An Unusual Case of Mixed Respiratory Capillariosis in a Dog
Abstract
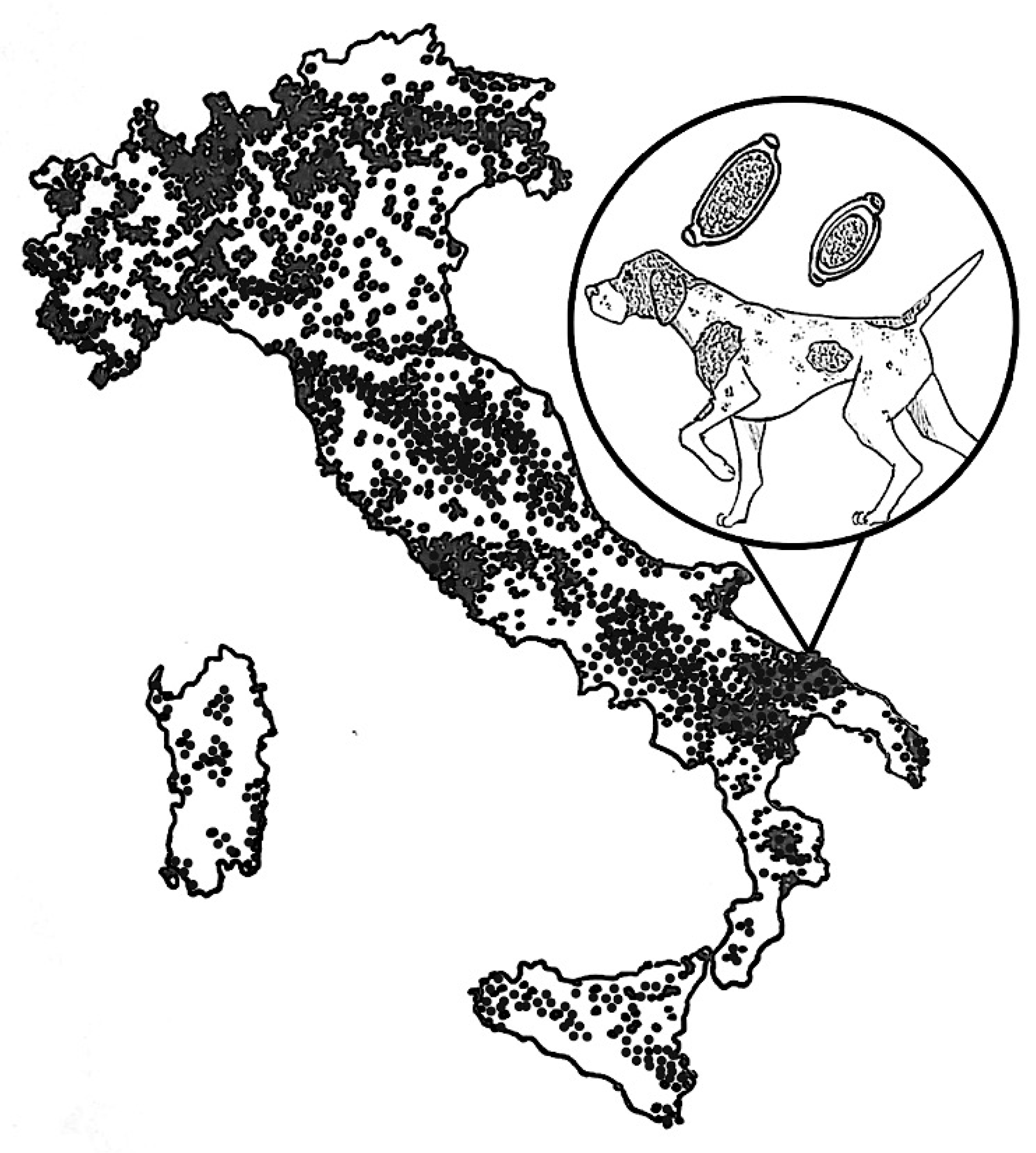
1. Introduction
2. Case Details
2.1. Clinical Case
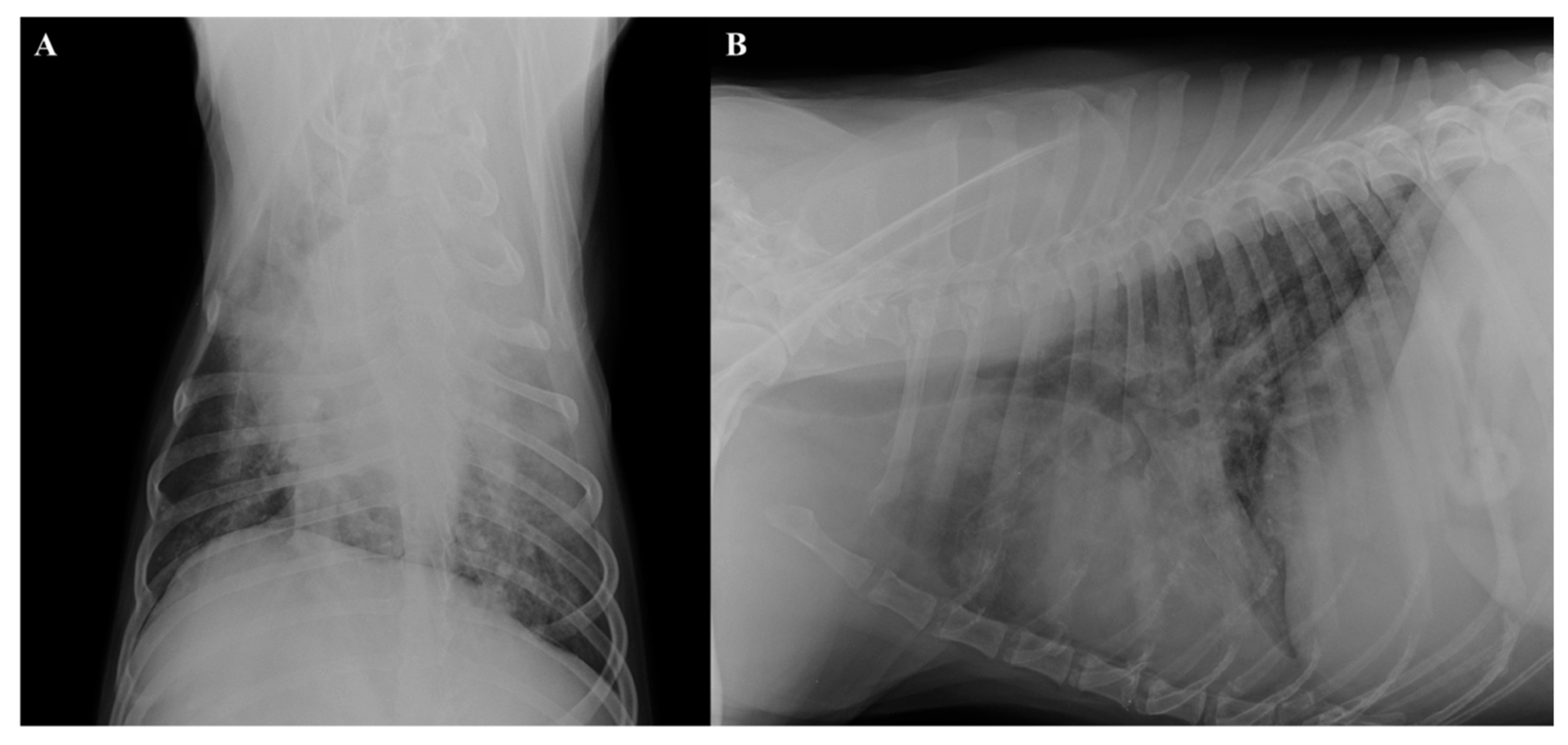
2.2. Radiographic and Ultrasound Examinations
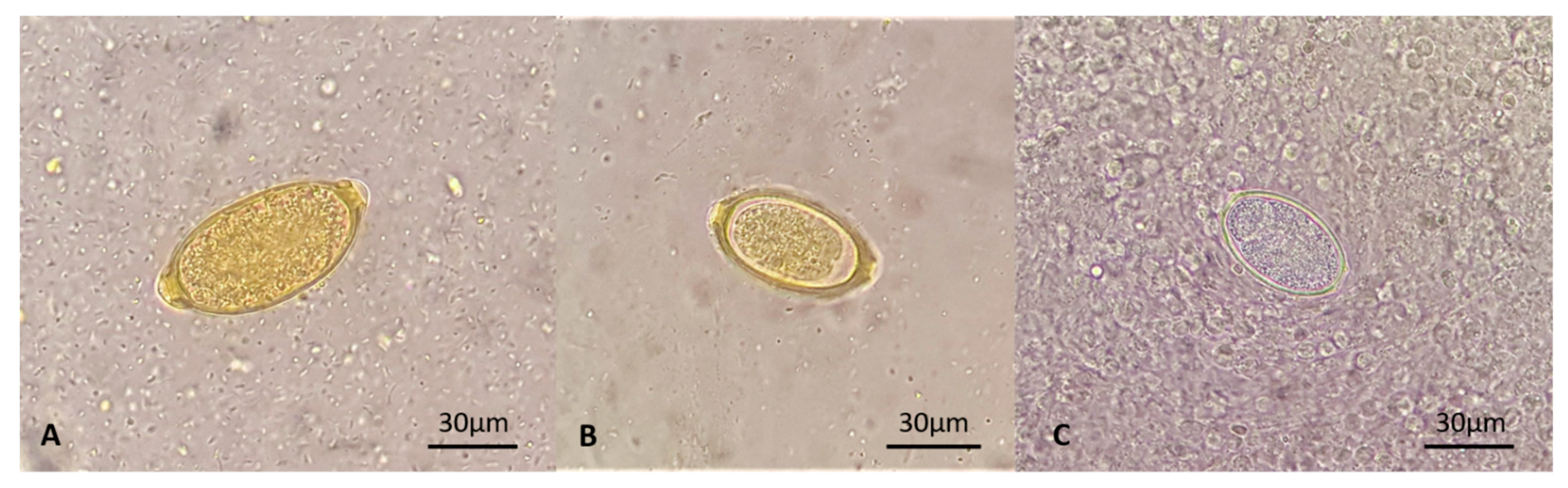
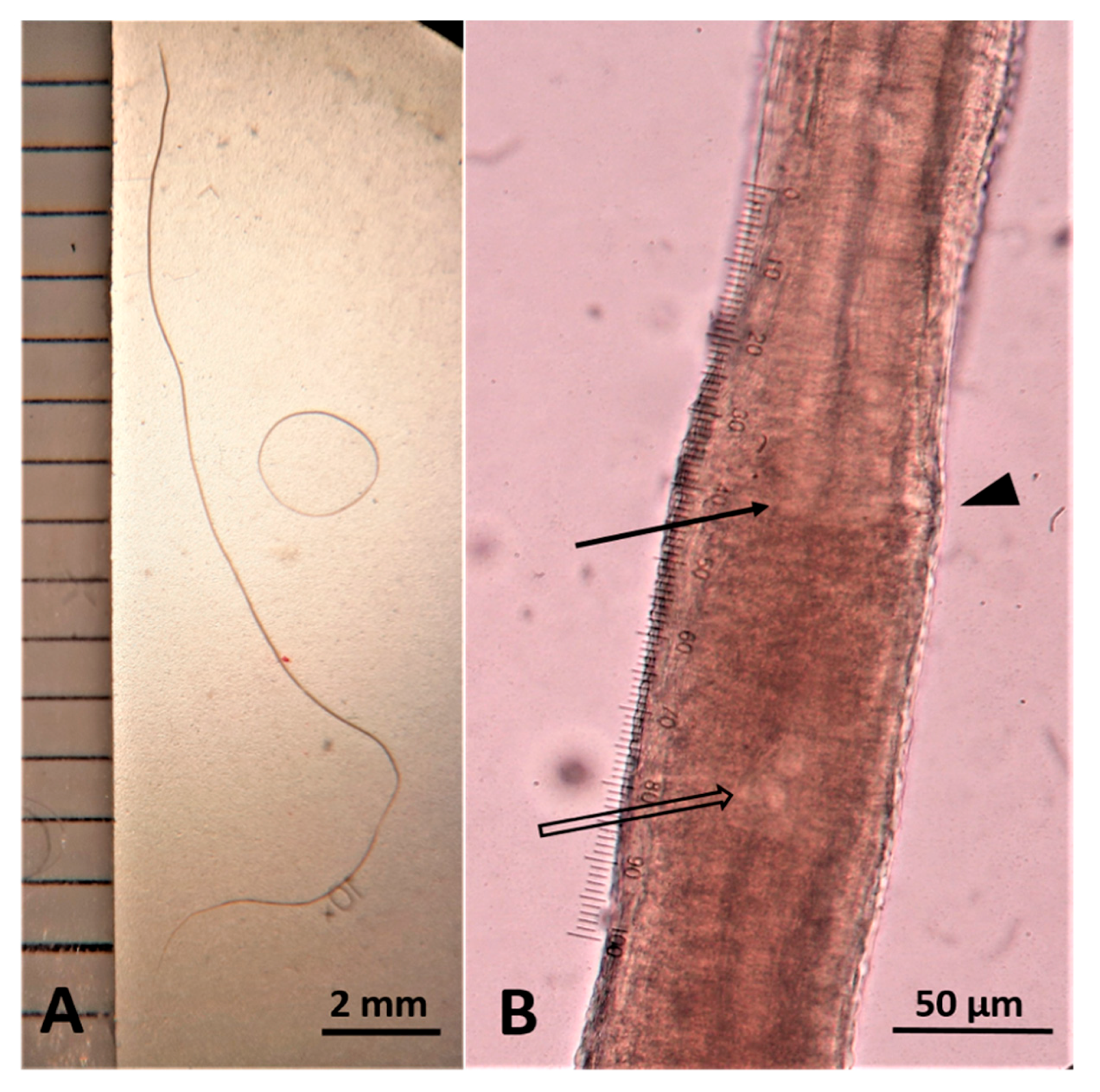
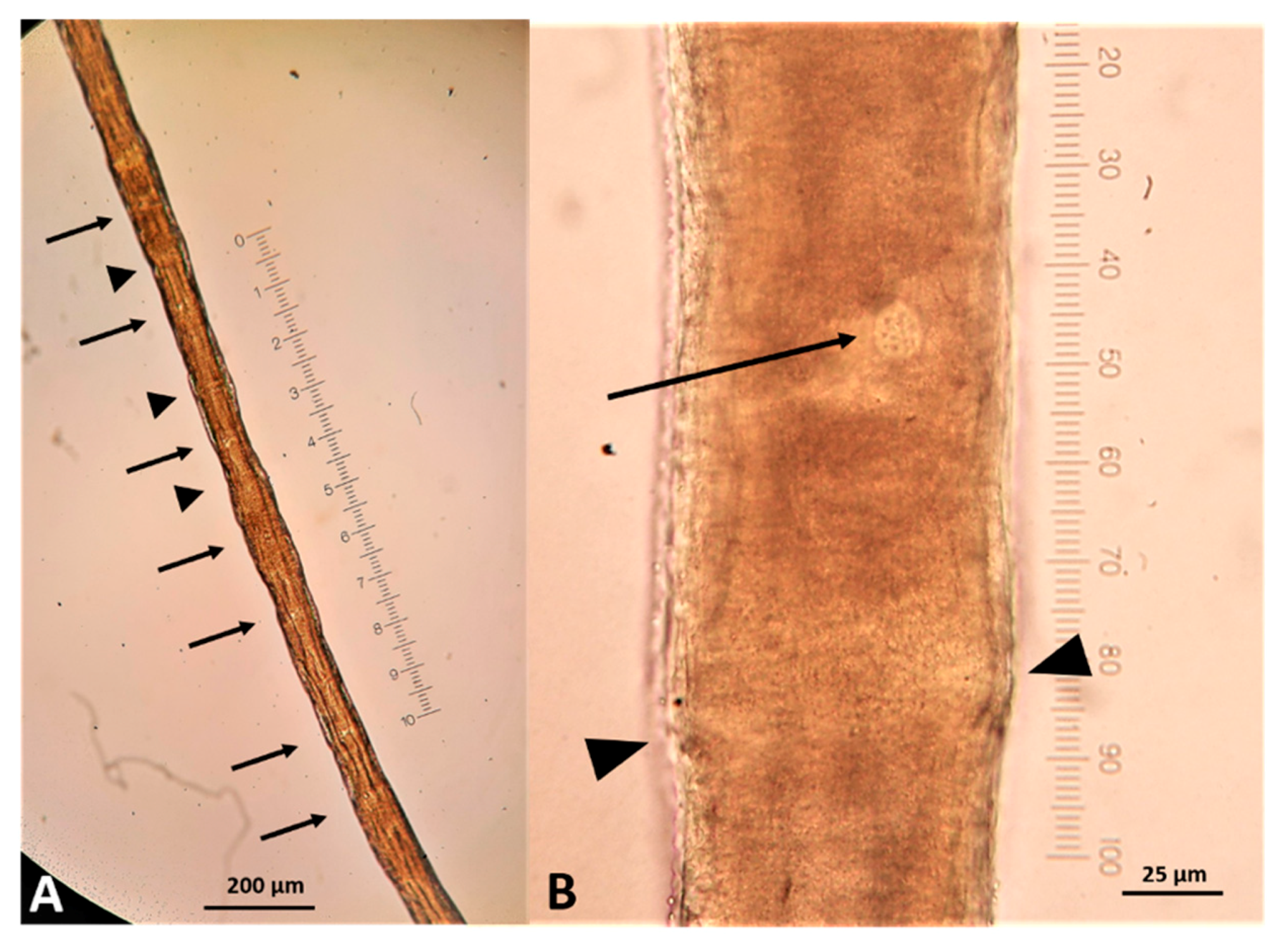
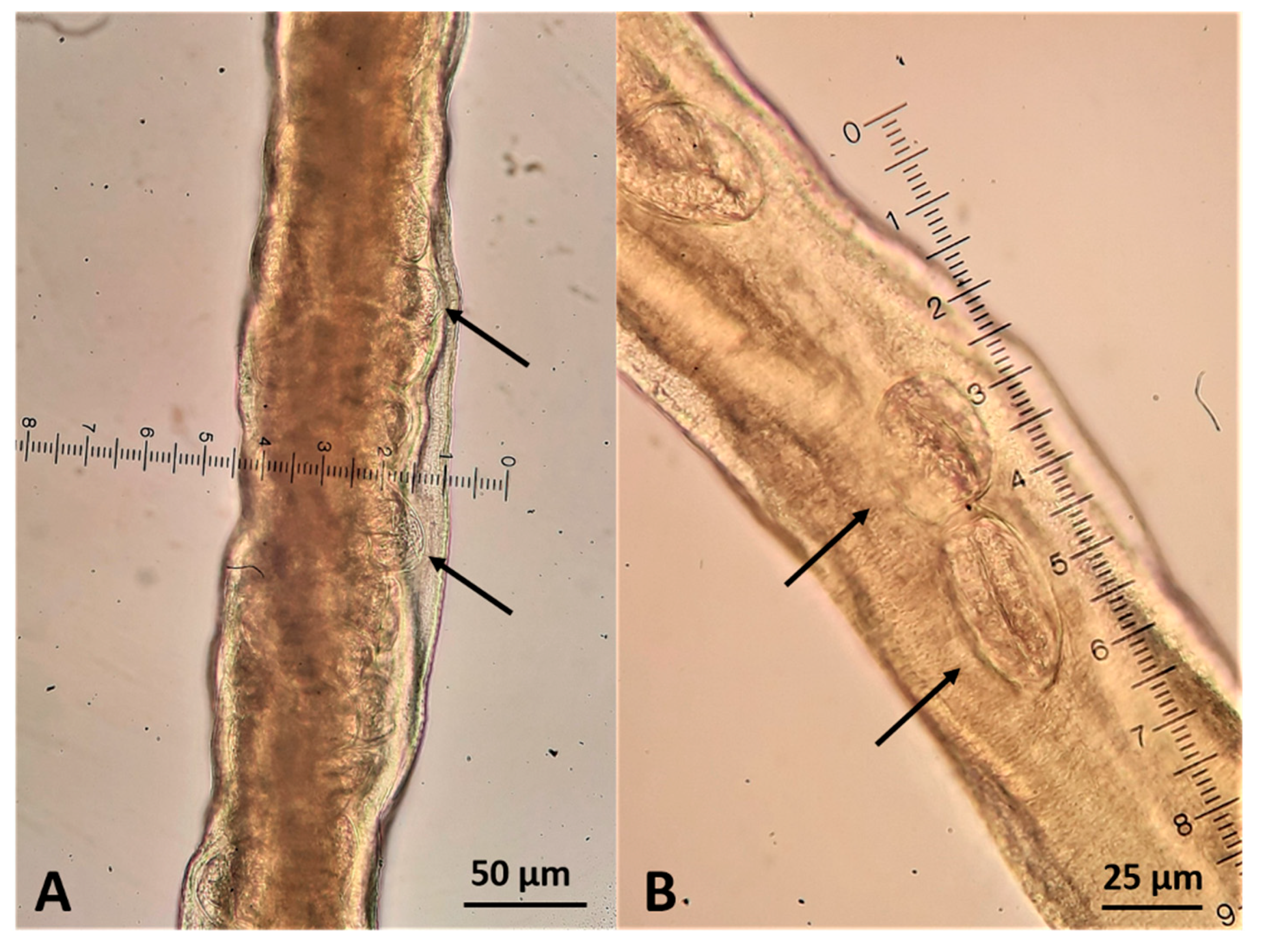
2.3. Parasitological Findings
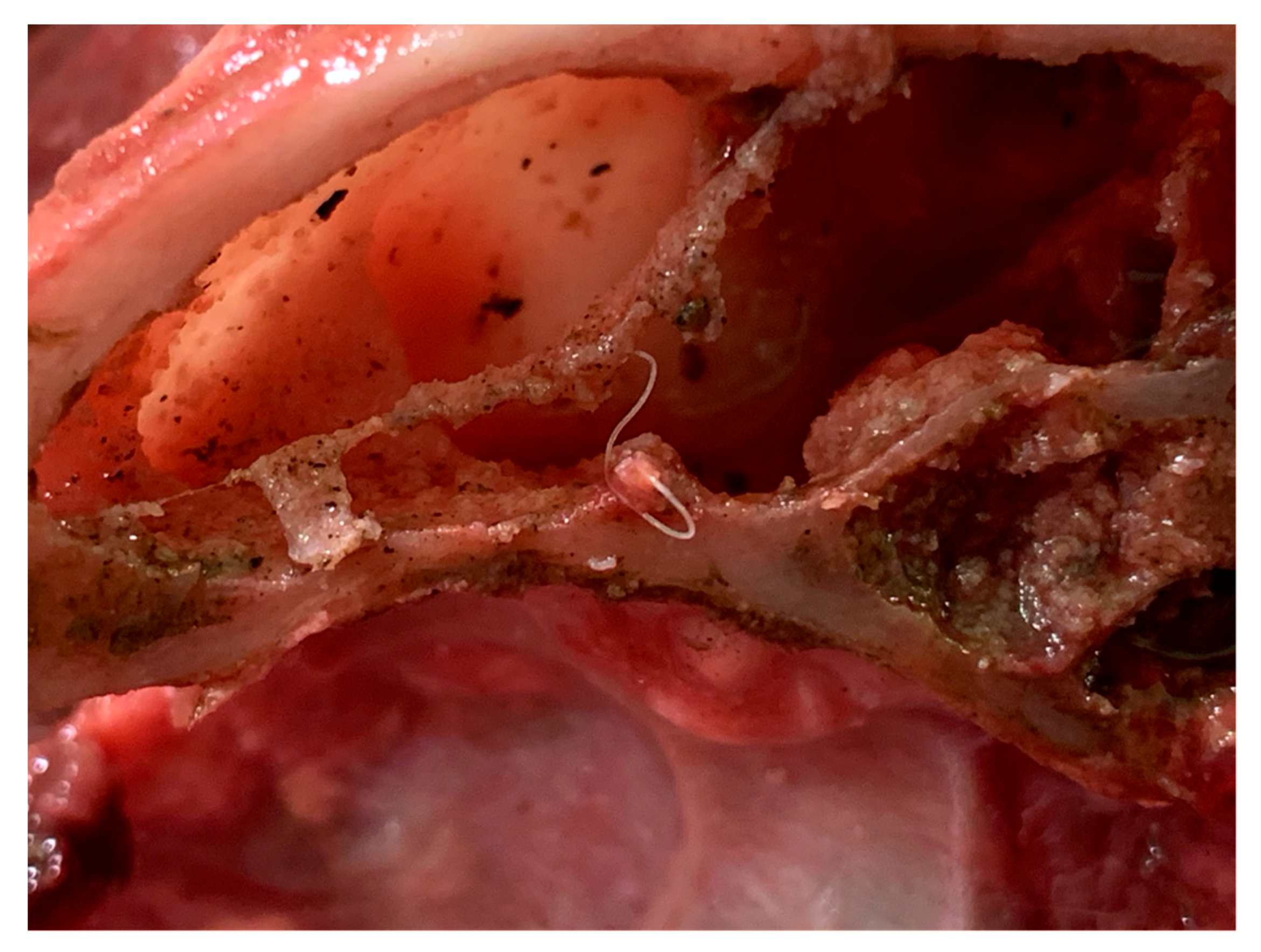
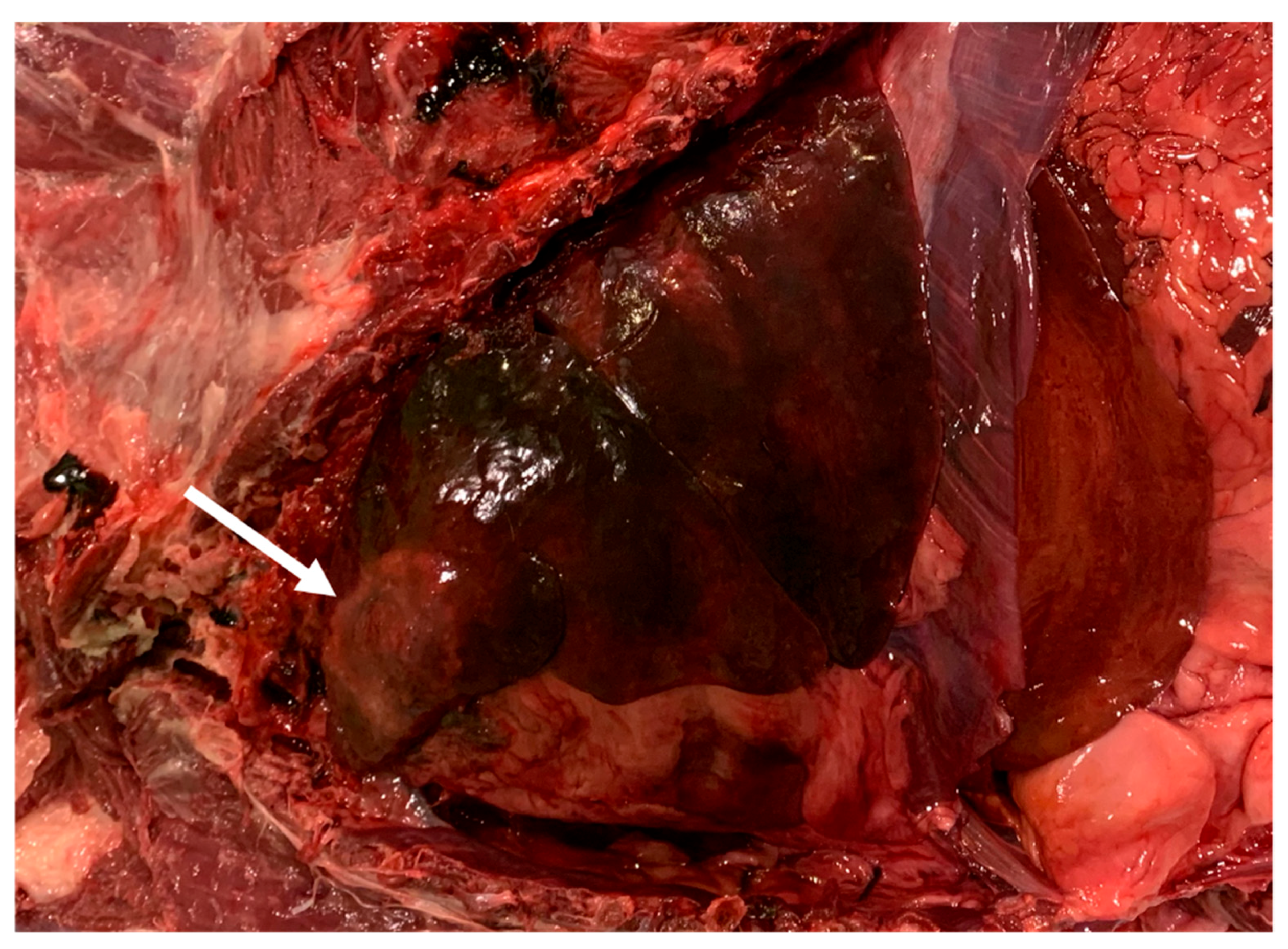
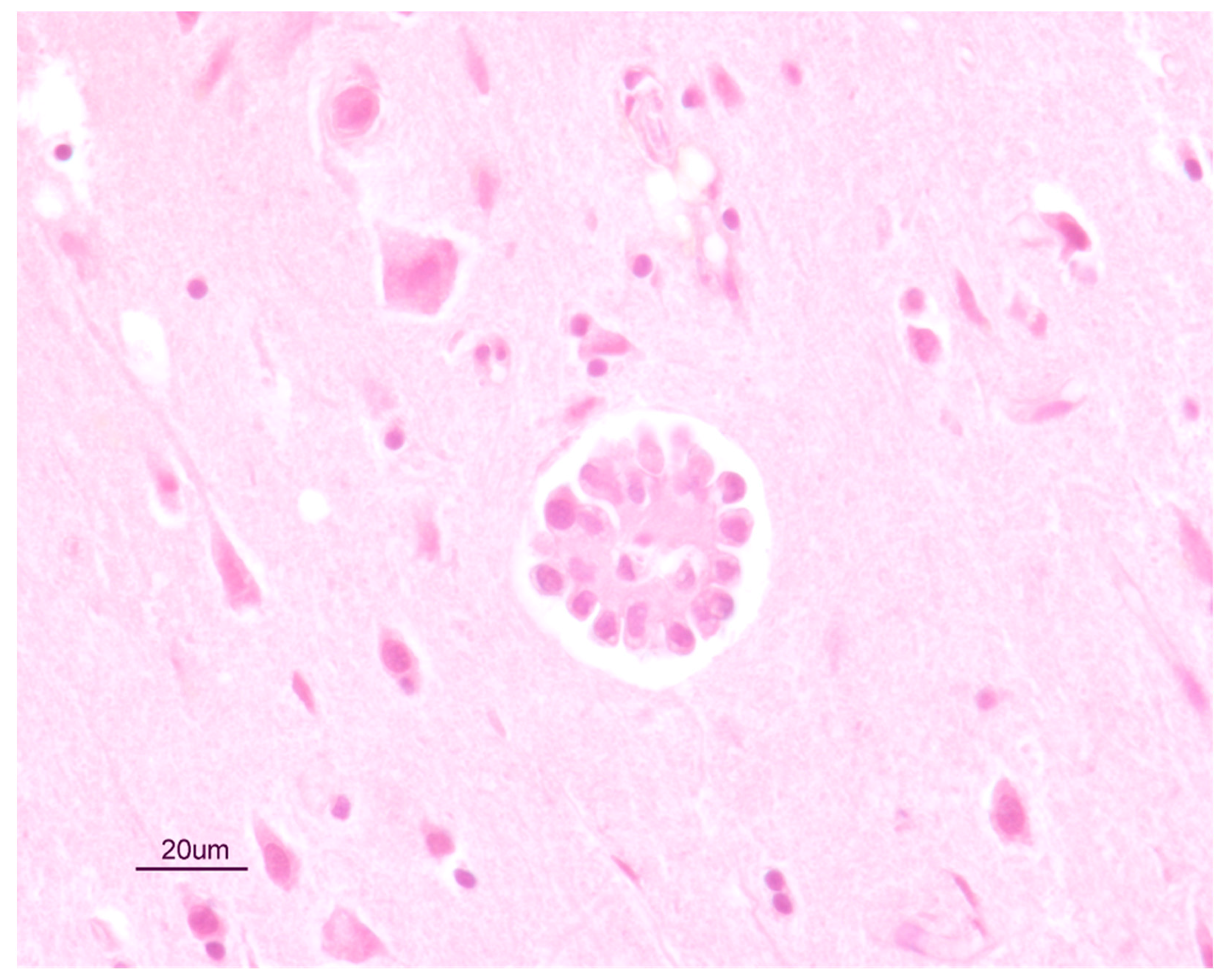
2.4. Necroscopic Examination and Histopathology
2.5. Molecular Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, B.G.; Little, M.B. Identification of eggs of a nematode (Eucoleus boehmi) from the nasal mucosa of North American dogs. J. Am. Vet. Med. Assoc. 1991, 54, 145–154. [Google Scholar]
- Lalosević, D.; Lalosević, V.; Klem, I.; Stanojev-Jovanović, D.; Pozio, E. Pulmonary capillariasis miming bronchial carcinoma. Am. J. Trop Med. Hyg. 2008, 78, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Conboy, G.A. Helminth parasites of the canine and feline respiratory tract. