The Vector-Borne Zoonotic Nematode Thelazia callipaeda in the Eastern Part of Europe, with a Clinical Case Report in a Dog in Poland
Abstract
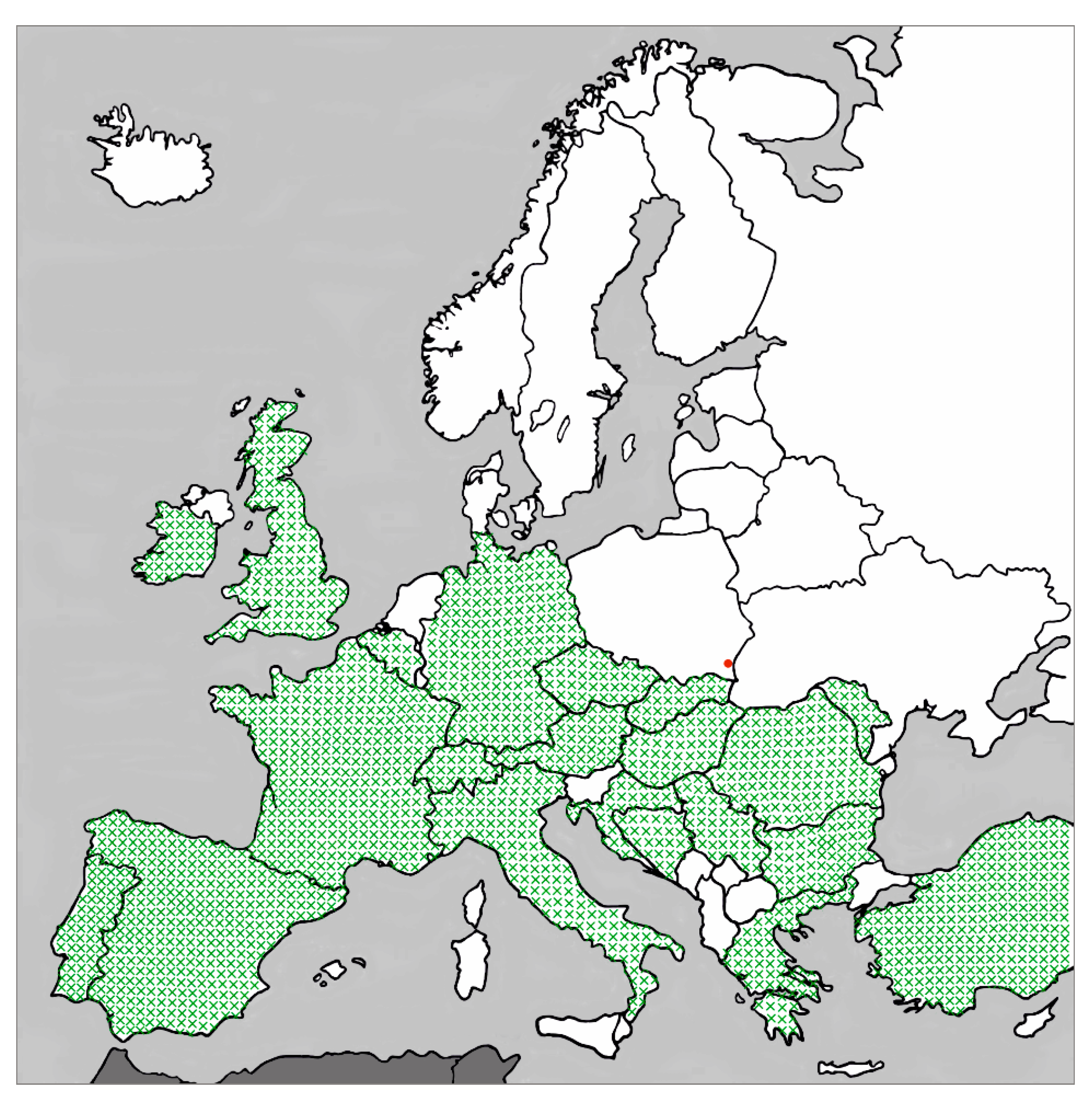
1. Introduction
2. Results
2.1. Case Report
2.2. Description of the Nematode
3. Discussion
4. Materials and Methods
4.1. Description of the Host
4.2. Parasitological Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Skrjâbin, K.I.; Sobolev, A.A.; Ivaškin, V.M. Spiruraty životnyh i čeloveka i vyzyvaemye imi zabolevaniâ, Telâsioidei. In Osnovy Nematodologii, 16, 4; Izdale’stvo Nauka: Moskva, Russia, 1967; pp. 12–58. [Google Scholar]
- Otranto, D.; Dutto, M. Human thelaziasis, Europe. Emerg. Infect. Dis. 2008, 14, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Traversa, D. Thelazia eyeworm: An original endo and ecto- parasitic nematode. Trends Parasitol. 2005, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- do Vale, B.; Lopes, A.P.; da Conceição Fontes, M.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Thelaziosis due to Thelazia callipaeda in Europe in the 21st century—A review. Vet. Parasitol. 2019, 275, 108957. [Google Scholar] [CrossRef]
- Railliet, A.; Henry, A. Nouvelles observations sur les Thélazies, Nématodes parasites de l’oeil. C. R. Seances Soc. Biol. Fil 1910, 68, 783–785. [Google Scholar]
- Bhaibulaya, M.; Prasertsilpa, S.; Vajrasthira, S. Thelazia callipaeda Railliet and Henry, 1910, in man and dog in Thailand. Am. J. Trop. Med. Hyg. 1970, 19, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Kosin, E.; Kosman, M.L.; Depary, A.A. First case of human thelaziosis in Indonesia. Southeast Asian J. Trop. Med. Public Health 1989, 20, 233–236. [Google Scholar] [PubMed]
- Hong, S.T.; Park, Y.K.; Lee, S.K.; Yoo, J.H.; Kim, A.S.; Chung, Y.H.; Hong, S.J. Two human cases of Thelazia callipaeda infection in Korea. Korean J. Parasitol. 1995, 33, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; pp. 404–407. [Google Scholar]
- Yang, C.-H.; Tung, K.-C.; Wang, M.-Y.; Chang, S.-C.; Tu, W.-C.; Wang, K.-S.; Shyu, C.-L.; Lee, W.-M. First Thelazia callipaeda infestation report in a dog in Taiwan. J. Vet. Med. Sci. 2006, 68, 103–104. [Google Scholar] [CrossRef] [PubMed]
- De, N.V.; Le, T.H.; Chai, J.-Y. The first human case of Thelazia callipaeda infection in Vietnam. Korean J. Parasitol. 2012, 50, 221–223. [Google Scholar] [CrossRef]
- Akhanda, A.H.; Akonjee, A.R.; Hossain, M.M.; Rahman, M.A.; Mishu, F.A.; Hasan, M.F.; Akhanda, T.H. Thelazia callipaeda infestation in Bangladesh: A case report. Mymensingh Med. J. 2013, 22, 581–584. [Google Scholar]
- Sah, R.; Khadka, S.; Adhikari, M.; Niraula, R.; Shah, A.; Khatri, A.; Suznne Donovan, S. Human thelaziasis: Emerging ocular pathogen in Nepal. Open Forum Infect. Dis. 2018, 5, ofy237. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2020, 1–2. [Google Scholar] [CrossRef] [PubMed]
- do Vale, B.; Lopes, A.P.; da Conceição Fontes, M.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Systematic review on infection and disease caused by Thelazia callipaeda in Europe: 2001–2020. Parasite 2020, 27, 52. [Google Scholar] [CrossRef]
