Developing a Vaccine to Block West Nile Virus Transmission: In Silico Studies, Molecular Characterization, Expression, and Blocking Activity of Culex pipiens mosGCTL-1
Abstract
1. Introduction
2. Results
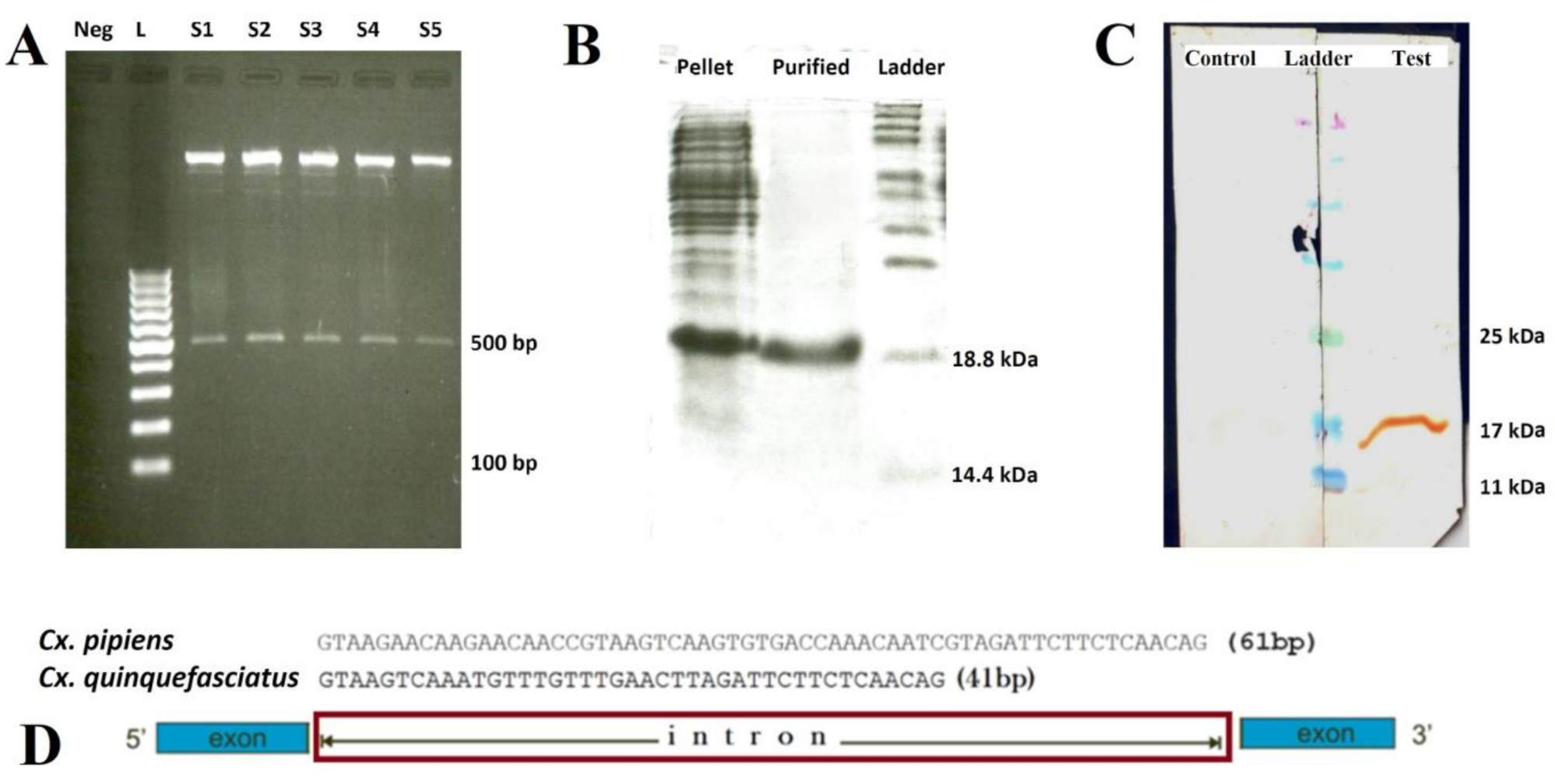
2.1. Morphological and Molecular Identification of Cx. pipiens
2.2. Characterization of Cx. pipiens mosGCTL-1
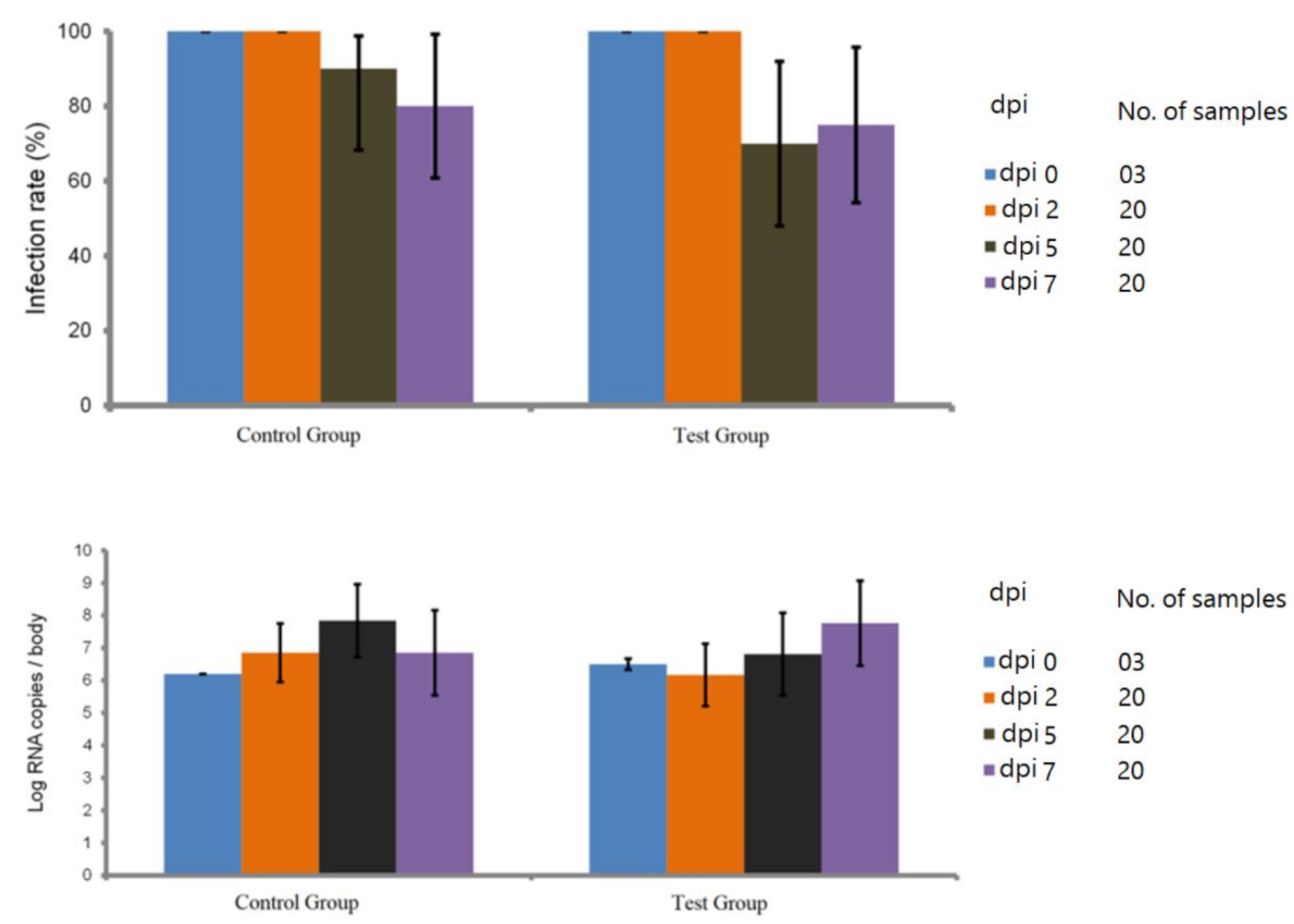
