First report of Leishmania infantum infection in the endangered orangutan (Pongo pygmaeus pygmaeus) in Madrid, Spain. Parasit. Vectors 2018, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Navea-Pérez, H.M.; Díaz-Sáez, V.; Corpas-López, V.; Merino-Espinosa, G.; Morillas-Márquez, F.; Martín-Sánchez, J. Leishmania infantum in wild rodents: Reservoirs or just irrelevant incidental hosts? Parasitol. Res. 2015, 114, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Helhazar, M.; Leitão, J.; Duarte, A.; Tavares, L.; da Fonseca, I.P. Natural infection of synathropic rodent species Mus musculus and Rattus norvegicus by Leishmania infantum in Sesimbra and Sintra—Portugal. Parasit. Vectors 2013, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakidis, Ι.; Angelopoulou, K.; Dovas, C.I.; Dokianakis, Ε.; Tamvakis, A.; Symeonidou, I.; Antoniou, Μ.; Diakou, A. Leishmania infection in rodents in Greece. Trop. Med. Int. Health 2017, 22, 1523–1532. [Google Scholar] [CrossRef]
- Martín-Sánchez, J.; Torres-Medina, N.; Corpas-López, V.; Morillas-Márquez, F.; Díaz-Sáez, V. Vertical transmission may play a greater role in the spread of Leishmania infantum in synanthropic Mus musculus rodents than previously believed. Transbound. Emerg. Dis. 2020, 67, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Psaroulaki, A.; Antoniou, M.; Toumazos, P.; Mazeris, A.; Ioannou, I.; Chochlakis, D.; Christophi, N.; Loukaides, P.; Patsias, A.; Moschandrea, I.; et al. Rats as indicators of the presence and dispersal of six zoonotic microbial agents in Cyprus, an island ecosystem: A seroepidemiological study. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 733–739. [Google Scholar] [CrossRef]
- Papadogiannakis, E.; Spanakos, G.; Kontos, V.; Menounos, P.G.; Tegos, N.; Vakalis, N. Molecular detection of Leishmania infantum in wild rodents (Rattus norvegicus) in Greece. Zoonoses Public Health 2010, 57, e23–e25. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Gómez-Samblás, M.; Suárez-Morán, J.M.; Osuna, A.; Sanxis-Furió, J.; Pascual, J.; Bueno-Marí, R.; Franco, S.; Peracho, V.; Montalvo, T.; et al. Leishmaniasis in Norway rats in sewers, Barcelona, Spain. Emerg. Infect. Dis. 2019, 25, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Sposimo, P.; Trisciuoglio, A.; Giannini, F.; Strumia, F.; Ferroglio, E. Epidemiology of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Rattus rattus in absence of domestic reservoir and definitive hosts. Vet. Parasitol. 2014, 199, 247–249. [Google Scholar] [CrossRef]
- Otranto, D.; Testini, G.; Buonavoglia, C.; Parisi, A.; Brandonisio, O.; Circella, E.; Dantas-Torres, F.; Camarda, A. Experimental and field investigations on the role of birds as hosts of Leishmania infantum, with emphasis on the domestic chicken. Acta Trop. 2010, 113, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Carrillo, E.; Moreno, J.; Cruz, I. What is responsible for a large and unusual outbreak of leishmaniasis in Madrid? Trends Parasitol. 2013, 29, 579–580. [Google Scholar] [CrossRef]
- The European Pet Food Industry. FEDIAF Annual Report 2020. Available online: https://fediaf.org/images/FEDIAF_Annual_Report_2020_cor.pdf (accessed on 15 February 2021).