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 3rd ed.; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Traversa, D.; Di Cesare, A.; Lia, R.P.; Castagna, G.; Meloni, S.; Heine, J.; Strube, K.; Milillo, P.; Otranto, D.; Meckes, O.; et al. New insights into morphological and biological features of Capillaria aerophila (Trichocephalida, Trichuridae). Parasitol. Res. 2011, 1, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Magi, M.; Guardone, L.; Prati, M.C.; Mignone, W.; Macchioni, F. Extraintestinal nematodes of the red fox Vulpes vulpes in north-west Italy. J. Helminthol. 2015, 89, 506–511. [Google Scholar] [CrossRef]
- Veronesi, F.; Morganti, G.; Di Cesare, A.; Lepri, E.; Cassini, R.; Zanet, S.; Deni, D.; Chiari, M.; Ferroglio, E. Eucoleus boehmi infection in red fox (Vulpes vulpes) from Italy. Vet. Parasitol. 2014, 206, 232–239. [Google Scholar] [CrossRef]
- Hodžić, A.; Alić, A.; Klebić, I.; Kadrić, M.; Brianti, E.; Duscher, G.G. Red fox (Vulpes vulpes) as a potential reservoir host of cardiorespiratory parasites in Bosnia and Herzegovina. Vet. Parasitol. 2016, 223, 63–70. [Google Scholar] [CrossRef]
