Updates on Geographical Dispersion of Leishmania Parasites Causing Cutaneous Affections in Algeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Clinic
2.2. Molecular Diagnosis and Typing
2.3. Sequencing and Typing of Leishmania Isolates
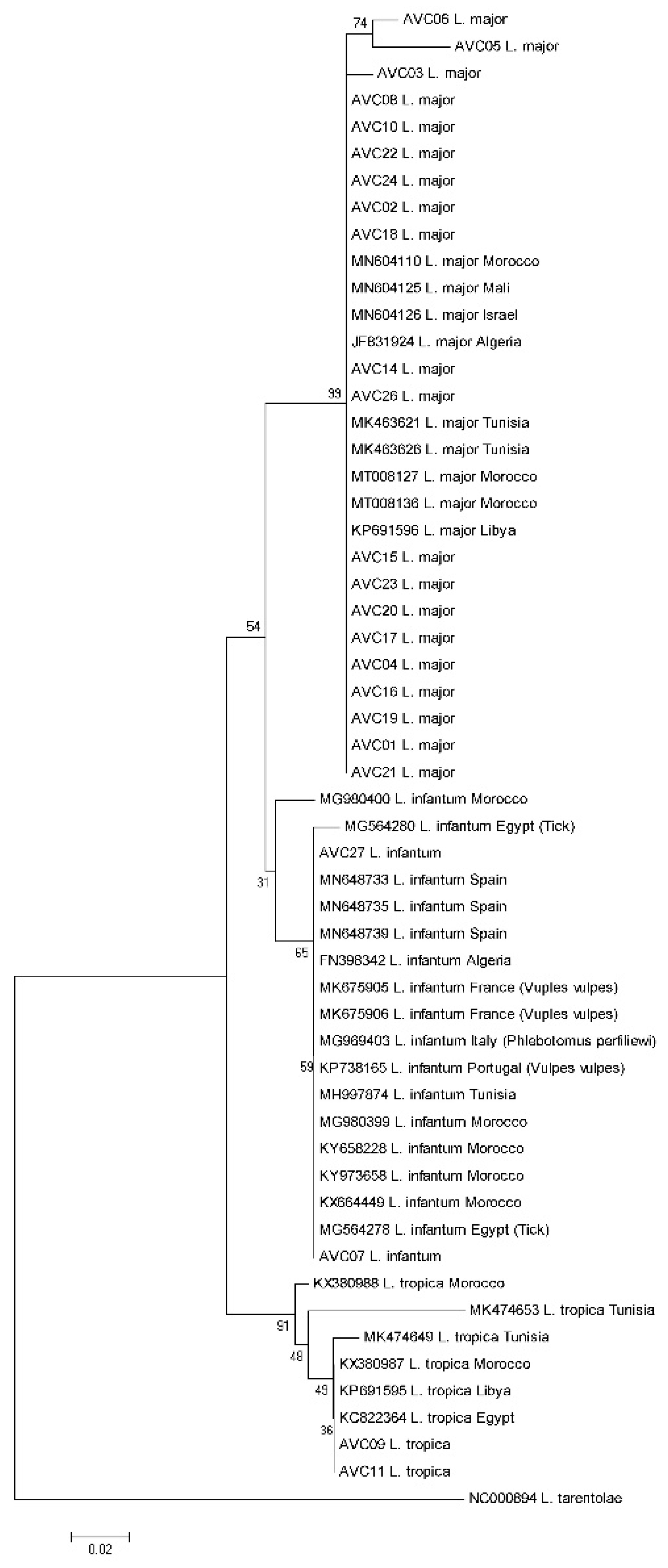
3. Results
4. Discussion
| Clinico-Epidemiological Form | Zymodemes | Reference | ||
|---|---|---|---|---|
| Human | Vector | Reservoir | ||
| ZCL | MON-25 (L. major) MON-269 (L. major) | MON-25 (P. papatasi) MON-269 (P. papatasi) | MON-25 (Psammomys obesus) MON-269 (Psammomys obesus, Meriones shawi) | [9,10,45,58,59] |
| ACL | MON-301 (L. tropica) MON-306 (L. tropica) | - | - | [13,45,60] |
| SCL | MON-1 * (L. infantum) MON-24 * (L. infantum) MON-80 * (L. infantum) | MON-24 * (P. perfilliewi) | - | [22,61] |
| ZVL | MON-1 (L. infantum) MON-24 (L. infantum) MON-33 (L. infantum) MON-34 (L. infantum) MON-77 (L. infantum) MON-78 (L. infantum) MON-80 (L. infantum) MON-281 (L. infantum) | MON-1 (P. perniciosus) MON-24 (P. perfilliewi) | MON-1 (Canis familiaris, Canis aureus) MON-24 (Canis familiaris) MON-34 (Canis familiaris) MON-77 (Canis familiaris) MON-80 (Canis familiaris) MON-281 (Canis familiaris) | [6,61,62,63,64] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- World Health Organization. 2018. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 30 May 2018).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, 5671. [Google Scholar] [CrossRef]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kholoud, K.; Bounoua, L.; Sereno, D.; El Hidan, M.; Messouli, M. Emerging and Re-Emerging Leishmaniases in the Mediterranean Area: What Can Be Learned from a Retrospective Review Analysis of the Situation in Morocco during 1990 to 2010? Microorganisms 2020, 8, 1511. [Google Scholar] [CrossRef] [PubMed]
- Chaara, D.; Haouas, N.; Dedet, J.P.; Babba, H.; Pratlong, F. Leishmaniases in Maghreb: An endemic neglected disease. Acta Trop. 2014, 132, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.; Boughoufalah, A.; Saegerman, C.; De Deken, R.; Bouchene, Z.; Soukehal, A.; Berkvens, D.; Boelaert, M. Epidemiology of Visceral Leishmaniasis in Algeria: An Update. PLoS ONE 2014, 9, e99207. [Google Scholar] [CrossRef]
- Eddaikra, N.; Ait-Oudhia, K.; Kherrachi, I.; Oury, B.; Moulti-Mati, F.; Benikhlef, R.; Harrat, Z.; Sereno, D. Antimony susceptibility of Leishmania isolates collected over a 30-year period in Algeria. PLoS Negl. Trop. Dis. 2018, 12, e0006310. [Google Scholar] [CrossRef]
- Belazzoug, S. The new focus of cutaneous leishmaniasis of M’sila (Algeria). Natural infection cf Psammomys obesus (Rodentia, Gerbillidae). Bull. Soc. Pathol. Exot. Filiales. 1983, 76, 146–149. [Google Scholar] [PubMed]
- Harrat, Z.; Pratlong, F.; Belazzoug, S.; Dereure, J.; Deniau, M.; Rioux, J.A.; Belkaid, M.; Dedet, J.P. Leishmania infantum and L. major in Algeria. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 625–629. [Google Scholar] [CrossRef]