- Soares, C.S.; Duarte, S.C.; Sousa, S.R. What do we know about feline leishmaniosis? J. Feline. Med. Surg. 2016, 18, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, S.; Fakhar, M.; Teshnizi, S.H. Is the cat an important reservoir host for visceral leishmaniasis? A systematic review with meta-analysis. J. Venom Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190012. [Google Scholar] [CrossRef]
- Otranto, D.; Cantacessi, C.; Pfeffer, M.; Dantas-Torres, F.; Brianti, E.; Deplazes, P.; Genchi, C.; Guberti, V.; Capelli, G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet. Parasitol. 2015, 213, 12–23. [Google Scholar] [CrossRef]
- Karkamo, V.; Kaistinen, A.; Näreaho, A.; Dillard, K.; Vainio-Siukola, K.; Vidgrén, G.; Tuoresmäki, N.; Anttila, M. The first report of autochthonous non-vector-borne transmission of canine leishmaniosis in the Nordic countries. Acta Vet. Scand. 2014, 56, 84. [Google Scholar] [CrossRef] [PubMed]
- Gradoni, L.; Pozio, E.; Gramiccia, M.; Maroli, M.; Bettini, S. Leishmaniasis in Tuscany (Italy): VII. Studies on the role of the black rat, Rattus rattus, in the epidemiology of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 427–431. [Google Scholar] [CrossRef]
- Peixoto, H.M.; de Oliveira, M.R.; Romero, G.A. Serological diagnosis of canine visceral leishmaniasis in Brazil: Systematic review and meta-analysis. Trop. Med. Int. Health 2015, 20, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, M.F.; Lachaud, L.; Simon, L.; Battery, E.; Marty, P.; Pomares, C. Place of serology in the diagnosis of zoonotic leishmaniases with a focus on visceral leishmaniasis due to Leishmania infantum. Front. Cell Infect. Microbiol. 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.D.; Iniguez, E.; Oliveira, F. Leishmania infantum. Trends Parasitol. 2020, 36, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Hasker, E.; Boelaert, M.; Sacks, D.; Sundar, S. Xenodiagnosis to address key questions in visceral leishmaniasis control and elimination. PLoS Negl. Trop. Dis. 2020, 14, e0008363. [Google Scholar] [CrossRef] [PubMed]
| Country (Area) | Period of Sampling (Month or Season and Year) | No. of Cats (Lifestyle) | Positivity % | % of Non-Healthy Positive Cats (Abnormalities) | Variables Associated with Positivity (Test) | References | ||
|---|---|---|---|---|---|---|---|---|
| (Immunological Test) | (Parasitological Test: Sample) | Overall § | ||||||
| Albania (Tirana) | 2008–2010 | 146 (free roaming) | 0.7 (IFAT) | 0.0 (PCR: blood) | 0.7 | NA | NA | [51] |
| Cyprus (all six districts) | March–September 2014 | 174 (feral, outdoors, indoors) | 4.4 (ELISA #) | 2.3 (PCR: blood) | 5.8 # | Ab = 86.0 (NA) PCR = 100 (NA) | Hepatozoon spp., CMt infections (Ov) | [52] |
| Greece (Thessaloniki) | NA | 284 (stray) | 3.9 (ELISA) | NA | NA | NA | No | [53] |
| Greece (Macedonia and Thessaly) | January 2009–September 2011 | 100 (indoors, outdoors) | 11.0 (IFAT IgG and IgM, ELISA) | 0.0 (cysm: lynd, skin, bm, conj) 41.0 (PCR: skin, bm, blood, cjsw) | 46.0 | Ab = 45.5 (skin, ocular, systemic) PCR = 48.8 (skin, ocular, systemic) | Season, FeL compatible signs (PCR) | [48,49] |
| Greece (Crete, Mykonos, Skopelos and Athens) | Summer 2015 | 148 (colony, free roaming) | 6.1 (IFAT) | 6.1 (PCR: blood) | 6.1 | NA | NA | [54] |