2.3. Blocking Efficacy of Cx. Pipiens rmosGCTL-1
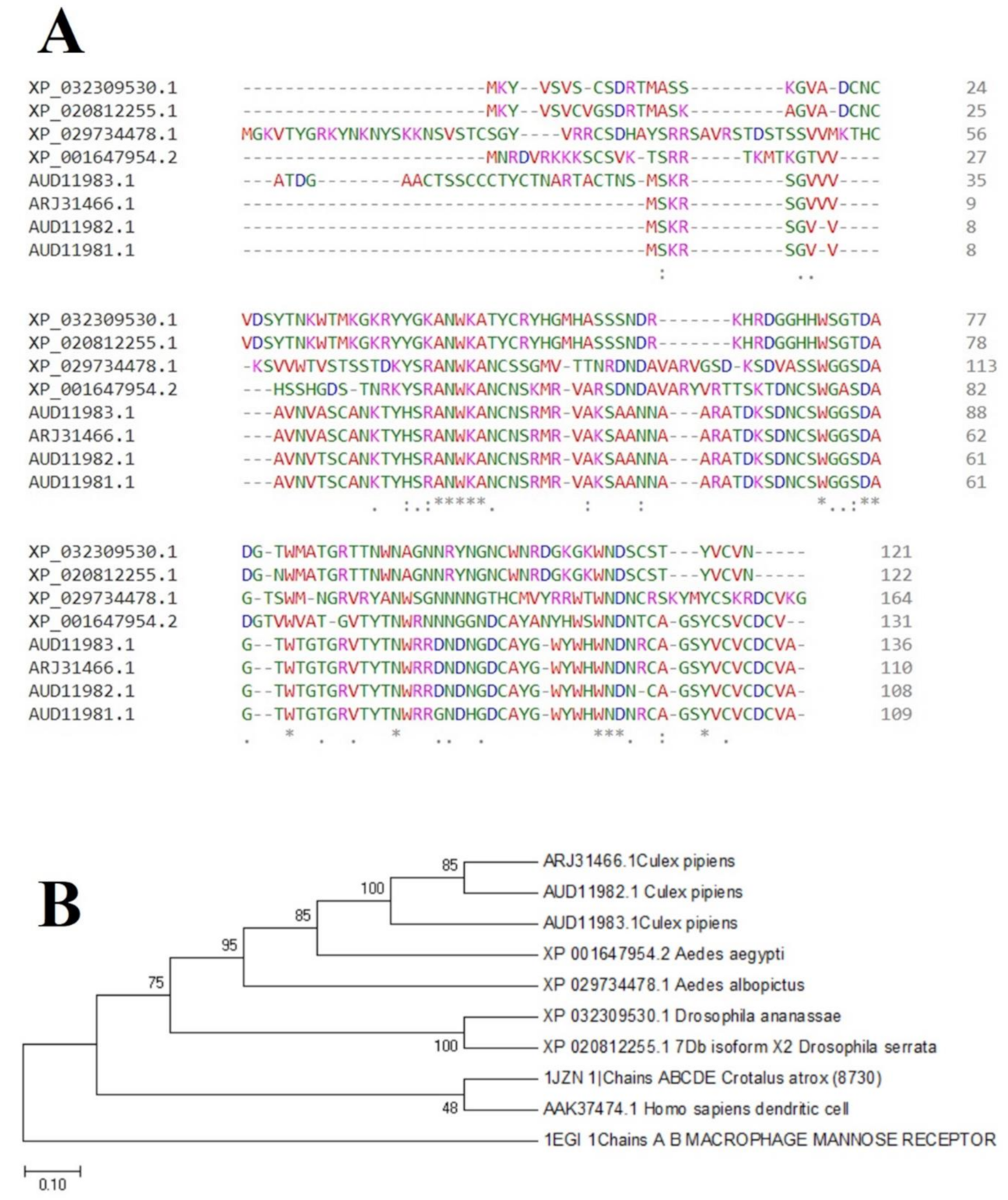
2.4. Phylogenetic Prediction and Conserved Sequence
2.5. Prediction of the Pitope Avidity
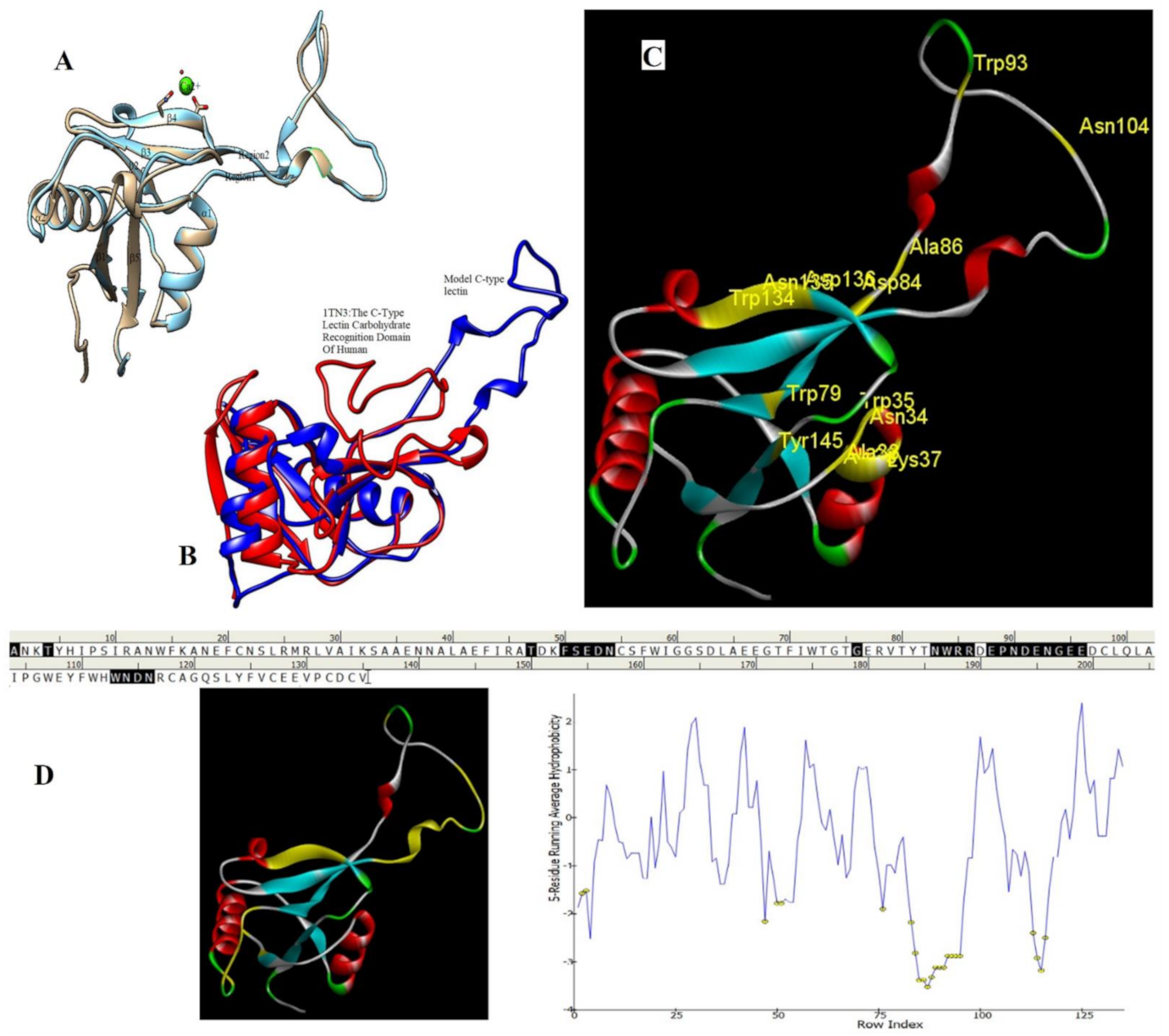
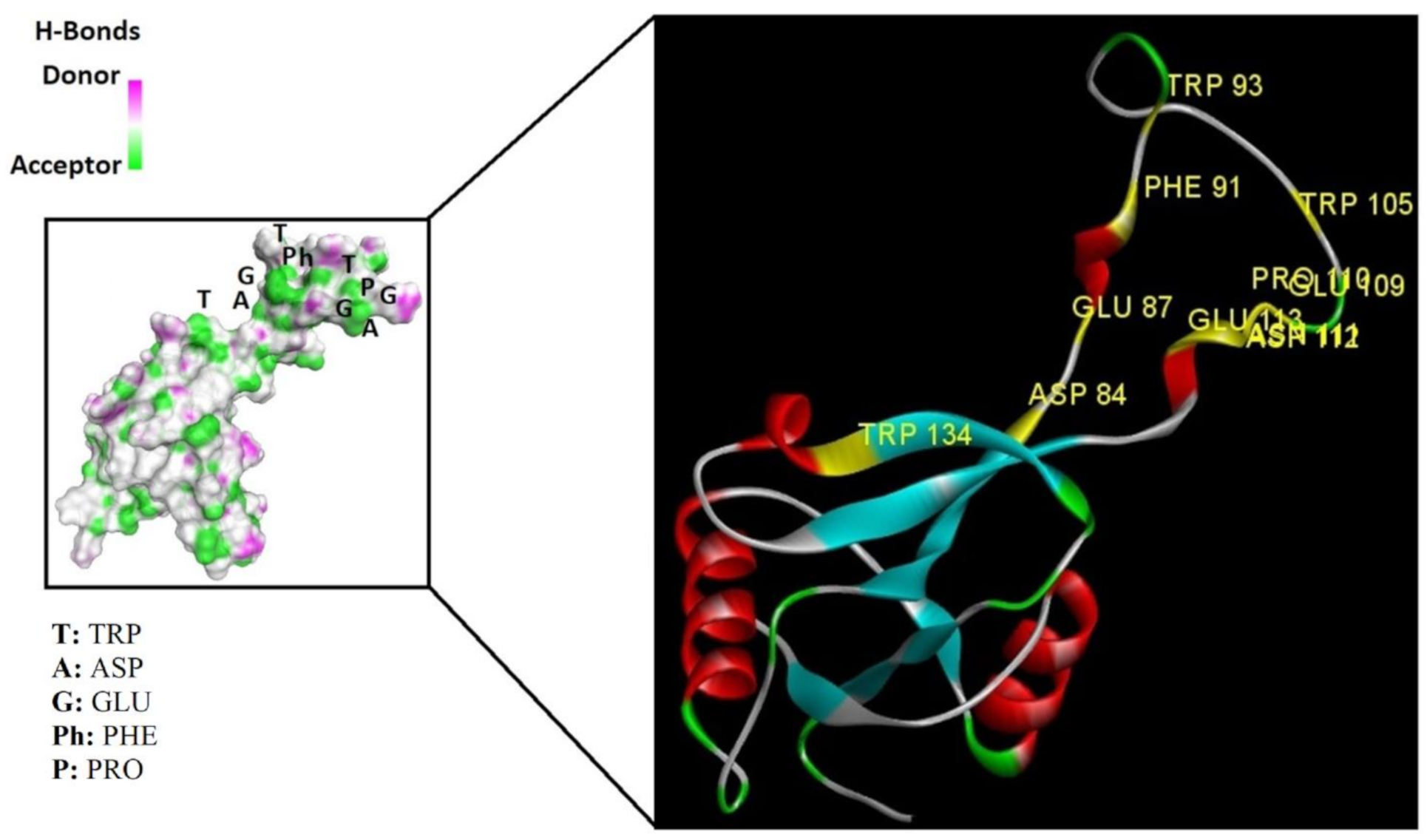
2.6. Homology Modeling and Superimposition
2.7. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Mosquito Collection, Identification, and Rearing
4.2. Characterization of the Cx. pipiens mosGCTL-1
4.3. Cloning, Expression, and Purification of the Cx. pipiens Recombinant mosGCTL-1 (rmosGCTL-1) and Immunization of the Rabbits
4.4. Blocking Assay
4.5. In Silico Studies
4.5.1. Phylogenetic Prediction and Conserved Sequence Studies
4.5.2. Prediction of the Epitope Avidity
4.5.3. Prediction of Protein Structure, Homology Modeling, and Molecular Interaction
4.5.4. Molecular Docking Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell-Lendrum, D.; Manga, L.; Bagayoko, M.; Sommerfeld, J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130552. [Google Scholar] [CrossRef]
- World Health Organization. Vector-Borne diseases (Fact sheet N°387). Available online: http://www.who.int/mediacentre/factsheets/fs387/en/ (accessed on 5 March 2015).