- De Waal, T.; Lawlor, A.; Roulston, S. Case study: Ocular thelaziosis in a travelled dog. Vet. Irel. J. 2019, 9, 367–368. [Google Scholar]
- Hermosilla, C.; Bauer, C.; Herrmann, B. First case of Thelazia callipaeda infection in a dog in Germany. Vet. Rec. 2004, 154, 568–569. [Google Scholar] [CrossRef]
- Soaresa, C.; Sousaa, S.R.; Anastácioa, S.; Matiasd, M.G.; Marquêsd, I.; Mascarenhasd, S.; Vieiraa, M.J.; de Carvalhob, L.M.; Otranto, D. Feline thelaziosis caused by Thelazia callipaeda in Portugal. Vet. Parasitol. 2013, 196, 528–531. [Google Scholar] [CrossRef]
- Hammond, A. Thelazia callipaeda in a travelled dog in England. Vet. Rec. 2018, 183, 477. [Google Scholar] [CrossRef]
- Hodžić, A.; Payer, A.; Duscher, G. The first autochthonous case of feline ocular thelaziosis in Austria. Parasitol. Res. 2019, 118, 1321–1324. [Google Scholar] [CrossRef]
- Sargo, R.; Loureiro, F.; Catarino, A.L.; Valente, J.; Silva, F.; Cardoso, L.; Otranto, D.; Maia, C. First report of Thelazia callipaeda in red foxes (Vulpes vulpes) from Portugal. J. Zoo Wildl. Med. 2014, 45, 458–460. [Google Scholar] [CrossRef]
- Odoevskaya, I.; Khrustalev, A.V.; Shaĭtanov, V.M.; Seriodkin, I.V.; Panayotova-Pencheva, M. Occurrence of the nematode Thelazia callipaeda Railliet and Henry, 1910 (Spirurida, Thelaziidae) in wild carnivores in the Russian Far East. Acta Zool. Bulg. 2015, 67, 561–566. [Google Scholar]
- Mihalca, A.D.; Ionică, A.M.; D’Amico, G.; Daskalaki, A.A.; Deak, G.; Matei, I.A.; Șimonca, V.; Iordache, D.; Modrý, D.; Gherman, C.M. Thelazia callipaeda in wild carnivores from Romania: New host and geographical records. Parasites Vectors 2016, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Seixas, F.; Travassos, P.; Coutinho, T.; Lopes, A.P.; Latrofa, M.S.; dos Pires, M.A.; Cardoso, L.; Otranto, D. The eyeworm Thelazia callipaeda in Portugal: Current status of infection in pets and wild mammals and case report in a beech marten (Martes foina). Vet. Parasitol. 2018, 15, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Gajić, B.; Bugarski-Stanojević, V.; Penezić, A.; Kuručki, M.; Bogdanović, N.; Ćirović, D. First report of eyeworm infection by Thelazia callipaeda in gray wolf (Canis lupus) from Serbia. Parasitol. Res. 2019, 118, 3549–3553. [Google Scholar] [CrossRef]
- Ionică, A.M.; Deak, G.; D’Amico, G.; Stan, G.F.; Chișamera, G.B.; Constantinescu, I.C.; Adam, C.; Lefkaditis, M.; Gherman, C.M.; Mihalca, A.D. Thelazia callipaeda in mustelids from Romania with the European badger, Meles meles, as a new host for this parasite. Parasites Vectors 2019, 12, 370. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Malli, E.; DiGeronimo, P.M.; Brianti, E.; Testini, G.; Traversa, D.; Lia, R.P. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: Report of new host species and ecological implications. Vet. Parasitol. 2009, 166, 262–726. [Google Scholar] [CrossRef]
