Abstract
Nakalanga syndrome is a clinical manifestation of onchocerciasis-associated epilepsy characterized by stunting, delayed or absent secondary sexual development and skeletal deformities, and is often accompanied by epileptic seizures. The pathophysiology of Nakalanga syndrome is unknown. Here, we describe the post-mortem findings of a 17-year-old female who died with Nakalanga syndrome in northern Uganda. Macroscopic and histopathological examination of all major organs (liver, lungs, kidney and heart), including the brain and the pituitary gland, was performed. The suspected cause of death was malaria, and all major organs and pituitary gland appeared normal, except the lungs, which were edematous consistent with the malaria. Neuropathological changes include signs of neuro-inflammation (gliosis and activated microglia), which co-localized with tau-reactive neurofibrillary tangles and threads. The pathology was most abundant in the frontal cortex, thalamic and hypothalamic regions, and mesencephalon. The choroid plexus showed psammoma bodies. These findings indicate accelerated aging, probably due to repeated seizures. The neuropathological findings were similar to other persons who died with onchocerciasis-associated epilepsy. Examination of the pituitary gland did not reveal new information concerning the underlying pathophysiological mechanism of Nakalanga syndrome. Therefore, more post-mortem studies should be performed.
The Nakalanga syndrome has been reported in many onchocerciasis-endemic regions, including South America [1], Uganda [2,3], Tanzania [4], Burundi [5], the Democratic Republic of Congo [6], and Cameroon [7]. The syndrome is characterized by stunted growth, delayed or absence of external signs of secondary sexual development, wasting, mental retardation, epilepsy, and thoracic and spinal abnormalities [3,5]. Nakalanga syndrome occurs in the same places where persons with epilepsy live who meet the criteria of onchocerciasis-associated epilepsy (OAE), and Nakalanga features are often overlapping with nodding seizures and other forms of OAE [2,8,9]. Epidemiological evidence has linked onchocerciasis with the Nakalanga syndrome [8], but the underlying pathophysiological mechanism remains unknown. Thus far, only one post-mortem exam has been performed in a person with Nakalanga syndrome in 1956, in a 30-year-old man who lived in the Mabira forest in Uganda [10]. His thyroid gland showed some evidence of hypofunction and his testes did not produce germ cells or the male sex hormone [10]. Examination of the pituitary gland showed few basophils but normal numbers of acidophils, which are not explaining the underlying growth retardation. Furthermore, an autopsy study on persons who died with nodding syndrome and other forms of OAE also included three individuals with a combination of nodding syndrome and Nakalanga features [11]. However, in these cases, only an extensive histopathological examination of the brain was performed, whereas the other organs were only investigated macroscopically and the pituitary gland was not taken [11]. Another autopsy study on persons who died with nodding syndrome identified tau-reactive neurofibrillary tangles and threads in the brain, but no information was available on potential Nakalanga features and the pituitary gland was also not investigated [12].
In this case report, we describe the post-mortem findings of a 17-year-old female with Nakalanga syndrome who died during an acute febrile illness in northern Uganda. A complete post-mortem exam was performed as before [11]. Samples taken from the visceral organs (lungs, liver, kidney and heart) and the complete brain were formalin fixed, embedded in paraffin (brain regions Table 1) and stained by haematoxylin-eosin. Peroxidase-based immunohistochemistry with haematoxylin counterstaining was performed to stain astrocytes (Polyclonal Rabbit Anti-Glial Fibrillary Acidic Protein), macrophages (Monoclonal Mouse Anti-Human CD68 KP1), and phosphorylated tau (AT8) as before [11]. Cresyl violet (Nissl staining), TDP-43, alpha-synuclein, Kluyver-barrera, Ubiquitin and p62 staining were performed on a selection of slides showing abnormalities, such as inclusions, pseudo-inclusions and vacuolizations in neuronal bodies. The pituitary gland was investigated for general abnormalities (Hematoxylin & Eosin, H&E) and immunohistochemistry of all pituitary hormones. Ethical approval was obtained from Lacor hospital, the Uganda National Council for Science and Technology (UNCST) and the ethics committee from the University hospital of Antwerp.