- Caraballo, H.; King, K. Emergency department management of mosquito-borne illness: Malaria, dengue, and West Nile virus. Emerg. Med. Pr. 2014, 16, 1–23. [Google Scholar]
- Bakhshi, H.; Failloux, A.-B.; Zakeri, S.; Raz, A.; Djadid, N.D. Mosquito-borne viral diseases and potential transmission blocking vaccine candidates. Infect. Genet. Evol. 2018, 63, 195–203. [Google Scholar] [CrossRef]
- Wieten, R.; Goorhuis, A.; Jonker, E.; de Bree, G.; de Visser, A.; van Genderen, P.; Remmerswaal, E.; ten Berge, I.; Visser, L.; Grobusch, M. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and im-mune-compromised patients. J. Infect. 2016, 72, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Tetravalent Dengue Vaccine: A Review in the Prevention of Dengue Disease. Drugs 2016, 76, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, A.; Maleki-Ravasan, N.; Djadid, N.D.; Alipour, H.; Ahmadvand, R.; Karimian, F.; Yousefinejad, S. GC–MS analysis and anti–mosquito activities of Juniperus virginiana essential oil against Anopheles stephensi (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2019, 9, 168. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Èntomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Rener, J.; Graves, P.M.; Carter, R.; Williams, J.L.; Burkot, T.R. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J. Exp. Med. 1983, 158, 976–981. [Google Scholar] [CrossRef]
- Diosa-Toro, M.; Hernández, J.C. Immunopathogenesis of West Nile Virus Infection. Curare 2014, 1, 17–23. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Garcia, M.-D.; Mazzon, M.; Jacobs, M.; Amara, A. Pathogenesis of Flavivirus Infections: Using and Abusing the Host Cell. Cell Host Microbe 2009, 5, 318–328. [Google Scholar] [CrossRef]
- Hwang, J.; Jurado, K.A.; Fikrig, E. Genetics of War and Truce between Mosquitos and Emerging Viruses. Cell Host Microbe 2016, 19, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, F.; Liu, J.; Xiao, X.; Zhang, S.; Qin, C.; Xiang, Y.; Wang, P.; Cheng, G. Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention. PLOS Pathog. 2014, 10, e1003931. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cox, J.; Wang, P.; Krishnan, M.N.; Dai, J.; Qian, F.; Anderson, J.F.; Fikrig, E. A C-Type Lectin Collaborates with a CD45 Phosphatase Homolog to Facilitate West Nile Virus Infection of Mosquitoes. Cell 2010, 142, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda 1. Am. J. Trop. Med. Hyg. 1940, 1, 471–492. [Google Scholar] [CrossRef]
- Andreadis, T.G. The Contribution of Culex pipiens Complex Mosquitoes to Transmission and Persistence of West Nile Virus in North America. J. Am. Mosq. Control. Assoc. 2012, 28, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Roehrig, J.T.; Sejvar, J.J. West Nile Virus in the Americas. In New and Evolving Infections of the 21st Century; Springer International Publishing: New York, NY, USA, 2006; pp. 3–56. [Google Scholar]
- Rizzoli, A.; Jiménez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Eurosurveillance 2015, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.K.; Fuller, D.O.; Haddad, N.; Hassan, A.N.; Gad, A.M.; Beier, J.C. Modeling the distribution of the West Nile and Rift Valley Fever vector Culex pipiens in arid and semi-arid regions of the Middle East and North Africa. Parasites Vectors 2014, 7, 289. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Pignol, D.; Bernard, J.-P.; Verdier, J.-M.; Dagorn, J.-C.; Fontecilla-Camps, J.C. Crystal structure of human lithostathine, the pancreatic inhibitor of stone formation. EMBO J. 1996, 15, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.B.; Kastrup, J.S.; Rasmussen, H.; Holtet, T.L.; Graversen, J.H.; Etzerodt, M.; Thøgersen, H.C.; Larsen, I.K. Crystal structure of tetranectin, a trimeric plasminogen-binding protein with an α-helical coiled coil. FEBS Lett. 1997, 412, 388–396. [Google Scholar] [CrossRef]
