Toxocara cati and Other Parasitic Enteropathogens: More Commonly Found in Owned Cats with Gastrointestinal Signs Than in Clinically Healthy Ones
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Animals, Samples, Questionnaires, and Samples Analysis
4.2. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, S.B.; Wolen, A.R. The benefits of human–companion animal interaction: A review. J. Med. Vet. Educ. 2008, 35, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.statista.com/statistics/516041/cat-population-europe-europe/ (accessed on 28 October 2020).
- Available online: https://www.statista.com/statistics/515464/cat-ownership-european-union-eu-by-country/ (accessed on 28 October 2020).
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Kolar, L.M.; Klausnor, J.S. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J. Am. Vet. Med. Assoc. 1999, 214, 1336–1341. [Google Scholar]
- Jones, P.H.; Dawson, S.; Gaskell, R.M.; Coyne, K.P.; Tierney, A.; Setzkorn, C.; Radford, A.D.; Noble, P.J. Surveillance of diarrhoea in small animal practice through the Small Animal Veterinary Surveillance Network (SAVSNET). Vet. J. 2014, 201, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Sabshin, S.J.; Levy, J.K.; Tupler, T.; Tucker, S.J.; Greiner, E.C.; Leutenegger, C.M. Enteropathogens identified in cats entering a Florida animal shelter with normal feces or diarrhea. J. Am. Vet. Med. Assoc. 2012, 241, 331–337. [Google Scholar] [CrossRef]
- Tello, L.; Perez-Freytes, R. Fluid and electrolyte therapy during vomiting and diarrhea. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 505–519. [Google Scholar] [CrossRef]
- Deplazes, P.; Eckert, J.; Mathis, A.; Samson-Himmelstjerna, G.V.; Zahner, H. Parasitology in Veterinary Medicine; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; ISBN 978-90-8686-247-0. [Google Scholar]
- Marks, S.L. Chapter 11—Diarrhea. In Canine and Feline Gastroenterology; Washabau, R.J., Day, M.J., Eds.; W.B. Saunders: London, UK, 2012; pp. 99–108. ISBN 9781416036616. [Google Scholar]
- Matz, M.E.; Guilford, W.G. Laboratory procedures for the diagnosis of gastrointestinal tract diseases of dogs and cats. N. Z. Vet. J. 2003, 51, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Symeonidou, I.; Gelasakis, A.I.; Arsenopoulos, K.; Angelou, A.; Beugnet, F.; Papadopoulos, E. Feline gastrointestinal parasitism in Greece: Emergent zoonotic species and associated risk factors. Parasites Vectors 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.D.; Thompson, R.C. Enteric parasitic zoonoses of domesticated dogs and cats. Microbes Infect. 2002, 48, 867–873. [Google Scholar] [CrossRef]
- Nijsse, R.; Ploeger, H.W.; Wagenaar, J.A.; Mughini-Gras, L. Prevalence and risk factors for patent Toxocara infections in cats and cat owners’ attitude towards deworming. Parasitol. Res. 2016, 115, 4519–4525. [Google Scholar] [CrossRef]
- Overgaauw, P.A.; Nederland, V. Aspects of Toxocara epidemiology: Toxocarosis in dogs and cats. Crit. Rev. Microbiol. 1997, 23, 233–251. [Google Scholar] [CrossRef]
- Glickman, L.T.; Schantz, P.M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol. Rev. 1981, 3, 230–250. [Google Scholar] [CrossRef]
- Macpherson, C.N. The epidemiology and public health importance of toxocariasis: A zoonosis of global importance. Int. J. Parasitol. 2013, 43, 999–1008. [Google Scholar] [CrossRef]
- Fisher, M. Toxocara cati: An underestimated zoonotic agent. Trends Parasitol. 2003, 19, 167–170. [Google Scholar] [CrossRef]
