Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19
Abstract
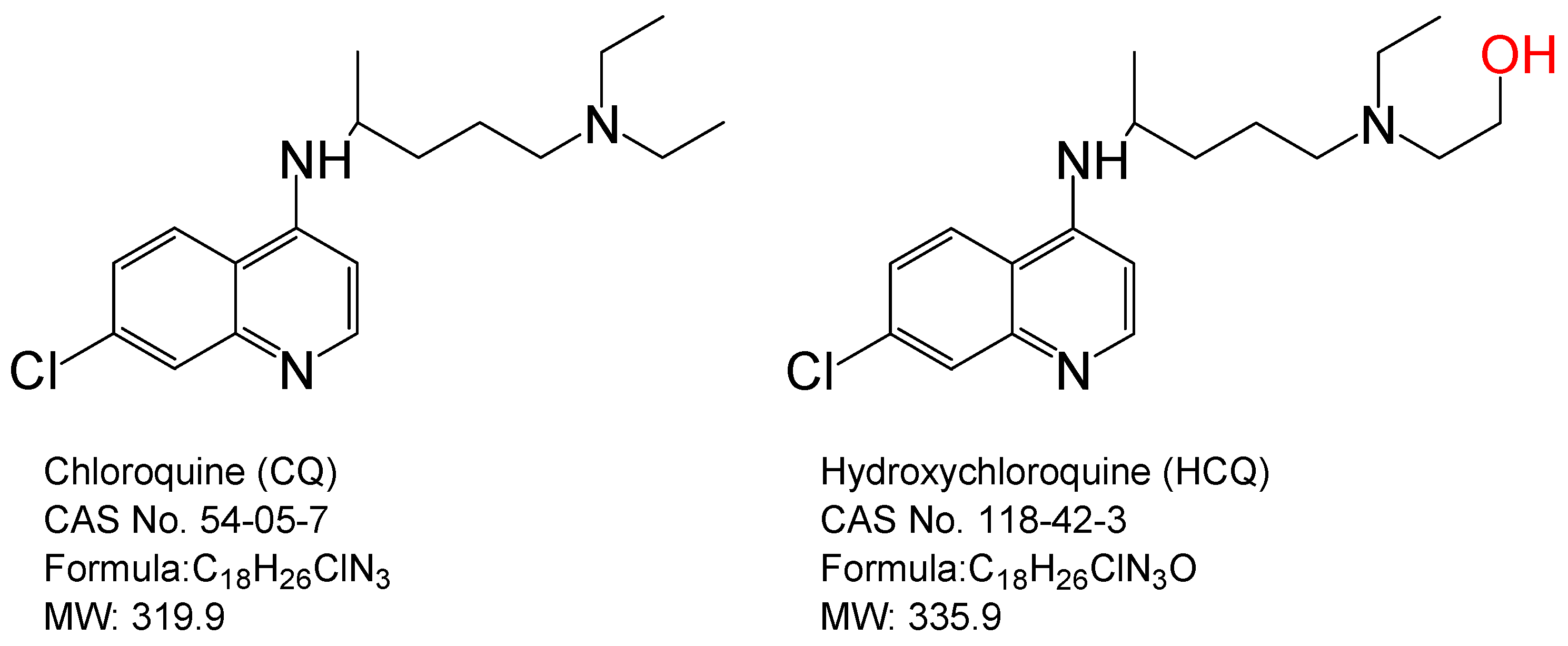
1. Introduction
2. Method
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Disclosure Statement
Conflicts of Interest
References
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, L. Human coronaviruses: What do they cause? Antivir. Ther. 2007, 12, 651–658. [Google Scholar] [PubMed]
- Zhong, N.S.; Zheng, B.J.; Li, Y.M.; Poon, L.; Xie, Z.H.; Chan, K.H.; Li, P.H.; Tan, S.Y.; Chang, Q.; Xie, J.P.; et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef]
- Zaki, A.; Van Boheemen, S.; Bestebroer, T.; Osterhaus, A.; Fouchier, R. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Shi, N.; Shan, F.; Zhang, Z.; Shen, J.; Lu, H.; Ling, Y.; Jiang, Y.; Shi, Y. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020, 295, 210–217. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 11 February 2020).