- Hodžić, A.; Bruckschwaiger, P.; Duscher, G.G.; Glawischnig, W.; Fuehrer, H.P. High prevalence of Eucoleus boehmi (syn. Capillaria boehmi) in foxes from western Austria. Parasitol. Res. 2016, 115, 3275–3278. [Google Scholar] [CrossRef]
- Schug, K.; Krämer, F.; Schaper, R.; Hirzmann, J.; Failing, K.; Hermosilla, C. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in central Germany. Parasit Vectors 2018, 11, 85. [Google Scholar] [CrossRef]
- Gillis-Germitsch, N.; Müller, S.; Gori, F.; Schnyder, M. Capillaria boehmi (syn. Eucoleus boehmi): Challenging treatment of a rarely diagnosed nasal nematode in dogs and high prevalence in Swiss foxes. Vet. Parasitol. 2020, 281, 109103. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Milillo, P.; Iorio, R.; Otranto, D. Infection by Eucoleus aerophilus in dogs and cats: Is another extra-intestinal parasitic nematode of pets emerging in Italy? Res. Vet. Sci. 2009, 87, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasit Vectors 2010, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Baan, M.; Kidder, A.C.; Johnson, S.E.; Sherding, R.G. Rhinoscopic Diagnosis of Eucoleus boehmi Infection in a Dog. J. Am. Anim. Hosp. Assoc. 2011, 47, 60–63. [Google Scholar] [CrossRef]