Table 1.
Detailed histological distribution of gliosis (GFAP), activated macrophages (CD68) and tau-reactive neurofibrillary tangles (AT8).
The deceased belonged to the Acholi tribe and lived in the Pader district of northern Uganda. She had an older sister who suffered from nodding syndrome. The deceased had head nodding seizures induced by the sight of food or by cold weather which started in 2005, at the age of 4 years. Several years later, the disease progressed to generalized tonic–clonic seizures and episodes of blank staring (absences) and wandering away from home were reported. There was no history of any severe disease, such as severe malaria or febrile illness, head trauma or abnormal development before the onset of the seizures, which could explain the development of seizures and the disease. Despite treatment with sodium valproate, carbamazepine and folic acid, three epileptic episodes weekly (nodding and generalized tonic–clonic seizures) were reported, as well as an episode of status epilepticus. She did not present onchocerciasis skin lesions or nodules, but she took ivermectin treatment during the bi-annual mass distributions in the region.
Prior to death, the deceased was unable to perform normal household tasks, had a poor appetite and sometimes needed assistance with eating. She was very weak but able to walk without assistance, dress, wash and groom herself and was not bedridden. Around the time of death, she developed fever, headache and cough and was started on antimalarial treatment after a positive rapid diagnostic test. However, her clinical condition deteriorated and she was admitted to the hospital where she died three days later.