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 4 February 2021).
- Lei, Z.-N.; Wu, Z.-X.; Dong, S.; Yang, D.-H.; Zhang, L.; Ke, Z.; Zou, C.; Chen, Z.-S. Chloroquine and hydroxychloroquine in the treatment of malaria and repurposing in treating COVID-19. Pharmacol. Ther. 2020, 216, 107672. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection In Vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) In Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Romero-Ortuno, R.; Carvajal, A.; Savarino, A. A pharmacological perspective of chloroquine in SARS-CoV-2 infection: An old drug for the fight against a new coronavirus? Int. J. Antimicrob. Agents. 2020, 56, 106078. [Google Scholar] [CrossRef] [PubMed]
- Hickley, N.M.; Al-Maskari, A.; McKibbin, M. Chloroquine and hydroxychloroquine toxicity. Arch. Ophthalmol. 2011, 129, 1506–1507. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.C.; Parola, P.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; Seng, P.; et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithro-mycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020, 34, 101663. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associ-ated pneumonia in clinical studies. Biosci. Trends. 2020, 14, 72–73. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E.; et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, m1849. [Google Scholar] [CrossRef]
- Mahévas, M.; Tran, V.T.; Roumier, M.; Chabrol, A.; Paule, R.; Guillaud, C.; Gallien, S.; Lepeule, R.; Szwebel, T.A.; Lescure, X.; et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalised for COVID-19 infection and requiring oxygen: Results of a study using routinely collected data to emulate a target trial. medRxiv 2020, 20060699. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.H.; Hardin, J.W.; Sutton, S.S.; Ambati, J. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. medRxiv 2020, 1, 114–127. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Molina, J.; Delaugerre, C.; Le Goff, J.; Mela-Lima, B.; Ponscarme, D.; Goldwirt, L.; De Castro, N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Méd. Mal. Infect. 2020, 50, 384. [Google Scholar] [CrossRef]
- Chen, Z.W.; Hu, J.J.; Zhang, Z.W.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. Preprint. medRxiv 2020, 20040758. [Google Scholar] [CrossRef]
- Borba, M.G.S.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Brito, M.; Mourão, M.P.G.; Brito-Sousa, J.D.; Baía-da-Silva, D.; Guerra, M.V.F.; et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e208857. [Google Scholar] [CrossRef]
- COVID-19 RISK and Treatments (CORIST) Collaboration. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur. J. Intern. Med. 2020, 82, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Catteau, L.; Dauby, N.; Montourcy, M.; Bottieau, E.; Hautekiet, J.; Goetghebeur, E.; Van Ierssel, S.; Duysburgh, E.; Van Oyen, H.; Wyndham-Thomas, C.; et al. Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: A nationwide observational study of 8075 participants. Int. J. Antimicrob. Agents 2020, 56, 106144. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Mösbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Krüger, N.; Gassen, N.C.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nat. Cell Biol. 2020, 585, 1–5. [Google Scholar] [CrossRef]