- Di Cesare, A.; Castagna, G.; Meloni, S.; Otranto, D.; Traversa, D. Mixed trichuroid infestation in a dog from Italy. Parasit Vectors 2012, 5, 128. [Google Scholar] [CrossRef]
- Di Cesare, A.; Castagna, G.; Otranto, D.; Meloni, S.; Milillo, P.; Latrofa, M.S.; Paoletti, B.; Bartolini, R.; Traversa, D. Molecular detection of Capillaria aerophila, an agent of canine and feline pulmonary capillariosis. J. Clin. Microbiol. 2012, 50, 1958–1963. [Google Scholar] [CrossRef]
- Veronesi, F.; Lepri, E.; Morganti, G.; Di Palma, S.; Mechelli, L.; Moretti, A.; Traversa, D. Nasal capillariosis in a symptomatic dog from Italy. Vet. Parasitol. 2013, 195, 187–191. [Google Scholar] [CrossRef]
- Piperisova, I.; Neel, J.A.; Tarigo, J. What is your diagnosis? Nasal discharge from a dog. Vet. Clin. Pathol. 2010, 39, 121–122. [Google Scholar] [CrossRef]
- Veronesi, F.; Morganti, G.; Di Cesare, A.; Schaper, R.; Traversa, D. A pilot trial evaluating the efficacy of a 10% imidacloprid/2:5% moxidectin spot-on formulation in the treatment of natural nasal capillariosis in dogs. Vet. Parasitol. 2014, 200, 133–138. [Google Scholar] [CrossRef]