External examination showed general wasting, with reduced subcutaneous fat and muscle mass, poor secondary sexual development, crippled legs, kyphosis and stunting (short stature). There was severe pallor, dehydration, mild jaundice and anemia but no palpable peripheral lymph nodes. Internal organs were normal, but smaller than expected for her age. The lungs were heavy and edematous with thick mucus secretions in the parenchyma and petechiae on the serous surfaces, which may have been related to the malaria infection prior to death [13].
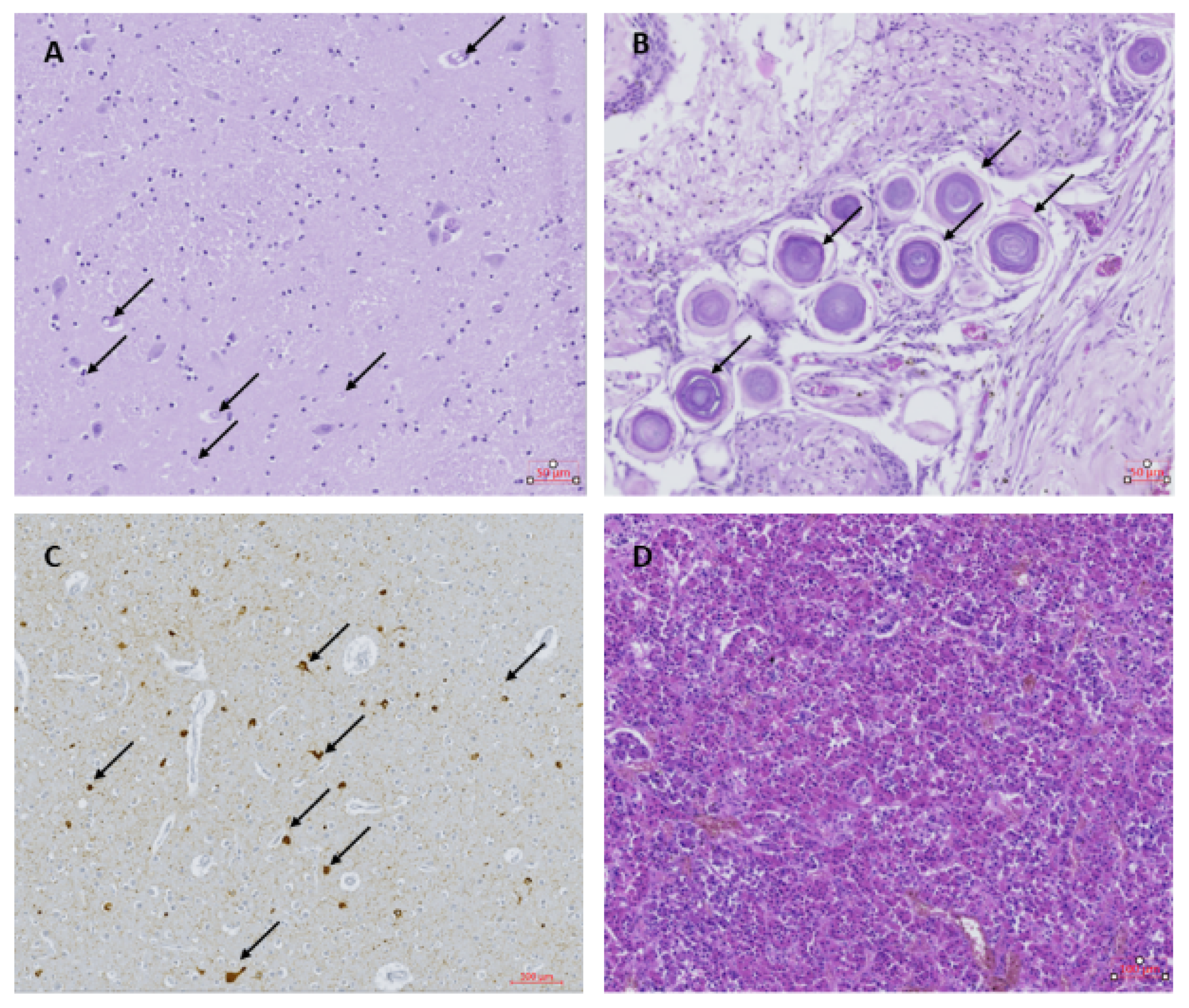
Examination of the visceral organs (lungs, liver, kidney and heart) showed no abnormalities. Histological examination of the brain showed neuronal loss, especially the Purkinje cells and granular cells in the tops of the folia in the cerebellum. Spongiosis and an increased amount of glial cells were observed in the sub-pial layer at the top of the gyri of the left and right frontal cortex. Large neuronal bodies with inclusions, pseudo-inclusions and vacuolization were observed in the paraventricular nuclei on the right side (Figure 1A). The nature of these inclusions could not be determined by the additional staining performed. The choroid plexus showed strong fibrotic zones with strongly dilated blood vessels and psammoma bodies (Figure 1B). The ependymal layer of the right ventricle was interrupted locally, with some signs of gliosis and spongiosis. The hippocampus appeared normal in both the left and right side, with no signs of hippocampus sclerosis despite repeated seizures. Overlapping foci of tau-reactive neurofibrillary tangles, gliosis and activated microglia were observed as previously described in other OAE cases, although at a relatively higher abundance (Figure 1C). Tau-reactive tangles, threads and dots were found with variable abundance in almost all samples investigated, except for the choroid plexus and the left putamen (Table 1). The pathology was most abundant in the frontal cortex, thalamic and hypothalamic regions and mesencephalon (Table 1). The pituitary gland was normal (Figure 1D), with no signs of hormonal dysfunction.
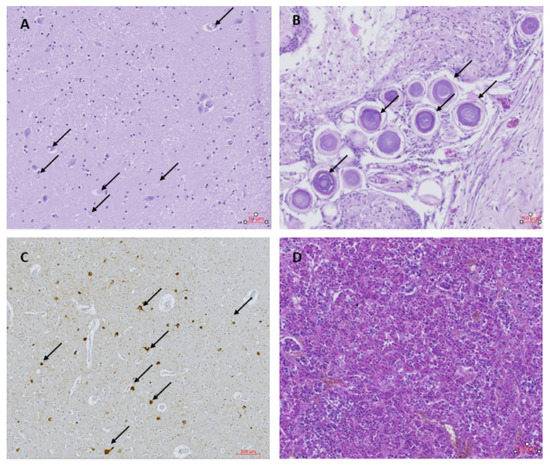
Figure 1.