- Glickman, L.T.; Shofer, F.S. Zoonotic visceral and ocular larva migrans. Vet. Clin. N. Am. Small Anim. Pract. 1987, 17, 39–53. [Google Scholar] [CrossRef]
- Taira, K.; Saeed, I.; Permin, A.; Kapel, C.M.O. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet. Parasitol. 2004, 121, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, R.W.; Jessup, D.A. Zoonotic diseases associated with free-roaming cats. Zoonoses Public Health 2013, 60, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nedisan, M.E.; Ursache, A.L.; Dumitrache, M.O.; Mircean, V.; Cozma-Petruț, A.; Györke, A. Intestinal toxoplasmosis in cats treated with Procox (case report). Sci. Parasitol. 2019, 20, 19–24. [Google Scholar]
- Györke, A.; Dumitrache, M.O.; Kalmár, Z.; Paştiu, A.I.; Mircean, V. Molecular survey of metastrongyloid lungworms in domestic cats (Felis silvestris catus) from Romania: A retrospective study (2008–2011). Pathogens 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Schnyder, M.; Colombo, M.; Strube, C.; Dimzas, D.; Latino, R.; Traversa, D. Feline lungworms in Greece: Copromicroscopic, molecular and serological study. Parasitol. Res. 2020, 119, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Crisi, P.E.; Di Cesare, A.; De Santis, F.; Barlaam, A.; Santoprete, G.; Parrinello, C.; Palermo, S.; Mancini, P.; Traversa, D. Exposure of client-owned cats to zoonotic vector-borne pathogens: Clinic-pathological alterations and infection risk analysis. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101344. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Bourdeau, P.; Chalvet-Monfray, K.; Cozma, V.; Farkas, R.; Guillot, J.; Halos, L.; Joachim, A.; Losson, B.; Miró, G.; et al. Parasites of domestic owned cats in Europe: Co-infestations and risk factors. Parasites Vectors 2014, 7, 291. [Google Scholar] [CrossRef]
- Giannelli, A.; Capelli, G.; Joachim, A.; Hinney, B.; Losson, B.; Kirkova, Z.; René-Martellet, M.; Papadopoulos, E.; Farkas, R.; Napoli, E.; et al. Lungworms and gastrointestinal parasites of domestic cats: A European perspective. Int. J. Parasitol. 2017, 47, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Sepidarkish, M.; Ma, G.; Wang, T.; Ebrahimi, M.; Fakhri, Y.; Mirjalali, H.; Hofmann, A.; Macpherson, C.N.; Hotez, P.J.; et al. Global prevalence of Toxocara infection in cats. Adv. Parasitol. 2020, 109, 615. [Google Scholar] [CrossRef]
- Mircean, V.; Titilincu, A.; Vasile, C. Prevalence of endoparasites in household cat (Felis catus) populations from Transylvania (Romania) and association with risk factors. Vet. Parasitol. 2010, 171, 163–166. [Google Scholar] [CrossRef]
- Näreaho, A.; Puomio, J.; Saarinen, K.; Jokelainen, P.; Juselius, T.; Sukura, A. Feline intestinal parasites in Finland: Prevalence, risk factors and anthelmintic treatment practices. J. Feline Med. Surg. 2012, 14, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, Y.; Gasser, R.B.; Rostami, A.; Fan, C.K.; Ghasemi, S.M.; Javanian, M.; Bayani, M.; Armoon, B.; Moradi, B. Toxocara eggs in public places worldwide-A systematic review and meta-analysis. Environ. Pollut. 2018, 242, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Borecka, A.; Gawor, J. Modification of gDNA extraction from soil for PCR designed for the routine examination of soil samples contaminated with Toxocara spp. eggs. J. Helminthol. 2008, 82, 119–122. [Google Scholar] [CrossRef]
- Simonato, G.; Cassini, R.; Morelli, S.; Di Cesare, A.; La Torre, F.; Marcer, F.; Traversa, D.; Pietrobelli, M.; di Regalbono, A.F. Contamination of Italian parks with canine helminth eggs and health risk perception of the public. Prev. Vet. Med. 2019, 172, 104788. [Google Scholar] [CrossRef]