- Clark, A.C.; López, F.R.; Levine, J.M.; Cooper, J.J.; Craig, T.M.; Voges, A.K.; Johnson, M.C.; Porter, B.F. Intracranial migration of Eucoleus (Capillaria) boehmi in a dog. J. Small Anim. Pract. 2013, 54, 99–103. [Google Scholar] [CrossRef]
- Di Cesare, A.; Castagna, G.; Meloni, S.; Milillo, P.; Latrofa, S.; Otranto, D.; Traversa, D. Canine and feline infections by cardiopulmonary nematodes in central and southern Italy. Parasitol. Res. 2011, 1, 87–96. [Google Scholar] [CrossRef]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop 2019, 193, 227–235. [Google Scholar] [CrossRef]
- Magi, M.; Guardone, L.; Prati, M.C.; Torracca, B.; Macchioni, F. First report of Eucoleus boehmi (syn. Capillaria boehmi) in dogs in north-western Italy, with scanning electron microscopy of the eggs. Parasite 2012, 19, 433–435. [Google Scholar] [CrossRef]
- Di Cesare, A.; Veronesi, F.; di Regalbono, A.F.; De Liberato, C.; Perrucci, S.; Iorio, R.; Morganti, G.; Marangi, M.; Simonato, G.; Traversa, D. PCR-based assay for the mitochondrial cox1 specific amplification of Eucoleus böhmi. Vet. Parasitol 2015, 211, 67–70. [Google Scholar] [CrossRef]