(A) Thalamus with inclusions and pseudo-inclusions (black arrows); Hematoxylin & Eosin (H&E) staining. (B) Choroid plexus containing psammoma bodies (black arrows); H&E. (C) Frontal cortex with tau-reactive neurofibrillary tangles and threads (black arrows); AT8. (D) Pituitary gland; H&E.
The pathophysiology of Nakalanga syndrome remains unknown. Two main characteristics of the syndrome include stunted growth and delayed or absence of secondary sexual development, pointing towards an endocrinological cause. Endocrinological investigations were performed earlier in eight persons with nodding syndrome in Uganda [14]. Here, serum hormone levels were normal in all but two individuals with severe stunting and one with moderate stunting, who had low levels of somatomedin C (insuline like growth factor (IGF1)) and/or IGF binding protein 3 (IGBP3), which are mediators of growth hormone function [14]. Furthermore, a linear relationship was observed between serum IGF1 and the height for age score [14].
In this case report, macroscopic investigation of the brain of the 17-year-old woman did not show any abnormalities; microscopic investigation of the brain revealed overlapping foci of tau-reactive neurofibrillary tangles, gliosis and activated microglia, as was previously observed in persons with OAE [11], further supporting the epidemiological evidence that Nakalanga syndrome is one of the clinical phenotypes of OAE. Furthermore, the abundance of tau-reactive neurofibrillary tangles, inclusions and psammoma bodies indicate accelerated aging [15], which might be associated with uncontrolled seizures [16,17].
One major drawback of post-mortem studies is that the disease is in its end stage, and factors underlying the pathological mechanism might no longer be present. However, the normal appearance of the pituitary gland in this case report and an earlier post-mortem study suggest that Nakalanga syndrome may not be caused by a structural dysfunction or lesion of the pituitary gland. Our post-mortem examination of the brain and pituitary gland did not provide further indication for the underlying pathophysiological mechanism. In agreement with Marshal and Cherry (1962), there was no evidence supporting an earlier suggestion that pituitary dysfunction in Nakalanga syndrome is the result of accumulating O. volvulus microfilariae [10]. Additional hormonal studies, such as pituitary stimulation tests, are needed to identify the pathophysiological mechanism. Moreover, as no height and no detailed Tanner scale [18] information was available in this patient, additional post-mortem studies on persons with clinically well-documented Nakalanga syndrome should be done.
Author Contributions
Conceptualization, A.H. and R.C.; Methodology, A.H., M.L. and R.C.; Software, A.H.; Validation, M.L., R.C.; Formal Analysis, M.L., S.K.-S., A.H.; Investigation, F.O., S.O., R.L., R.I.; Resources, R.C., R.I.; Data Curation, A.H.; Writing—Original Draft Preparation, A.H., R.C., S.V.H.; Writing—Review and Editing, A.H., M.L., S.K.-S., R.I., R.C., S.V.H.; Visualization, A.H.; Supervision, M.L., S.K.-S., R.C.; Project Administration, R.C.; Funding Acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European Research Council, project NSETHIO (671055).