- Otero, D.; Alho, A.M.; Nijsse, R.; Roelfsema, J.; Overgaauw, P.; de Carvalho, L.M. Environmental contamination with Toxocara spp. eggs in public parks and playground sandpits of Greater Lisbon, Portugal. J. Infect. Public Health 2018, 11, 94–98. [Google Scholar] [CrossRef]
- Tyungu, D.L.; McCormick, D.; Lau, C.L.; Chang, M.; Murphy, J.R.; Hotez, P.J.; Mejia, R.; Pollack, H. Toxocara species environmental contamination of public spaces in New York City. PLoS Negl. Trop. Dis. 2020, 14, e0008249. [Google Scholar] [CrossRef]
- Bakhshani, A.; Maleki, M.; Haghparast, A.; Shirvan, S.P.; Borji, H. A survey on Toxocara cati eggs on the hair of stray cats: A potential risk factor for human toxocariasis in Northeastern Iran. Comp. Immunol. Microbiol. Infect Dis. 2019, 64, 10–13. [Google Scholar] [CrossRef]
- ESCCAP Worm Control in Dogs and Cats ESCCAP Guideline 01 Sixth Edition—February 2020. Available online: https://www.esccap.org/guidelines/gl1/ (accessed on 18 January 2021).
- Overgaauw, P.A.; van Knapen, F. Veterinary and public health aspects of Toxocara spp. Vet. Parasitol. 2013, 193, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Barutzki, D.; Schaper, R. Endoparasites in dogs and cats in Germany 1999–2002. Parasitol. Res. 2003, 90, S148–S150. [Google Scholar] [CrossRef]
- Strube, C.; Heuer, L.; Janecek, E. Toxocara spp. infections in paratenic hosts. Vet. Parasitol. 2013, 193, 375–389. [Google Scholar] [CrossRef]
- Zanzani, S.A.; Gazzonis, A.L.; Scarpa, P.; Berrilli, F.; Manfredi, M.T. Intestinal parasites of owned dogs and cats from metropolitan and micropolitan areas: Prevalence, zoonotic risks, and pet owner awareness in northern Italy. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- López, J.; Abarca, K.; Paredes, P.; Inzunza, E. Intestinal parasites in dogs and cats with gastrointestinal symptoms in Santiago, Chile. Rev. Med. Chile 2006, 134, 193–200. [Google Scholar] [CrossRef] [PubMed]
- López-Arias, Á.; Villar, D.; López-Osorio, S.; Calle-Vélez, D.; Chaparro-Gutiérrez, J.J. Giardia is the most prevalent parasitic infection in dogs and cats with diarrhea in the city of Medellín, Colombia. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100335. [Google Scholar] [CrossRef] [PubMed]
- Sauda, F.; Malandrucco, L.; De Liberato, C.; Perrucci, S. Gastrointestinal parasites in shelter cats of central Italy. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100321. [Google Scholar] [CrossRef] [PubMed]
- Symeonidou, I.; Gelasakis, A.Ι.; Miliotou, A.N.; Angelou, A.; Arsenopoulos, K.V.; Loukeri, S.; Papadopoulos, E. Rapid on-site diagnosis of canine giardiosis: Time versus performance. Parasites Vectors 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Riggio, F.; Mannella, R.; Ariti, G.; Perrucci, S. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet. Parasitol. 2013, 193, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.A.; Levy, J.K.; McManus, C.M.; McGorray, S.P.; Leutenegger, C.M.; Piccione, J.; Blackwelder, L.K.; Tucker, S.J. Prevalence of enteropathogens in cats with and without diarrhea in four different management models for unowned cats in the southeast United States. Vet. J. 2018, 236, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mircean, V.; Cozma, V.; Gyorke, A. Coproscopical Diagnostic of Parasitic Diseases in Animals; Risoprint: Cluj-Napoca, Romania, 2011; ISBN 978-973-53-0542-0. (In Romanian) [Google Scholar]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 3rd ed.; Blackwell Publishing: Oxford, UK; ISBN 978-1-118-68711-6.