- Di Cesare, A.; Traversa, D. Canine angiostrongylosis: Recent advances in diagnosis, prevention, and treatment. Vet. Med. 2014, 5, 181–192. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent advances in the epidemiology, clinical and diagnostic features, and control of canine cardio-pulmonary angiostrongylosis. Vet. Res. 2014, 45, 92. [Google Scholar] [CrossRef]
- Macchioni, F.; Guardone, L.; Prati, M.C.; Magi, M. Eucoleus aerophilus (syn. Capillaria aerophila) and other trichinelloid nematodes in dogs from Liguria (Northwest Italy). In Trends in Veterinary Science; Boiti, C., Ferlazzo, A., Gaiti, A., Pugliese, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 85–89. [Google Scholar] [CrossRef]
- Burgess, H.; Ruotsalo, K.; Peregrine, A.S.; Hanselman, B.; Abrams-Ogg, A. Eucoleus aerophilus respiratory infection in a dog with Addison’s disease. Can. Vet. J. 2008, 49, 389–392. [Google Scholar]
- Schnieder, T.; Laabs, E.M.; Welz, C. Larval development of Toxocara canis in dogs. Vet. Parasitol. 2011, 175, 193–206. [Google Scholar] [CrossRef]
- Adalid-Peralta, L.; Sáenz, B.; Fragoso, G.; Cárdenas, G. Understanding host–parasite relationship: The immune central nervous system microenvironment and its effect on brain infections. Parasitology 2018, 145, 988–999. [Google Scholar] [CrossRef]