- Yam, J.C.S.; Kwok, A.K.H. Ocular toxicity of hydroxychloroquine. Hong Kong Med. J. 2006, 12, 294–304. [Google Scholar]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; Felix, S.E.B.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, 22 January–30 May 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Hamed, I.; Shaban, N.; Nassar, M.; Cayir, D.; Love, S.; Curran, M.D.; Webb, S.; Yang, H.; Watson, K.; Rostron, A.; et al. Paired Nasopharyngeal and Deep Lung Testing for Severe Acute Respiratory Syndrome Coronavirus-2 Reveals a Viral Gradient in Critically Ill Patients: A Multicenter Study. Chest 2021, S0012-3692(20)34909-6. [Google Scholar] [CrossRef] [PubMed]

| Reference | Institution/Country Study Conducted | Design | No. of Participants | Intervention | Results |
|---|---|---|---|---|---|
| Gao, J. et al. (2020) [16] | 10 hospitals in China in the cities of Wuhan, Jingzhou, Guangzhou, Beijing, Shanghai, Chungging, and Ningbo | Observational study | N = 100 Control group: unlisted Experimental group: unlisted | Unknown | Compared to the control group, CQ improves lung imaging findings, inhibits the exacerbation of pneumonia, and promotes a virus-negative conversion |
| Magagnoli, J. et al. (2020) [19] | Veterans Health Administration medical centers across the USA | Observational study | N = 807 Control group: no HCQ (n = 395) Experimental group: HCQ alone (n = 198) HCQ + AZ (n = 214) | HCQ alone: 400 mg/daily for 5 days HCQ + AZ: 422.2 mg/daily for 5 days. | Most of participants have chronic disease, such as diabetes and cancer. Compared to the control group, mortality risk is no significantly different in the HCQ group or in the HCQ + AZ group. The HCQ + AZ group has an increased risk of cardiac arrest. |
| Zhaowei, C et al. (2020) [22] | Hospital of Wuhan University, Wuhan, China | RCT | N = 62 Control group: No HCQ + SOC (n = 31) Experimental group: HCQ + SOC (n = 31) | HCQ, 200 mg, twice daily for 5 days | Severe COVID-19 patients are not enrolled in this study. Compared to the control group, the HCQ group (80.6%, 25/31) have pneumonia improvement and a shorter recovery time for clinical symptoms such as fever and cough. 2 patients in the HCQ group have mild adverse reactions such as rashes and headaches. |
| Mahevas, M. et al. (2020) [18] | 4 French tertiary care centers, France | Observational study | N = 181 Control group: no HCQ (n = 97) Experimental group: HCQ (n = 84) | HCQ, 600 mg/daily for 5 days (starting within 48 h after hospital admission) | The ratios of ICU admission, morality and ARDS development are not significantly different between the no HCQ group and the HCQ group. 8 patients in the HCQ group have electrocardiogram modifications and then HCQ discontinuation. |
| Gautret, P. et al. (2020) [15] | University Hospital Institute Méditerranée Infection in Marseille, France. | Observation study | N = 80 Control group: Not recruited Experimental group: HCQ + AZ (n = 80, 6 patients from a pervious study) | HCQ, 200 mg thrice daily for 10 days AZ, 500mg/daily for D1 and 250mg/daily for the D2 to D5 | 81.3% (65/80) of patients have a favorable outcome and are rapidly discharged from the hospital (mean of the discharged day: 4.1 days). |
| Gautret, P. et al. (2020) [20] | 4 centers in Southern France in cities of Marseille, Nice, Avignon and Briançon | Open-label, non-RCT | N = 32 Control group: no HCQ (n = 16) Experimental group: HCQ (n = 20) All group are further classified into three subgroups: asymptomatic, URTI and LRTI. | HCQ, 200 mg, thrice daily for 10 days 6 patients in HCQ group with combination of AZ (500 mg on D1 followed by 250 mg/daily for the D2 to D5) for prevention of bacterial infection | 6 days after treatment, the ratio of viral clearance in the HCQ + AZ group, HCQ alone group, and a control group is 100%, 57.1%, and 12.5%, respectively. |