| Greece (Thessaly) | NA | 150 (stray, NA^) | NA | 13.3 (PCR: blood) | NA | NA | No | [55] |
| Greece and Italy | NA | 269 (indoors, outdoors) | 3.0 (IFAT) | NA | NA | NA | Cohabitation with dogs | [56] |
| Italy (Abruzzo) | September 2002–March 2004 | 203 (stray, indoors, outdoors) | 16.3 (IFAT) | 45.5 (PCR: blood) a 100 (PCR: lynd) a | NA | 66.4 (heterogeneous) | NA | [50] |
| Italy (Greater Milan) | January 2008–January 2010 | 233 (colony) | 25.3 (IFAT) | 0.0 (PCR: blood) | 25.3 | 79.7 (heterogeneous) | Neutrophilia, FIV positivity (Ab) | [57] |
| Italy (Milan) | June–December 2014 | 90 (stray) | 12.2 (IFAT) | 1.1 (PCR: blood) 1.1 (PCR: lynd) 0.0 (PCR: cjsw) | 12.2 | 100 (lymphadenomegaly, stomatitis, skin) | FCoV positivity | [58] |
| Italy (Milan City, northern Italy) | June 2016–December 2018 | 117 | 4.9 (IFAT) | 4.3 (PCR: lynd) 0 (PCR: blood) 0 (PCR: cjsw) | 8.6 | NA | Hypergammaglobulinemia | [59] |
| Italy (Umbria, Tuscany and Marche) | 2010–2016 | 286 (cattery, colony) | 10.8 (IFAT) | 15.73 (PCR: cjsw) 0.0 (PCR: bc) | 21.7 | 0.0 | Geographical area (Ab, PCR) Age class (Ab) Lifestyle (Ov) | [60] |
| Italy (Sardinia) | October 2011–January 2013 | 90 (outdoors, indoors) | 10.0 (IFAT) | 5.5 (PCR: bc) | 14.4 | 38.5 (heterogeneous) | No | [61] |
| Italy (Sicily and Calabria) | March 2012–January 2013 | 76 (outdoors, indoors, stray) | 30.3 (IFAT, ELISA, WB) | NA | NA | NA (heterogeneous) | NA | [62] |
| Italy (Sicily and Calabria) | March 2012–January 2013 | 197 cats (outdoors, indoors, stray) | 9.6 (IFAT) | 6.6 (PCR: cjsw, osw, blood, lynd, urine) | 14.7 | NA | No | [63] |
| Italy (Sicily) | March 2016–April 2017 | 66 (indoors, outdoors, stray) | 21.0 (IFAT, ELISA) 17.0 (LSA-IFNγ &) | 4.0 (PCR: blood) | 36.0 | NA | NA | [47] |
| Italy (Aeolian Islands, Sicily) | January 2015–June 2016 | 330 (outdoors) | 25.7 (IFAT) | 2.1 (PCR: blood) 1.8 (PCR: cjsw) | 25.7 | NA (systemic, skin, ocular) | Age class (Ab) | [64] |
| Italy (Aeolian Islands, Sicily) | March–April 2016 | 159 (outdoors) | 9.4 (IFAT) | 7.5 (PCR: blood) 3.7 (PCR: cjsw) | 15.7 | NA | NA | [65] |
| Italy (North, Centre and South) | June 2017–August 2018 | 2659 (NA) | 3.3 (IFAT) | 0.8 (PCR: blood) | 3.9 | NA | Geographical area, age class, neutering status, FIV infection (Ov) | [66] |
| Italy (Emilia Romagna) | June–November 2017 | 152 (indoors, outdoors) | 11.8 (IFAT) | 0.7 (PCR: hair) 0.0 (PCR: blood, cjsw) | 12.5 | NA | Serum total proteins, β2- and γ-globulins (Ov) | [67] |
| Italy (Emilia Romagna, northern Italy) | February 2018–October 2019 | 85 (stray) | 2.4 (IFAT) | 5.9 (PCR: blood) | 2.4 | NA | NA | [68] |
| Portugal (Lisbon) | Spring–autumn 2004 | 23 (stray) | 20.0 (IFAT) | 30.4 (PCR: blood) | NA | NA | NA | [69] |
| Portugal (Lisbon) | November 2003–July 2005 | 180 (stray) | 0.6 (IFAT) | NA | NA | NA | NA | [70] |
| Portugal (Lisbon) | January 2007–August 2008 | 142 (NA^, stray) | 1.3 (IFAT) | 20.3 (PCR: blood) | 20.4 | NA | NA | [71] |
| Portugal (Northeast) | May 2004–July 2008 | 316 (indoors, outdoors) | 2.8 (ELISA, DAT) | NA | NA | 11.1 (NA) | No | [72] |
| Portugal (North and Centre) | NA | 320 (outdoors, indoors) | NA | 0.3 (PCR: blood) | NA | 0.0 | NA | [73] |