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Fagan, R.F. Epi Info™, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2011.
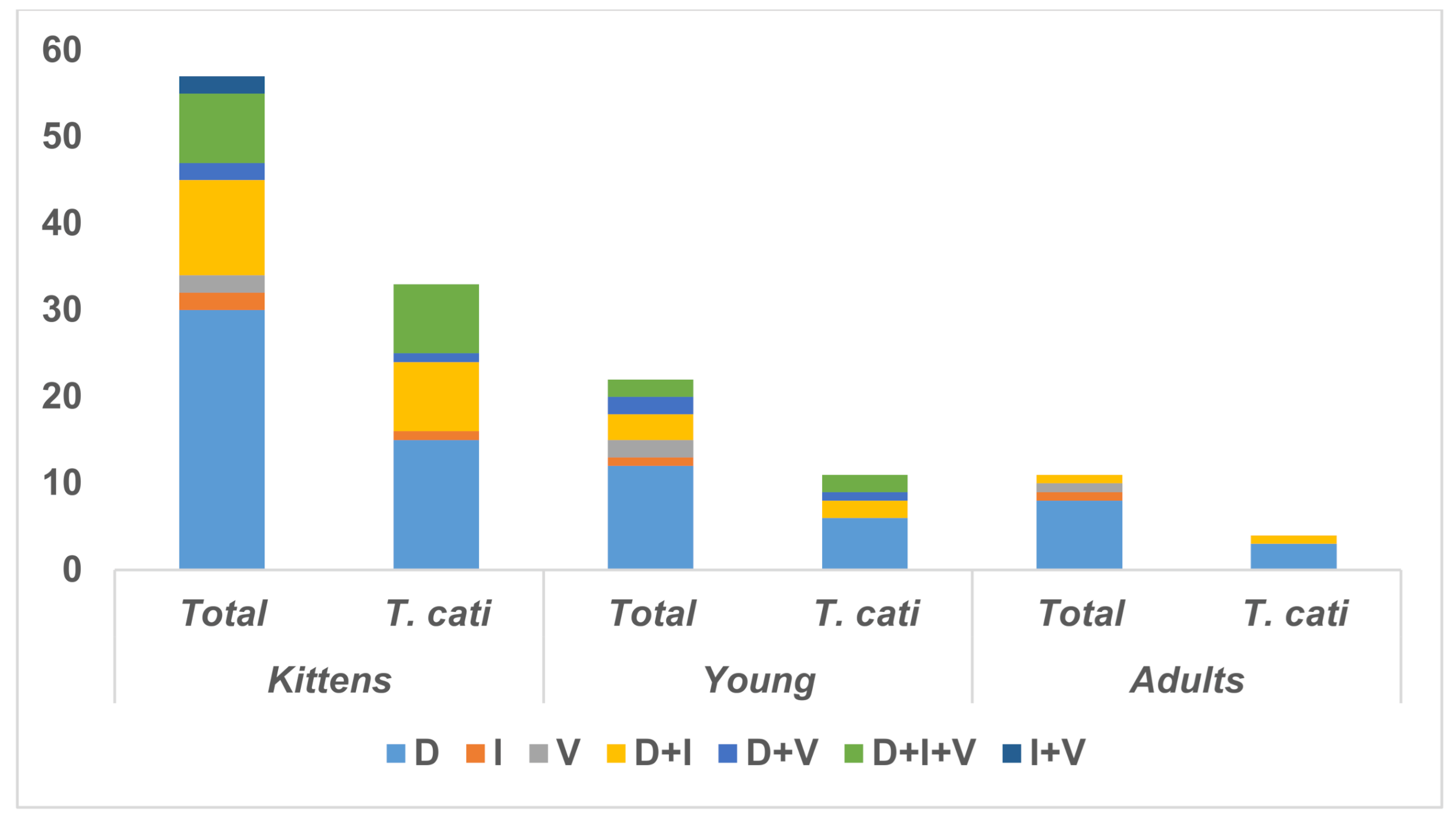
| Frequency | Prevalence | 95% Confidence Interval | |
|---|---|---|---|
| Intestinal parasites | 69 | 50.4 | 42.1–58.6 |
| T. cati | 55 | 40.2 *** | 31.9–48.9 |
| Cystoisospora spp. | 14 | 10.2 | 5.7–16.6 |
| Giardia duodenalis | 3 | 2.2 | 0.5–6.3 |
| Hookworms | 5 | 3.7 | 1.2–8.3 |
| Taeniidae | 3 | 2.2 | 0.5–16.6 |
| Toxoplasma gondii | 1 | 0.7 | 0.0–4.0 |
| Respiratory parasites | 7 | 5.1 | 2.5–10.2 |
| Lungworms | 6 | 4.4 | 1.6–9.3 |
| Eucoleus aerophilus | 1 | 0.7 | 0.0–4.0 |
| Total | 71 | 51.8 | 43.5–60.0 |
| Single infection | 54 | 39.4 | 31.6–47.8 |
| Mixed infection | 17 | 12.4 | 7.4–19.1 |
| T.cati + Cystoisospora spp. | 8 | 5.8 | 2.6–11.2 |
| T.cati + Taeniidae | 2 | 1.5 | 0.2–5.2 |
| T.cati + lungworms | 2 | 1.5 | 0.2–5.2 |
| T. cati + G. duodenalis | 1 | 0.7 | 0.0–4.0 |
| T.cati + T. gondii | 1 | 0.7 | 0.0–4.0 |
| T. cati + E. aerophilus | 1 | 0.7 | 0.0–4.0 |
| Cystoisospora spp. + lungworms | 1 | 0.7 | 0.0–4.0 |
| Hookworms + lungworms | 1 | 0.7 | 0.0–4.8 |
| Parasite | Cats with Digestive Signs (n = 90) | Clinically Healthy Cats (n = 47) | p | ||
|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | ||
| T. cati | 48 (53.3) | 42.5–64.0 | 7 (14.9) | 6.2–28.3 | 0.0000 |
| Cystoisospora spp. | 14 (15.6) | 8.8–24.7 | 0 | 0.0–7.6 | 0.0009 |
| Giardia duodenalis | 3 (3.3) | 0.7–9.4 | 0 | 0.0–7.6 | 0.1401 |
| Hookworms | 4 (4.4) | 1.2–11.0 | 1 (2.1) | 0.1–11.3 | 0.2783 |
| Taeniidae | 1 (1.1) | 0.0–6.0 | 2 (4.3) | 0.5–14.5 | 0.1547 |
| Toxoplasma gondii | 1 (1.1) | 0.0–6.0 | 0 | 0.0–7.6 | 0.3284 |