- Kabbout, N.; Merzoug, D.; Chenchouni, H. Ecological Status of Phlebotomine Sand flies (Diptera: Psychodidae) in Rural Communities of North-eastern Algeria. J. Arthropod Borne Dis. 2016, 10, 24–38. [Google Scholar]
- Boudrissa, A.; Cherif, K.; Kherrachi, I.; Benbetka, S.; Bouiba, L.; Boubidi, S.C.; Benikhlef, R.; Arrar, L.; Hamrioui, B.; Harrat, Z. Extension de Leishmania major au nord de l’Algérie. Spread of Leishmania major to the north of Algeria. Bull. Soc. Pathol. Exot. 2012, 105, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Harrat, Z.; Boubidi, S.C.; Pratlong, F.; Benikhlef, R.; Selt, B.; Dedet, J.P.; Ravel, C.; Belkaid, M. Description of Leishmania close to Leishmania killicki (Rioux, Lanotte et Pratlong, 1986) in Algeria. Trans. Roy. Soc. Trop. Med. Hyg. 2009, 103, 716–720. [Google Scholar] [CrossRef]
- Garni, R.; Tran, A.; Guis, H.; Baldet, T.; Benallal, K.; Boubibi, S.; Harrat, Z. Remote sensing, land cover changes, and vector-borne diseases: Use of high spatial resolution satellite imagery to map the risk of occurrence of cutaneous leishmaniasis in Ghardaïa, Algeria. Infect. Genet. Evol. 2014, 28, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Mihoubi, I.; Picot, S.; Hafirassou, N.; de Monbrison, F. Cutaneous leishmaniasis caused by Leishmania tropica in Algeria. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1157–1159. [Google Scholar] [CrossRef]
- Izri, A.; Bendjaballah, A.; Andriantsoanirina, V.; Durand, R. Cutaneous leishmaniasis caused by Leishmania killicki, Algeria. Emerg. Infect. Dis. 2014, 20, 502–504. [Google Scholar] [CrossRef]
- Jaouadi, K.; Haouas, N.; Chaara, D.; Gorcii, M.; Chargui, N.; Augot, D.; Pratlong, F.; Dedet, J.-P.; Ettlijani, S.; Mezhoud, H.; et al. First detection of Leishmania killicki (Kinetoplastida, Trypanosomatidae) in Ctenodactylus gundi (Rodentia, Ctnenodactilidae), a possible reservoir of human cutaneous leishmaniasis in Tunisia. Parasit Vectors 2011, 4, 159. [Google Scholar] [CrossRef] [PubMed]
- Boubidi, S.C.; Benallal, K.; Boudrissa, A.; Bouiba, L.; Bouchareb, B.; Garni, R.; Bouratbine, A.; Ravel, C.; Dvorak, V.; Votypka, J.; et al. Phlebotomus sergenti (Parrot, 1917) identified as Leishmania killicki host in Ghardaïa, south Algeria. Microbes Infect. 2011, 13, 691–696. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Sergent, E.; Gueidon, E. Chronique duboutond’Orient en Algérie. «Le clou de Mila». Arch. Institut. Pasteur Algerie 1923, 1, 1–3. [Google Scholar]
- Belazzoug, S.; Lanotte, G.; Maazoun, R.; Pratlong, F.; Rioux, J.A. Un nouveau variant enzymatique de Leishmania infantum Nicolle, 1908, agent de la leishmaniose cutanée du Nord de l’Algérie. Ann. Parasitol. Hum. Comp. 1985, 60, 1–3. [Google Scholar] [CrossRef]