- Gama, A.; Pires, I.; Canado, M.; Coutinho, T.; Lopes, A.P.; Latrofa, M.S.; Cardoso, L.; Dantas-Torres, F.; Otranto, D. First report of Thelazia callipaeda infection in wild European rabbits (Oryctolagus cuniculus) in Portugal. Parasites Vectors 2016, 9, 236. [Google Scholar] [CrossRef]
- Otranto, D.; Eberhard, M.L. Zoonotic helminths affecting the human eye. Parasites Vectors 2011, 4, 41. [Google Scholar] [CrossRef]
- Fuentes, I.; Montes, I.; Saugar, J.M.; Latrofa, S.; Gárate, T.; Otranto, D. Thelaziosis in humans, a zoonotic infection, Spain, 2011. Emerg. Infect. Dis. 2012, 18, 2073–2075. [Google Scholar] [CrossRef]
- Otranto, D.; Cantacessi, C.; Testini, G.; Lia, R.P. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: Evidence for pathogen transmission by a male arthropod vector. Int. J. Parasitol. 2006, 36, 1167–1173. [Google Scholar] [CrossRef]
- Otranto, D.; Brianti, E.; Cantacessi, C.; Lia, R.P.; Máca, J. The zoophilic fruitfly Phortica variegata: Morphology, ecology and biological niche. Med. Vet. Entomol. 2006, 20, 358–364. [Google Scholar] [CrossRef]
- Máca, J.; Otranto, D. Drosophilidae feeding on animals and the inherent mystery of their parasitism. Parasites Vectors 2014, 7, 516. [Google Scholar] [CrossRef]
- Huang, J.; Gong, L.; Tsaur, S.-C.; Zhu, L.; An, K.; Chen, H. Revision of the subgenus Phortica (sensu stricto) (Diptera, Drosophilidae) from East Asia, with assessment of species delimitation using DNA barcodes. Zootaxa 2019, 4678, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Bertaglia, P. Presence of Thelazia callipaeda Railliet and Henry, 1910, in Piedmont, Italy. Parassitologia 1989, 31, 167–172. [Google Scholar] [PubMed]
- Shtakelberg, A.A. Family Drosophilidae. In Keys to the Insects of the European Part of the USSR, Diptera and Siphonaptera; Bei-Bienko, G.Y., Steyskal, G.C., Eds.; E.J. Brill: Leiden, NY, USA; København, Danmark; Köln, Germany, 1989; Volume 5, pp. 393–665. [Google Scholar]
- Madany, J.; Wrześniewska, K.; Milczak, A.; Abramowicz, B.; Winiarczyk, D. Wzrastające ryzyko wystąpienia inwazji Thelazia callipaeda w Polsce, pasożyta powodującego objawy okulistyczne u psów i kotów. Życie Wet. 2018, 93, 175–178. [Google Scholar]
- Čabanová, V.; Kocák, P.; Víchová, B.; Miterpáková, M. First autochthonous cases of canine thelaziosis in Slovakia: A new affected area in Central Europe. Parasites Vectors 2017, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Balicka, A.; Lapšanská, M.; Halán, M.; Trbolová, A. Canine ocular thelaziosis in Slovakia a case report. Folia Vet. 2018, 62, 33–38. [Google Scholar] [CrossRef]
- Jirků, M.; Kuchta, R.; Gricaj, E.; Modry, D.; Pomajbikova, K.J. Canine thelaziosis in the Czech Republic: The northernmost autochthonous occurrence of the eye nematode Thelazia callipaeda Railliet et Henry, 1910 in Europe. Folia Parasitol. 2020, 67, 10. [Google Scholar] [CrossRef] [PubMed]
- Jańczak, D.; Wroński, K.; Słoński, A.; Przygodzka, M.; Lisowski, A.; Sałamatin, R.; Gołąb, E. Imported Thelazia callipaeda infection in German pointer dog in Poland. In Proceedings of the XXVth Congress of the Polish Parasitological Society, Warsaw, Poland, 9–12 September 2019; Volume 65 (Suppl. 1), p. 130. [Google Scholar]