- Mizuno, H.; Fujimoto, Z.; Koizumi, M.; Kano, H.; Atoda, H.; Morita, T. Structure of coagulation factors IX/X-binding protein, a heterodimer of C-type lectin domains. Nat. Genet. 1997, 4, 438–441. [Google Scholar] [CrossRef]
- Boyington, J.C.; Riaz, A.N.; Patamawenu, A.; Coligan, J.E.; Brooks, A.G.; Sun, P.D. Structure of CD94 reveals a novel C-type lectin fold: Implications for the NK cell–associated CD94/NKG2 receptors. Immunity 1999, 10, 75–82. [Google Scholar] [CrossRef]
- Tormo, J.; Natarajan, K.; Margulies, D.H.; Mariuzza, R.A. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nat. Cell Biol. 1999, 402, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.T.; Taylor, M.E.; Feinberg, H.; Park-Snyder, S.; Kolatkar, A.R.; Weis, W.I. Structure of a C-type Carbohydrate Recognition Domain from the Macrophage Mannose Receptor. J. Biol. Chem. 2000, 275, 21539–21548. [Google Scholar] [CrossRef] [PubMed]
- Mullin, N.P.; Hitchen, P.G.; Taylor, M.E. Mechanism of Ca2+ and monosaccharide binding to a C-type carbohy-drate-recognition domain of the macrophage mannose receptor. J. Biol. Chem. 1997, 272, 5668–5681. [Google Scholar] [CrossRef]
- Weis, W.I.; Kahn, R.; Fourme, R.; Drickamer, K.; Hendrickson, W.A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science 1991, 254, 1608–1615. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K.; Hendrickson, W.A. Structure of a C-type mannose-binding protein complexed with an oligo-saccharide. Nature 1992, 360, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Jain, R.; Vig, R.; Norgard-Sumnicht, K.E.; Matta, K.L.; Varki, A. Selectin inhibition: Synthesis and evaluation of novel sialylated, sulfated and fucosylated oligosaccharides, including the major capping group of GlyCAM-1. Glycobiology 1997, 7, 79–93. [Google Scholar] [CrossRef][Green Version]
- Fritz, M.; Walker, E.; Miller, J.; Severson, D.; Dworkin, I. Divergent host preferences of above-and below-ground C ulex pipiens mosquitoes and their hybrid offspring. Med. Vet. Entomol. 2015, 29, 115–123. [Google Scholar] [CrossRef]
- Hamer, G.L.; Goldberg, T.L.; Ruiz, M.O.; Brawn, J.D.; Kitron, U.D.; Hayes, D.B.; Loss, S.R.; Walker, E.D. Host Selection by Culex pipiens Mosquitoes and West Nile Virus Amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef]
- Tempelis, C.H. REVIEW ARTICLE1: Host-Feeding Patterns of Mosquitoes, with a Review of Advances in Analysis of Blood Meals by Serology2. J. Med. Èntomol. 1975, 11, 635–653. [Google Scholar] [CrossRef]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Vinogradova, E.B. Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control; Pensoft Publishers: Sofia, Bulgaria, 2000. [Google Scholar]
- Dehghan, H.; Sadraei, J.; Moosa-Kazemi, S.H.; Baniani, N.A.; Nowruzi, F. The molecular and morphological variations of Culex pipiens complex (Diptera: Culicidae) in Iran. J. Vector Borne Dis. 2013, 50, 111–120. [Google Scholar]
- Tham, H.-W.; Balasubramaniam, V.R.M.T.; Chew, M.-F.; Ahmad, H.; Hassan, S.S. Protein-protein interactions between A. aegypti midgut and dengue virus 2: Two-hybrid screens using the midgut cDNA library. J. Infect. Dev. Ctries. 2015, 9, 1338–1349. [Google Scholar] [CrossRef]
- Liu, K.; Qian, Y.; Jung, Y.-S.; Zhou, B.; Cao, R.; Shen, T.; Shao, D.; Wei, J.; Ma, Z.; Chen, P.; et al. mosGCTL-7, a C-Type Lectin Protein, Mediates Japanese Encephalitis Virus Infection in Mosquitoes. J. Virol. 2017, 91, e01348-16. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Myles, K.M. The C-Type Lectin Domain Gene Family in Aedes aegypti and Their Role in Arbovirus Infection. Viruses 2018, 10, 367. [Google Scholar] [CrossRef]
- Pakdel, J.D.; Zakeri, S.; Raz, A.; Djadid, N.D. Identification, molecular characterization and expression of aminopeptidase N-1 (APN-1) from Anopheles stephensi in SF9 cell line as a candidate molecule for developing a vaccine that interrupt malaria transmission. Malar. J. 2020, 19, 79–119. [Google Scholar] [CrossRef]