- Izri, M.A.; Belazzoug, S. Phlebotomus (Larroussius) perfiliewi naturally infected with dermotropic Leishmania infantum at Tenes, Algeria. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 399. [Google Scholar] [CrossRef]
- Addadi, K.; Dedet, J. Epidemiology of leishmaniasis in Algeria. 6- Survey of clinical cases of infantile visceral leishmaniasis from 1965 to 1974. Bull. Soc. Path. Exot. 1976, 69, 68–75. (In French) [Google Scholar] [PubMed]
- Barchiche, A.N.; Madiou, M. Recrudescence des leishmanioses cutanées: À propos de 213 cas dans la wilaya de Tizi-Ouzou. Outbreak of cutaneous leishmaniasis: About 213 cases in the province of Tizi-Ouzou. Pathol. Biol. 2009, 57, 65–70. [Google Scholar] [CrossRef]
- Evans, D. UNDP/WORLD BANK/WHO. In Handbook on Isolation, Characterization and Cryopreservation of Leishmania, 1st ed.; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Kelly, J.M. Method in Molecular Biology. In Isolation of RNA & DNA from Leishmania; Hyde, J.E., Ed.; Humana Press: Totowa, NJ, USA, 1993. [Google Scholar]
- Schönian, G.; Nasereddin, A.; Dinse, N.; Schweynoh, C.; Schalling, H.D.; Presbe, W.; Jaffe, C. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diag. Microbiol. Infect. 2003, 47, 349–358. [Google Scholar] [CrossRef]
- Akhoundi, M.; Hajjaran, H.; Baghaei, A.; Mohebali, M. Geographical distribution of leishmania species of human cutaneous leishmaniasis in fars province, southern iran. Iran J. Parasitol. 2013, 8, 85–91. [Google Scholar] [PubMed]
- Akhoundi, M.; Mohebali, M.; Asadi, M.; Mahmodi, M.R.; Amraei, K.; Mirzaei, A. Molecular characterization of Leishmania spp. in reservoir hosts in endemic foci of zoonotic cutaneous leishmaniasis in Iran. Folia Parasitologica 2013, 60, 218–224. [Google Scholar] [CrossRef]
- Sergent, E.; Parrot, L. Chronique du Bouton d’Orient. Arch. Inst. Pasteur Alger. 1926, 4, 26–29. [Google Scholar]
- Sergent, E.; Parrot, I.; Donatien, A.; Beguet, M. Transmission du clou de Biskra par le phlébotome Phlebotomus papatasi (Scop). CR Acad. Sci. 1921, 8, 1030–1032. [Google Scholar]
- Louzir, H.; Aoun, K.; Späth, G.F.; Laouini, D.; Prina, E.; Victoir, K.; Bouratbine, A. Leishmania epidemiology, diagnosis, chemotherapy and vaccination approaches in the international network of Pasteur Institutes. Med. Sci. 2013, 29, 1151–1160. [Google Scholar]
- Bachi, F.; Icheboudene, K.; Benzitouni, A.; Taharboucht, Z.; Zemmouri, M. Epidemiology of Cutaneous Leishmaniasis in Algeria through Molecular Characterization. Bull. Soc. Pathol. Exot. 2019, 112, 147–152. [Google Scholar] [CrossRef]
- Hamel, H. Etude comparée des boutons d’Alep et de Biskra. Rec. Mém. Med.Chir. Pharm. Milit. 1860, 4, 314–339. [Google Scholar]
- Elhadj, H.; Kerboua Ziari, Y.S.; Selmane, S. Cutaneous Leishmaniasis Modeling: The case of Msila Province in Algeria. Int. J. Innov. Appl. Stud. 2015, 10, 149–154. [Google Scholar]