- Ferroglio, E.; Rossi, L.; Tomio, E.; Schenker, R.; Bianciardi, P. Therapeutic and prophylactic efficacy of milbemycin oxime (interceptor) against Thelazia callipaeda in naturally exposed dogs. Vet. Parasitol. 2008, 154, 351–353. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liag, T.H.; Lin, S.H.; Chen, H.C.; Lai, S.C. Human thelaziasis occurrence in Taiwan. Clin. Exp. Optom. 2006, 89, 40–44. [Google Scholar] [CrossRef]
- Palfreyman, J.; Graham-Brown, J.; Caminade, C.; Gilmore, P.; Otranto, D.; Williams, D.J.L. Predicting the distribution of Phortica variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasites Vectors 2018, 11, 272. [Google Scholar] [CrossRef]
- Zatwarnicki, T. Wywilżniowate (Drosophilidae). In Fauna Polski. Charakterystyka i Wykaz Gatunków; Bogdanowicz, W., Chudzicka, E., Pilipiuk, I., Skibińska, E., Eds.; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 2007; pp. 130, 216–217. [Google Scholar]
- Pombi, M.; Marino, V.; Jaenike, J.; Graham-Brown, J.; Bernardini, I.; Lia, R.P.; Beugnet, F.; Miro, G.; Otranto, D. Temperature is a common climatic descriptor of lachryphagous activity period in Phortica variegata (Diptera: Drosophilidae) from multiple geographical locations. Parasites Vectors 2020, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Fauna Europaea. Available online: http://www.faunaeur.org (accessed on 11 December 2020).
- Falck, M. Phortica variegata (Fallén, 1823) (Diptera, Drosophilidae) new to Norway. Nor. J. Entomol. 2007, 54, 113–114. [Google Scholar]
- Gompper, M.E. The dog-human-wildlife interface: Assessing the scope of the problem. In Free-Ranging Dogs and Wildlife Conservation; Gompper, M.E., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 9–54. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolbiecki, L.; Izdebska, J.N.; Franke, M.; Iliszko, L.; Fryderyk, S. The Vector-Borne Zoonotic Nematode Thelazia callipaeda in the Eastern Part of Europe, with a Clinical Case Report in a Dog in Poland. Pathogens 2021, 10, 55. https://doi.org/10.3390/pathogens10010055
Rolbiecki L, Izdebska JN, Franke M, Iliszko L, Fryderyk S. The Vector-Borne Zoonotic Nematode Thelazia callipaeda in the Eastern Part of Europe, with a Clinical Case Report in a Dog in Poland. Pathogens. 2021; 10(1):55. https://doi.org/10.3390/pathogens10010055
Chicago/Turabian StyleRolbiecki, Leszek, Joanna N. Izdebska, Marta Franke, Lech Iliszko, and Sławomira Fryderyk. 2021. "The Vector-Borne Zoonotic Nematode Thelazia callipaeda in the Eastern Part of Europe, with a Clinical Case Report in a Dog in Poland" Pathogens 10, no. 1: 55. https://doi.org/10.3390/pathogens10010055
APA StyleRolbiecki, L., Izdebska, J. N., Franke, M., Iliszko, L., & Fryderyk, S. (2021). The Vector-Borne Zoonotic Nematode Thelazia callipaeda in the Eastern Part of Europe, with a Clinical Case Report in a Dog in Poland. Pathogens, 10(1), 55. https://doi.org/10.3390/pathogens10010055