| Total intestinal parasites | 60 (66.7) | 56.4–75.6 | 9 (19.2) | 10.4–32.5 | <0.00001 |
| Single infection | 49 (54.4) | 44.2–64.3 | 8 (17.0) | 7.7–30.8 | 0.00003 |
| Mixed infection | 11 (12.2) | 7.0–20.6 | 1 (2.1) | 0.4–11.1 | 0.05 |
| T.cati + Cystoisospora spp. | 8 (8.9) | 3.9–16.8 | 0 | 0.0–7.6 | 0.0155 |
| T.cati + Taeniidae | 1 (1.1) | 0.0–6.0 | 1 (2.1) | 0.1–11.3 | 0.3430 |
| T. cati + G. duodenalis | 1 (1.1) | 0.0–6.0 | 0 | 0.0–7.6 | 0.3284 |
| T.cati + T. gondii | 1 (1.1) | 0.0–6.0 | 0 | 0.0–7.6 | 0.3284 |
| Total (n = 137) | Cats with Digestive Signs (n = 90) | Clinically Healthy Cats (n = 47) | p | ||||
|---|---|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | ||
| Age | |||||||
| <6 months | 34 (51.5) ** | 38.9–64.0 | 33 (57.9) | 44.1–70.9 | 1 (11.1) | 0.3–48.3 | 0.006 |
| 6 months–2 years | 15 (38.5) | 23.4–55.4 | 11 (50.0) | 28.2–71.8 | 4 (23.5) | 6.8–49.9 | 0.05 |
| >2 years | 6 (18.8) | 7.2–36.4 | 4 (36.4) | 10.9–69.2 | 2 (9.5) | 1.2–30.4 | 0.05 |
| Gender | |||||||
| Females | 30 (47.8) | 32.6–57.4 | 26 (65.0) | 48.3–79.4 | 4 (14.8) | 4.2–33.7 | 0.0000 |
| Males | 25 (35.7) | 24.6–48.1 | 22 (44.0) | 30.0–58.8 | 3 (15.0) | 3.2–37.9 | 0.01 |
| Outdoor Access | |||||||
| Yes | 53 (55.2) *** | 44.7–65.4 | 46 (64.8) | 52.5–75.8 | 7 (28.0) | 12.1–49.4 | 0.0009 |
| No | 2 (4.9) | 0.6–16.5 | 2 (10.5) | 1.3–33.1 | 0 | - | 0.10 |
| Dewormed in the Last 3 Months | |||||||
| Yes | 10 (13.5) | 6.7–23.5 | 8 (19.1) | 8.6–34.1 | 2 (6.3) | 0.8–20.8 | 0.06 |
| No | 45 (71.4) *** | 58.7–82.1 | 40 (83.3) | 69.8–95.5 | 5 (33.3) | 11.8–61.6 | 0.0002 |
| OR | 95% CI | p | |
|---|---|---|---|
| Age | |||
| 0–24 months old (n = 105) | 4.2 | 1.03–17.15 | 0.05 |
| >24 months old (n = 32) | Ref. | ||