- Bennai, K. Surveillance et Contrôle des Leishmanioses dans le nord de l’Algérie. Ph.D. Thesis, Université M’Hamed Bougara-Boumerdes, Boumerdes, Algeria, 2019. [Google Scholar]
- Cherif, K.; Boudrissa, A.; Cherif, M.H.; Harrat, Z. A social program for the control of zoonotic cutaneous leishmaniasis in M’Sila, Algeria. Sante Publique 2012, 24, 511–522. [Google Scholar] [CrossRef]
- Bounoua, L.; Kahime, K.; Houti, L.; Blakey, T.; Ebi, K.L.; Zhang, P.; Imhoff, M.L.; Thome, K.J.; Dudek, C.; Sahabi, S.A.; et al. Linking climate to incidence of zoonotic cutaneous leishmaniasis (L. major) in pre-Saharan North Africa. Int. J. Environ. Res. Public Health 2013, 10, 3172–3191. [Google Scholar] [CrossRef]
- Tashakori, M.; Kuhls, K.; Al-Jawabreh, A.; Mauricio, I.L.; Schönian, G.; Farajnia, S.; Alimohammadian, M.H. Leishmania major: Genetic heterogeneity of Iranian isolates by single-strand conformation polymorphism and sequence analysis of ribosomal DNA internal transcribed spacer. Acta Trop. 2006, 98, 52–58. [Google Scholar]
- Attia, H.; Sghaier, R.M.; Gelanew, T.; Bali, A.; Schweynoch, C.; Guerfali, F.Z.; Mkannez, G.; Chlif, S.; Belhaj-Hamid, N.; Dellagia, K.; et al. Genetic micro-heterogeneity of Leishmania major in emerging foci of zoonotic cutaneous leishmaniasis in Tunisia. Infect. Gen. Evol. 2016, 43, 179–185. [Google Scholar] [CrossRef]
- Ait Kbaich, M.; Mhaidi, I.; Daoui, O.; Ait Maatallah, I.; Riyad, M.; Akarid, K.; Lemrani, M. Population structure of leishmania major in southeastern morocco. Acta Trop. 2020, 210, 105587. [Google Scholar] [CrossRef]
- Lanotte, G.; Rioux, J.A.; Maazoun, R.; Pasteur, N.; Pratlong, F.; Lepart, J. Application de la method numérique à la taxonomie du genre Leishmania Ross,1903. Apropos de 146 souches originaires de l’Ancien Monde Utilisation des allozymes. Corollaires épidémiologiques et phylétiques. Ann. Parasit. Hum. Comp. 1981, 56, 575–592. [Google Scholar] [CrossRef]
- Rioux, J.A.; Guilvard, E.; Dereure, J.; Lanotte, G.; Denial, M.; Pratlong, F.; Serres, E.; Belmonte, A. Infestation naturelle de Phlebotomus papatasi (Scopoli, 1786), par Leishmania major MON-25. A propos de 28 souches isolées dans un foyer du Sud marocain. In Leishmania. Taxonomie et Phylogenèse. Applications Éco-Épidémiologiques; Rioux, J.A., Ed.; IMEEE: Montpellier, France, 1986; pp. 471–480. [Google Scholar]
- Maazoun, R.; Pratlong, F.; Lanotte, G.; Rioux, J.A. Le complexe Leishmania major. A propos de l’analyse numérique de 35 souches identifiées par la méthode enzymatique. In Leishmania. Taxonomie et phylogenèse. Applications éco-épidémiologiques; Rioux, J.A., Ed.; IMEEE: Montpellier, France, 1986; pp. 119–128. [Google Scholar]
- Pratlong, F.; Dereure, J.; Ravel, C.; Lami, P.; Balard, Y.; Serres, G.; Lanotte, G.; Rioux, J.A.; Dedet, J.P. Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop. Med. Int. Health 2009, 14, 1071–1085. [Google Scholar] [CrossRef]