- Selin, L.K.; Cornberg, M.; Brehm, M.A.; Kim, S.-K.; Calcagno, C.; Ghersi, D.; Puzone, R.; Celada, F.; Welsh, R.M. CD8 memory T cells: Cross-reactivity and heterologous immunity. Semin. Immunol. 2004, 16, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Xu, Q.; Nelson, S.; Oliphant, T.; Nybakken, G.E.; Fremont, D.H.; Diamond, M.S. The stoichiometry of anti-body-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 2007, 1, 135–145. [Google Scholar]
- Azari-Hamidian, S.; Harbach, R.E. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa 2009, 2078, 1–33. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.R.; Fox, J.G. Institutional Policies and Guidelines on Adjuvants and Antibody Production. ILAR J. 1995, 37, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Vazeille-Falcoz, M.; Rodhain, F.; Chungue, E.; Failloux, A.B.; Mousson, L. Variation in oral susceptibility to dengue type 2 virus of populations of Aedes aegypti from the islands of Tahiti and Moorea, French Polynesia. Am. J. Trop. Med. Hyg. 1999, 60, 292–299. [Google Scholar] [CrossRef]
- Zakhia, R.; Mousson, L.; Vazeille, M.; Haddad, N.; Failloux, A.-B. Experimental transmission of West Nile Virus and Rift Valley Fever Virus by Culex pipiens from Lebanon. PLoS Negl. Trop. Dis. 2018, 12, e0005983. [Google Scholar] [CrossRef] [PubMed]
- Murgue, B.; Murri, S.; Zientara, S.; Durand, B.; Durand, J.-P.; Zeller, H. West Nile outbreak in horses in southern France, 2000: The return after 35 years. Emerg. Infect. Dis. 2001, 7, 692–696. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Amirzargar, A.; Mytilineos, J.; Farjadian, S.; Doroudchi, M.; Scherer, S.; Opelz, G.; Ghaderi, A. Human leukocyte antigen class II allele frequencies and haplotype association in Iranian normal population. Hum. Immunol. 2001, 62, 1234–1238. [Google Scholar] [CrossRef]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo-and het-ero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Reposito-ry—new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo—Distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, M. DC-SIGN antagonists–A paradigm of C-type lectin binding inhibition. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; IntechOpen: London, UK, 2012. [Google Scholar]
- Combs, S.A.; DeLuca, S.L.; DeLuca, S.H.; Lemmon, G.H.; Nannemann, D.P.; Nguyen, E.D.; Willis, J.R.; Sheehan, J.H.; Meiler, J. Small-molecule ligand docking into comparative models with Rosetta. Nat. Protoc. 2013, 8, 1277–1298. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, S.; Khar, K.; Meiler, J. Fully Flexible Docking of Medium Sized Ligand Libraries with RosettaLigand. PLoS ONE 2015, 10, e0132508. [Google Scholar] [CrossRef]
- Cheng, T.M.-K.; Blundell, T.L.; Fernandez-Recio, J. pyDock: Electrostatics and desolvation for effective scoring of rigid-body protein-protein docking. Proteins Struct. Funct. Bioinform. 2007, 68, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nguyen, M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef]
| No | MHC Alleles | Binding Situation | Highest Affinity (nM) | Number of Peptides |
|---|---|---|---|---|
| 1 | HLA-DPA10103-DPB10401 | high binders = 0 weak binders = 0 | - | 122 |
| 2 | HLA-DQA10101-DQB10501 | high binders = 1 weak binders = 7 | 72.2 | 122 |
| 3 | HLA-DQA10102-DQB10602 | high binders = 7 weak binders = 11 | 11.9 | 122 |
| 4 | HLA-DQA10501-DQB10201 | high binders = 0 weak binders = 0 | - | 122 |
| 5 | HLA-DQA10501-DQB10301 | high binders = 4 weak binders = 22 | 29.0 | 122 |
| 6 | HLA-DQA10501-DQB10303 | high binders = 2 weak binders = 8 | 81.8 | 122 |
| 7 | HLA-DQA10201-DQB10301 | high binders = 1 weak binders = 42 | 62.3 | 122 |
| 8 | HLA-DRB1_0101 | high binders = 0 weak binders = 0 | - | 122 |