| Portugal (Lisbon and Algarve) | January 2012–August 2013 | 649 (indoors, outdoors, sheltered) | NA | 9.9 (PCR: blood) | NA | NA | Age class, habitat, ectoparasiticide treatment | [74] |
| Portugal (Algarve) | November 2011–May 2014 | 271 (stray, NA^) | 3.7 (DAT) | NA | NA | NA | No | [75] |
| Portugal (Centre, Lisbon and Algarve) | April–December 2017 | 350 (stray, colony, outdoors) | 0.9 (IFAT) | 6.9 (PCR: blood) | 7.4 | 26.9 (NA) | No | [76] |
| Portugal (Centre, Lisbon and Algarve) | February 2017–August 2018 | 465 (stray, colony, NA^) | NA | 5.4 (PCR: bc) * | NA | NA | NA | [44] |
| Spain (South) | February 2003–December 2004 | 180 (NA^) | 28.3 (IFAT) | 25.7 (PCR: blood) 42.9 (cysm: bc of seven PCR positive cats | 48.3 | NA | FeLV (Ab) | [77] |
| Spain (NA) | 2004–2007 | 2632 (NA) | NA | 0.57 (IHC) | NA | NA | NA | [78] |
| Spain (Madrid) | September 2005–June 2006 | 233 (indoors, outdoors) | 1.3 (IFAT) | 0.4 (PCR: blood) | 1.7 | 75 (heterogeneous) | No | [79] |
| Spain (Madrid) | September 2005–August 2008 | 680 (indoors, outdoors, stray) | 3.7 (IFAT) | 0.6 (PCR: blood) | NA | NA | Age class (Ab), FIV (Ab), oral disease (Ab) | [80] |
| Spain (Madrid) | NA | 17 (breeding) | 17.6 (IFAT) | NA | NA | NA | NA | [81] |
| Spain (Madrid) | 2011–2012 | 55 | NA | 3.7 (PCR: spleen) | NA | NA | NA | [82] |
| Spain (Madrid) | NA | 43 (stray) | 9.3 (IFAT) | NA | NA | NA | NA | [83] |
| Spain (Centre) | Spring 2012 and spring 2013 | 346 (stray) | 3.2 (IFAT) | 0.0 (PCR: 57 blood samples) | NA | 9.1 (compatible with FeL) | No | [84] |
| Spain (Centre) | 2014–2017 | 249 (colony) | 4.8 (IFAT) | 0.0 (PCR: blood, skin) | 4.8 | 16.7 (compatible with FeL) | NA | [85] |
| Spain (Catalonia and Mallorca island) | NA | 445 (mixed) | 5.3–6.3 (ELISA) | NA | NA | NA | No | [86] |
| Spain (Barcelona) | January–Deccember 2006 | 100 (NA) | NA | 3.0 (PCR: blood) | NA | 100 (NA) | Age class (PCR) | [87] |
| Spain (Catalonia) | March 2016–April 2017 | 113 (indoors, outdoors, stray) | 22.0 (IFAT, ELISA) 18.0 (LSA-IFNγ &) | 5.0 (PCR: blood) | NA | 34 (heterogeneous) | NA | [47] |
| Spain (Ibiza island) | June–July 2008 | 105 (shelter outdoors) | 13.2 (ELISA) | 8.7 (PCR: blood) | 15.2 | 75.0 (heterogeneous) | Clinical signs (Ov) FeLV (Ab, PCR) FeLV, FIV (Ab, PCR) | [88] |
| Spain (Mallorca island) | June 2008–February 2009 | 86 (feral) | 15.7 (WB) | 26.0 (PCR: blood) | 25.6 | 0.0 | No | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, L.; Schallig, H.; Persichetti, M.F.; Pennisi, M.G. New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs. Pathogens 2021, 10, 307. https://doi.org/10.3390/pathogens10030307
Cardoso L, Schallig H, Persichetti MF, Pennisi MG. New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs. Pathogens. 2021; 10(3):307. https://doi.org/10.3390/pathogens10030307
Chicago/Turabian StyleCardoso, Luís, Henk Schallig, Maria Flaminia Persichetti, and Maria Grazia Pennisi. 2021. "New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs" Pathogens 10, no. 3: 307. https://doi.org/10.3390/pathogens10030307
APA StyleCardoso, L., Schallig, H., Persichetti, M. F., & Pennisi, M. G. (2021). New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs. Pathogens, 10(3), 307. https://doi.org/10.3390/pathogens10030307