- Sereno, D.; Harrat, Z.; Eddaikra, N. Meta-analysis and discussion on challenges to translate Leishmania drug resistance phenotyping into the clinic. Acta Trop. 2019, 191, 204–211. [Google Scholar] [CrossRef]
- Eddaikra, N.; Kherachi Djenad, I.; Benbetka, S.; Benikhlef, R.; Aït-Oudhia, K.; Moulti-Mati, F.; Oury, B.; Sereno, D.; Harrat, Z. Development of a Murine Infection Model with Leishmania killicki, Responsible for Cutaneous Leishmaniosis in Algeria: Application in Pharmacology. BioMed Res. Int. 2016, 2016, 7985104. [Google Scholar] [CrossRef] [PubMed]
- Marty, P.; Lacour, J.P.; Pratlong, F.; Perrin, C.; Del Giudice, P.; Le Fichoux, Y. Leishmaniose cutanée localisée due à Leishmania infantum MON-1 contractée dans le Nord de l’Algérie. Bull. Soc. Pathol. Exot. 1998, 91, 146–147. [Google Scholar]
- Dedet, J.P.; Belazzoug, S. Leishmaniasis in North Africa; Chang, K.P., Bray, R.S., Eds.; Leishmaniasis; Elsevier: Amsterdam, The Netherlands, 1985; pp. 353–375. [Google Scholar]
- Guerbouj, S.; Mkada–Driss, I.; Guizani, I. Molecular Tools for Understanding Eco-Epidemiology, Diversity and Pathogenesis of Leishmania Parasites. In Leishmaniasis—Trends in Epidemiology, Diagnosis and Treatment; InTech: Rijeka, Croatia, 2014; pp. 61–103. [Google Scholar]
- Seridi, N.; Amroc, A.; Kuhls, K.; Belkaid, M.; Zidane, C.; Al-Jawabreh, A.; Schonian, G. Genetic polymorphism of Algerian Leishmania infantum strains revealed by multilocus microsatellite analysis. Microb. Infec. 2008, 10, 1309e1315. [Google Scholar] [CrossRef]
- Chargui, N.; Amro, A.; Haouas, N.; Schönian, G.; Babba, H.; Schmidt, S.; Ravel, C.; Lefebvre, M.; Bastien, P.; Chaker, E.; et al. Population structure of Tunisian Leishmania infantum and evidence for the existence of hybrids and gene flow between genetically different populations. Int. J. Parasitol. 2009, 39, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Mouttaki, T.; Morales-Yuste, M.; Merino-Espinosa, G.; Chiheb, S.; Fellah, H.; Martin-Sanchez, J.; Riyad, M. Molecular diagnosis of cutaneous leishmaniasis and identification of the causative Leishmania species in Morocco by using three PCR-based assays. Parasit Vectors 2014, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Ahmadipour, F.; Cannet, A.; Marty, P.; Delaunay, P.; Perrin, P.; Dorkeld, F.; Sereno, D.; Akhoundi, M. Immunodetection and molecular determination of visceral and cutaneous Leishmania infection using patients’ urine. Infect. Genet. Evol. 2018, 63, 257–268. [Google Scholar] [CrossRef]
- Gherbi, R.; Bounechada, M.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Dantas-Torres, F.; Otranto, D. Phlebotomine sand flies and Leishmania species in a focus of cutaneous leishmaniasis in Algeria. PLoS Negl. Trop. Dis. 2020, 14, e0008024. [Google Scholar] [CrossRef]