| Gender | |||
| Males (n = 70) | 0.55 | 0.20–1.52 | 0.25 |
| Females (n = 67) | Ref. | ||
| Outdoor Access | |||
| Yes (n = 96) | 13.82 | 2.45–78.04 | 0.003 |
| No (n = 41) | Ref. | ||
| Dewormed in the Last 3 Months | |||
| No (n = 74) | 15.85 | 5.43–46.24 | 0.00001 |
| Yes (n = 63) | Ref. | ||
| Digestive Clinical Signs | |||
| No (n = 47) | Ref. | ||
| Yes (n = 90) | 5.37 | 1.60–18.01 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursache, A.L.; Györke, A.; Mircean, V.; Dumitrache, M.O.; Codea, A.R.; Cozma, V. Toxocara cati and Other Parasitic Enteropathogens: More Commonly Found in Owned Cats with Gastrointestinal Signs Than in Clinically Healthy Ones. Pathogens 2021, 10, 198. https://doi.org/10.3390/pathogens10020198
Ursache AL, Györke A, Mircean V, Dumitrache MO, Codea AR, Cozma V. Toxocara cati and Other Parasitic Enteropathogens: More Commonly Found in Owned Cats with Gastrointestinal Signs Than in Clinically Healthy Ones. Pathogens. 2021; 10(2):198. https://doi.org/10.3390/pathogens10020198
Chicago/Turabian StyleUrsache, Aurora L., Adriana Györke, Viorica Mircean, Mirabela O. Dumitrache, Andrei Răzvan Codea, and Vasile Cozma. 2021. "Toxocara cati and Other Parasitic Enteropathogens: More Commonly Found in Owned Cats with Gastrointestinal Signs Than in Clinically Healthy Ones" Pathogens 10, no. 2: 198. https://doi.org/10.3390/pathogens10020198
APA StyleUrsache, A. L., Györke, A., Mircean, V., Dumitrache, M. O., Codea, A. R., & Cozma, V. (2021). Toxocara cati and Other Parasitic Enteropathogens: More Commonly Found in Owned Cats with Gastrointestinal Signs Than in Clinically Healthy Ones. Pathogens, 10(2), 198. https://doi.org/10.3390/pathogens10020198