- Muchmore, C. A Study of the Nematode Capillaria boehmi (Supperer, 1953): A Parasite in the Nasal Passages of the Dog. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 1998. [Google Scholar]
- Hoskins, J.D. Pancreatitis: Clinical Signs Depend on Severity of Disease. 2003. Available online: https://www.dvm360.com/view/pancreatitis-clinical-signs-depend-severity-disease (accessed on 10 December 2020).
- O’Neill, E.J.; Merrett, D.; Jones, B. Granulomatous meningoencephalomyelitis in dogs: A review. Ir. Vet. J. 2005, 58, 86–92. [Google Scholar] [CrossRef]
- Lalošević, V.; Lalošević, D.; Capo, I.; Simin, V.; Galfi, A.; Traversa, D. High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia. Parasite 2013, 20, 3. [Google Scholar] [CrossRef]
- Simonato, G.; Cassini, R.; Morelli, S.; Di Cesare, A.; La Torre, F.; Marcer, F.; Traversa, D.; Pietrobelli, M.; di Regalbono, A.F. Contamination of Italian parks with canine helminth eggs and health risk perception of the public. Prev. Vet. Med. 2019, 172, 104788. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, S.; Marruchella, G.; Passarelli, A.; Diakou, A.; Di Cesare, A.; Colombo, M.; Frangipane di Regalbono, A.; Frate, A.; Traversa, D. An Unusual Case of Mixed Respiratory Capillariosis in a Dog. Pathogens 2021, 10, 117. https://doi.org/10.3390/pathogens10020117
Morelli S, Marruchella G, Passarelli A, Diakou A, Di Cesare A, Colombo M, Frangipane di Regalbono A, Frate A, Traversa D. An Unusual Case of Mixed Respiratory Capillariosis in a Dog. Pathogens. 2021; 10(2):117. https://doi.org/10.3390/pathogens10020117
Chicago/Turabian StyleMorelli, Simone, Giuseppe Marruchella, Alessandra Passarelli, Anastasia Diakou, Angela Di Cesare, Mariasole Colombo, Antonio Frangipane di Regalbono, Alessandro Frate, and Donato Traversa. 2021. "An Unusual Case of Mixed Respiratory Capillariosis in a Dog" Pathogens 10, no. 2: 117. https://doi.org/10.3390/pathogens10020117
APA StyleMorelli, S., Marruchella, G., Passarelli, A., Diakou, A., Di Cesare, A., Colombo, M., Frangipane di Regalbono, A., Frate, A., & Traversa, D. (2021). An Unusual Case of Mixed Respiratory Capillariosis in a Dog. Pathogens, 10(2), 117. https://doi.org/10.3390/pathogens10020117