- El Baidouri, F.; Diancourt, L.; Berry, V.; Chevenet, F.; Pratlong, F.; Marty, P.; Ravel, C. Genetic Structure and Evolution of the Leishmania Genus in Africa and Eurasia: What Does MLSA Tell Us. PLoS Negl. Trop. Dis. 2013, 7, e2255. [Google Scholar] [CrossRef]
- Schönian, G.; Kuhls, K.; Mauricio, I.L. Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania. Parasitology 2011, 138, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Izri, M.A.; Belazzoug, S.; Pratlong, F.; Rioux, J.A. Isolement de L. major chez Phlebotomus papatasi à Biskra (Algérie). Fin d’une épopée éco-épidémiologique. Ann. Parasitol. Hum. Comp. 1992, 67, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Belazzoug, S. Découverte d’un Meriones shawi (Rongeur, Gerbillidé) naturellement infesté par Leishmania dans le nouveau foyer de Ksar Chellal (Algérie). Bull. Soc. Pathol. Exot. 1986, 79, 630–633. [Google Scholar]
- Mansouri, R.; Pratlong, F.; Bachi, F.; Hamrioui, B.; Dedet, J.P. The first isoenzymatic characterizations of the Leishmania strains responsible for cutaneous leishmaniasis in the Area of Annaba (Eastern Algeria). Open Conf. Proc. J. 2012, 3, 6–11. [Google Scholar] [CrossRef]
- Benikhlef, R.; Harrat, Z.; Toudjine, M.; Djerbouh, A.; Bendali-Braham, S.; Belkaid, M. Présence de Leishmania infantum MON-24 chez le chien. Med. Trop. 2004, 64, 381–383. [Google Scholar]
- Ait-Oudhia, K.; Lami, P.; Lesceu, S.; Harrat, Z.; Hamrioui, B.; Dedet, J.P.; Pratlong, F. Increase in the prevalence of canine leishmaniasis in urban Algiers (Algeria) following the 2003 earthquake. Ann. Trop. Med. Parasitol. 2009, 103, 679–692. [Google Scholar] [CrossRef]
- Harrat, Z.; Belkaid, M. Leishmaniasis in Algiers: Epidemiologic data. Bull. Soc. Pathol. Exot. 2003, 96, 212–214. [Google Scholar] [PubMed]
- Harrat, Z.; Addadi, K.; Belkaid, M.; Tabet-Derraz, O. Visceral leishmaniasis in Algeria. Cases reported of visceral leishmaniasis (1985–1990). Bull. Soc. Pathol. Exot. 1992, 85, 296–301. [Google Scholar]


| Patients | Lesions | Leishmania Diagnosis | Geographical Location | Explanation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Code | Sex | Age (Year) | Number | Site | Microscopy | PCR–RFLP/Sequencing | City | Elevation (m) * | Rainfall (mm) | Bioclimatic Stage ** | Disease or Travel History |
| AVC1 | M | 39 | 1 | Neck | + | +/L. major | Ain skhouna | 995 | 87 | Cold semi-arid | |
| AVC2 | M | 30 | 2 | Forearm, hand | + | +/L. major | Biskra | 121 | 128 | Arid (Desertic) | |
| AVC3 | M | 41 | 1 | Ankle | + | +/L. major | Biskra | ||||
| AVC4 | F | 5 | 1 | Face | + | +/L. major | Bourkika | 203 | 642 | Mediterranean | |
| AVC5 | M | 59 | 1 | Ankle | + | +/L. major | Bou Kremissa | 328 | 201 | Cold semi-arid | |
| AVC6 | F | 9 | 1 | Foot | + | +/L. major | Bou Saada | 48 | 98 | Arid (Desertic) | |
| AVC7 | M | 69 | 1 | Forearm | + | +/L. infantum | Cherchell | 26 | 108 | Mediterranean | |