| Tang, W. et al. (2020) [17] | Ruijin Hospital in Shanghai, China | Open label, RCT, Multicenter | N = 150 Control group: no HCQ + SOC (n = 80) Experimental group: HCQ + SOC (n = 70) | HCQ, 1200 mg/daily on D1 to D3 followed by 800 mg/daily for 2 to 3 weeks SOC, treatment includes another antiviral drug such as arbidol, virazole, lopinavir-ritonavir, oseltamivir, entecavir | 98.6% (148/150) of patients have mild or moderate COVID-19 cases. Comparted to the control group, the rate of negative virus conversion is not significantly different in the HCQ + SOC group. The rate of adverse reaction is higher in the HCQ group than that in the control group (30% v.s. 9%). |
| Borba, MGS.et al. (2020) [23] | Fundação de Medicina Tropical Dr. Heitor Vieira Dourado, Manaus, Amazonas, Brazil | Double-blinded, phase IIb clinical trial | N = 440 (finally enrolled 81 patients for the study) Control group: no CQ from other countries Experimental group: High dosage CQ (n = 41) Low dosage CQ (n = 40) | High dosage CQ, 600 mg twice daily for 10 days Low dosage CQ, 450 mg twice daily on D1 and the 450mg/daily for remaining 4 days. | A high dosage of CQ for 10 days presented toxicity red flags, particularly affecting QTc prolongation. This study was terminated early because of the high dosage CQ resulted in a high rate of fatality. |
| Molina, J. M. et al. (2020) [21] | Infectious Diseases Department, AP–HP-Saint-Louis Hospital, Paris, France | Polit clinical trial | N = 11 Control group: Not recruited Experimental group: HCQ + AZ | HCQ, 200 mg thrice daily for 10 days; AZ, 500 mg on D1 followed by 250 mg/daily for the D2 to D5 | One patient died and another one discontinued treatment due to QTc prolongation. 20% of patients (2/10) have full viral clearance conversion on D6 after treatment. |
| Castelnuovo, D. A. et al. (2020) [24] | Mediterranea Cardiocentro, Napoli, Italy | Observational study, Multicenter | N = 3451 Control group: no HCQ (n = 817) Experimental group: HCQ (n = 2634) | HCQ, 400 mg twice daily or once daily on D1 and 200 mg/ daily on D2 to D5 or to D10 | HCQ treatment results in a 30% lower risk of death in COVID-19 hospitalized patients. |
| Catteau, L. et al. (2020) [25] | Department of Epidemiology and public health, Sciensano, Brussels, Belgium | Observational study, Multicenter | N = 8075 Control group: no HCQ (n = 3533) Experimental group: HCQ (n = 4542) | HCQ, 2400 mg in total over 5 days | Compared to the control group, the rate of mortality is significantly lower in the HCQ group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, T.-C.; Wang, Y.-H.; Chen, Y.-L.; Tsai, W.-C.; Lee, C.-H.; Chuang, K.-P.; Chen, Y.-M.A.; Yuan, C.-H.; Ho, S.-Y.; Yang, M.-H.; et al. Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19. Pathogens 2021, 10, 217. https://doi.org/10.3390/pathogens10020217
Ho T-C, Wang Y-H, Chen Y-L, Tsai W-C, Lee C-H, Chuang K-P, Chen Y-MA, Yuan C-H, Ho S-Y, Yang M-H, et al. Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19. Pathogens. 2021; 10(2):217. https://doi.org/10.3390/pathogens10020217
Chicago/Turabian StyleHo, Tzu-Chuan, Yung-Hsuan Wang, Yi-Ling Chen, Wan-Chi Tsai, Che-Hsin Lee, Kuo-Pin Chuang, Yi-Ming Arthur Chen, Cheng-Hui Yuan, Sheng-Yow Ho, Ming-Hui Yang, and et al. 2021. "Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19" Pathogens 10, no. 2: 217. https://doi.org/10.3390/pathogens10020217
APA StyleHo, T.-C., Wang, Y.-H., Chen, Y.-L., Tsai, W.-C., Lee, C.-H., Chuang, K.-P., Chen, Y.-M. A., Yuan, C.-H., Ho, S.-Y., Yang, M.-H., & Tyan, Y.-C. (2021). Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19. Pathogens, 10(2), 217. https://doi.org/10.3390/pathogens10020217