| 9 | HLA-DRB1_0301 | high binders = 0 weak binders = 0 | - | 122 |
| 10 | HLA-DRB1_0401 | high binders = 0 weak binders = 2 | 151.8 | 122 |
| 11 | HLA-DRB1_0402 | high binders = 0 weak binders = 3 | 977.3 | 122 |
| 12 | HLA-DRB1_0403 | high binders = 0 weak binders = 16 | 750.8 | 122 |
| 13 | HLA-DRB1_0404 | high binders = 2 weak binders = 9 | 26.5 | 122 |
| 14 | HLA-DRB1_0405 | high binders = 0 weak binders = 3 | 118.6 | 122 |
| 15 | HLA-DRB1_0701 | high binders = 0 weak binders = 0 | - | 122 |
| 16 | HLA-DRB1_1101 | high binders = 0 weak binders = 1 | 90.9 | 122 |
| 17 | HLA-DRB1_1501 | high binders = 0 weak binders = 0 | - | 122 |
| Sequence/ Amino Acid | Cys735 Cys 119 | Cys 759 Cys 148 | PHE 708 to ASN 728 PHE 91 to ASP 112 | 11 Different Residues |
|---|---|---|---|---|
| Sequence importance | Disulfide bridge Core stabilizer | Disulfide bridge Core stabilizer | calcium interactive loop | Ligand interactive residues |
| RMSD | 0.041 | 0.135 | 0.426 | 0.087 |
| Figure | n | 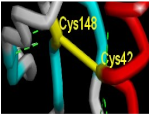 | 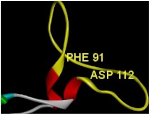 |  |
| Molecule | Coordinated Amino Acids 2D Pattern | Δ G of InterfaceKcal/mol | Total Δ G Score (Kcal/mol) | Graph Pattern of All Interaction and Highlighted Lowest Score | Graphical Interaction View |
|---|---|---|---|---|---|
| 1EGI | 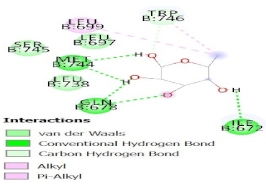 | −5.4 | −115.9 | 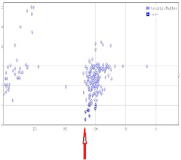 | 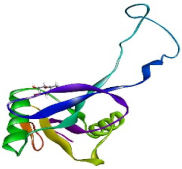 |
| CTLM | 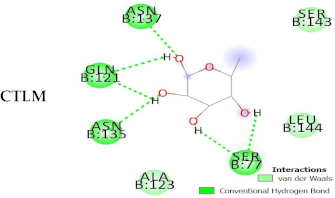 | −3.9 | −137.1 |  | 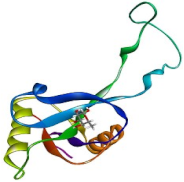 |
| Rank | 1EGI 2I69 Involved Residues | Total Δ G (Kcal/mol) | Graphical Interactions View |
|---|---|---|---|
| 1 | SER711:THR318 ASP712:GLY319 TYR718:SER07 ALA722:ASP28 TYR723:ASP28 | −98.762 | 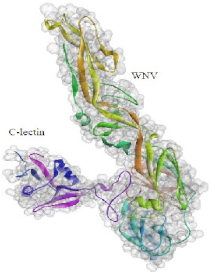 |
| 2 | THR700:SER15 6TYR723:LGY334 GLY724:ALA365 GLY724:THR366 | −93.892 |  |
| 3 | VAL732:SER156 TYR723:ARG166 SER705:ASN47 ARG663:GLY5 ALA664:GLU150 | −91.021 | 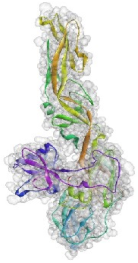 |
| Rank | CTLM: 2I69 Involved residues | Total Δ G( Kcal/mol) | Graphical interactions view |
| 1 | GLY141:SER156 GLU113:GLY334 Glu113:ALA367 GLU390:GLY95 | −102.560 | 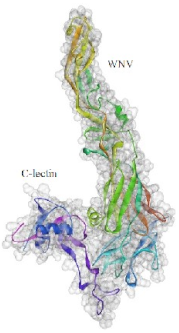 |
| 2 | GLU129:ASP317 GLU129:GLU150 PHE131:GLY5 GLU98:SER276 | −102.073 | 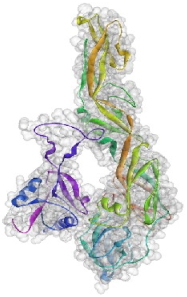 |
| 3 | ARG107:ALA365 TYR102:GLU150 GLU88:THR366 | −98.679 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshi, H.; Fazlalipour, M.; Dadgar-Pakdel, J.; Zakeri, S.; Raz, A.; Failloux, A.-B.; Dinparast Djadid, N. Developing a Vaccine to Block West Nile Virus Transmission: In Silico Studies, Molecular Characterization, Expression, and Blocking Activity of Culex pipiens mosGCTL-1. Pathogens 2021, 10, 218. https://doi.org/10.3390/pathogens10020218
Bakhshi H, Fazlalipour M, Dadgar-Pakdel J, Zakeri S, Raz A, Failloux A-B, Dinparast Djadid N. Developing a Vaccine to Block West Nile Virus Transmission: In Silico Studies, Molecular Characterization, Expression, and Blocking Activity of Culex pipiens mosGCTL-1. Pathogens. 2021; 10(2):218. https://doi.org/10.3390/pathogens10020218
Chicago/Turabian StyleBakhshi, Hasan, Mehdi Fazlalipour, Javad Dadgar-Pakdel, Sedigheh Zakeri, Abbasali Raz, Anna-Bella Failloux, and Navid Dinparast Djadid. 2021. "Developing a Vaccine to Block West Nile Virus Transmission: In Silico Studies, Molecular Characterization, Expression, and Blocking Activity of Culex pipiens mosGCTL-1" Pathogens 10, no. 2: 218. https://doi.org/10.3390/pathogens10020218
APA StyleBakhshi, H., Fazlalipour, M., Dadgar-Pakdel, J., Zakeri, S., Raz, A., Failloux, A.-B., & Dinparast Djadid, N. (2021). Developing a Vaccine to Block West Nile Virus Transmission: In Silico Studies, Molecular Characterization, Expression, and Blocking Activity of Culex pipiens mosGCTL-1. Pathogens, 10(2), 218. https://doi.org/10.3390/pathogens10020218