| AVC8 | M | 49 | 1 | Foot | + | +/L. major | Chlef | 114 | 394 | Mediterranean | Diabetic |
| AVC9 | F | 7 | 1 | Forehead | + | +/L. tropica | Constantine | 694 | 512 | Mediterranean | |
| AVC10 | F | 27 | 2 | Foot | + | +/L. major | El M’hir | 619 | 329 | Mediterranean | |
| AVC11 | F | 16 | 1 | Face | + | +/L. tropica | Ghardaïa | 497 | 68 | Arid (Desertic) | |
| AVC12 | F | 70 | 1 | Foot | - | - | Gouraya | 670 | 642 | Mediterranean | |
| AVC13 | M | 50 | 1 | Forearm | - | - | Hadjout | 81 | 635 | Warm temperate | |
| AVC14 | M | 29 | 1 | Hand | + | +/L. major | Hadjout | ||||
| AVC15 | M | 41 | 3 | Foot, hand | + | +/L. major | Hadjout | ||||
| AVC16 | M | 79 | 1 | Ankle | + | +/L. major | Maghnia | 374 | 365 | Warm temperate | Travel to Morocco |
| AVC17 | F | 74 | 1 | Forearm | + | +/L. major | Médéa | 981 | 736 | Warm temperate | Diabetic, nephrotic disorder |
| AVC18 | M | 10 | 1 | Cheek | + | +/L. major | Menaceur | 321 | 661 | Warm temperate | 2 months inhabitation in Biskra |
| AVC19 | M | 64 | 1 | Neck | + | +/L. major | Mesaad | 592 | 69 | Arid (Desertic) | |
| AVC20 | F | 4 | 1 | Foot | + | +/L. major | Mostaganem | 104 | 347 | Cold semi-arid | Travel to M’Sila |
| AVC21 | F | 3 | 2 | Cheek, foot | + | +/L. major | M’Sila | 471 | 229 | Cold semi-arid | |
| AVC22 | M | 18 | 1 | Cheek | + | +/L. major | Nador | 42 | 313 | Semi-arid | Travel to Biskra |
| AVC23 | M | 82 | 1 | Neck | - | +/L. major | Oran | 0.9 | 370 | Warm temperate | Travel to Tunisia |
| AVC24 | M | 28 | 1 | Forearm | + | +/L. major | Saida | 830 | 341 | Cold semi-arid | |
| AVC25 | F | 36 | 1 | Cheek | - | - | Sidi Ghiles | 30 | 634 | Mediterranean | |
| AVC26 | F | 14 | 1 | Hand | - | +/L. major | Sidi Okba | 54 | 127 | Arid (Desertic) | |
| AVC27 | M | 11 | 1 | Foot | + | +/L. infantum | Tizi Ouzu | 200 | 705 | Mediterranean | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izri, A.; Bendjaballah-Laliam, A.; Sereno, D.; Akhoundi, M. Updates on Geographical Dispersion of Leishmania Parasites Causing Cutaneous Affections in Algeria. Pathogens 2021, 10, 267. https://doi.org/10.3390/pathogens10030267
Izri A, Bendjaballah-Laliam A, Sereno D, Akhoundi M. Updates on Geographical Dispersion of Leishmania Parasites Causing Cutaneous Affections in Algeria. Pathogens. 2021; 10(3):267. https://doi.org/10.3390/pathogens10030267
Chicago/Turabian StyleIzri, Arezki, Amina Bendjaballah-Laliam, Denis Sereno, and Mohammad Akhoundi. 2021. "Updates on Geographical Dispersion of Leishmania Parasites Causing Cutaneous Affections in Algeria" Pathogens 10, no. 3: 267. https://doi.org/10.3390/pathogens10030267
APA StyleIzri, A., Bendjaballah-Laliam, A., Sereno, D., & Akhoundi, M. (2021). Updates on Geographical Dispersion of Leishmania Parasites Causing Cutaneous Affections in Algeria. Pathogens, 10(3), 267. https://doi.org/10.3390/pathogens10030267