Institutional Review Board Statement
Ethical approval was obtained from Lacor hospital, the Uganda National Council for Science and Technology (UNCST) and the ethics committee from the University hospital of Antwerp.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sacre, C. El sindrome epileptico y sus relaciones con la Oncocercosis. Bol. Salubr. Hig. 1938, 1, 11–31. [Google Scholar]
- Föger, K.; Gora-Stahlberg, G.; Sejvar, J.; Ovuga, E.; Jilek-Aall, L.; Schmutzhard, E.; Kaiser, C.; Winkler, A.S. Nakalanga Syndrome: Clinical Characteristics, Potential Causes, and Its Relationship with Recently Described Nodding Syndrome. PLoS Negl. Trop. Dis. 2017, 11, e0005201. [Google Scholar] [CrossRef] [PubMed]
- Kipp, W.; Bamuhiiga, J.; Burnham, G.; Leichsenring, M. The Nakalanga Syndrome in Kabarole District, Western Uganda. Am. J. Trop. Med. Hyg. 1996, 54, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Bhwana, D.; Mmbando, B.P.; Dekker, M.C.; Mnacho, M.; Kakorozya, A.; Matuja, W.; Makunde, W.H.; Weckhuysen, S.; Colebunders, R. Clinical presentation of epilepsy in six villages in an onchocerciasis endemic area in Mahenge, Tanzania. Epileptic Disord. 2019, 21, 425–435. [Google Scholar] [PubMed]
- Newell, E.; Vyungimana, F.; Bradley, J. Epilepsy, retarded growth and onchocerciasis, in two areas of different endemicity of onchocerciasis in Burundi. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 525–527. [Google Scholar] [CrossRef]
- Fodjo, J.N.S.; Mandro, M.; Mukendi, D.; Tepage, F.; Menon, S.; Nakato, S.; Nyisi, F.; Abhafule, G.; Wonya’Rossi, D.; Anyolito, A.; et al. Onchocerciasis-associated epilepsy in the Democratic Republic of Congo: Clinical description and relationship with microfilarial density. PLoS Negl. Trop. Dis. 2019, 13, e0007300. [Google Scholar] [CrossRef]
- Siewe, J.F.; Ngarka, L.; Tatah, G.; Mengnjo, M.K.; Nfor, L.N.; Chokote, E.S.; Boullé, C.; Nkouonlack, C.; Dema, F.; Nkoro, G.A.; et al. Clinical presentations of onchocerciasis-associated epilepsy (OAE) in Cameroon. Epilepsy Behav. 2019, 90, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Colebunders, R.; Fodjo, J.N.S.; Hopkins, A.; Hotterbeekx, A.; Lakwo, T.L.; Kalinga, A.; Logora, M.Y.; Basáñez, M.-G. From river blindness to river epilepsy: Implications for onchocerciasis elimination programmes. PLoS Negl. Trop. Dis. 2019, 13, e0007407. [Google Scholar] [CrossRef] [PubMed]
- Tumwine, J.K.; Vandemaele, K.; Chungong, S.; Richer, M.; Anker, M.; Ayana, Y.; Opoka, M.L.; Klaucke, D.N.; Quarello, A.; Spencer, P.S. Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr. Health Sci. 2013, 12, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Cherry, J. Endocrine dysfunction in a Nakalanga dwarf. Trans. R. Soc. Trop. Med. Hyg. 1961, 55, 188–191. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Lammens, M.; Idro, R.; Akun, P.R.; Lukande, R.; Akena, G.; Nath, A.; Taylor, J.; Olwa, F.; Kumar-Singh, S.; et al. Neuroinflammation and Not Tauopathy Is a Predominant Pathological Signature of Nodding Syndrome. J. Neuropathol. Exp. Neurol. 2019, 78, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Pollanen, M.S.; Onzivua, S.; Robertson, J.; McKeever, P.M.; Olawa, F.; Kitara, D.L.; Fong, A. Nodding syndrome in Uganda is a tauopathy. Acta Neuropathol. 2018, 136, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.J.; Cañon, V.; White, N.J. Pulmonary Manifestations of Malaria: Recognition and management. Treat. Respir. Med. 2006, 5, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Piloya, T.; Odongkara, B.; Namusoke, H.; Idro, R. Physical growth, puberty and hormones in adolescents with Nodding Syndrome; A pilot study. BMC Res. Notes 2014, 7, 858. [Google Scholar] [CrossRef]
- Jovanović, I.; Stefanović, N.; Antić, S.; Ugrenović, S.; Djindjić, B.; Vidović, N. Morphological and morphometric characteristics of choroid plexus psammoma bodies during the human aging. Ital. J. Anat. Embryol. 2004, 109, 19–33. [Google Scholar] [PubMed]
- Sharma, K.; Kalakoti, P.; Shaughnessy, J.E.; De La Cruz, N.; Dossani, R.H.; Zhu, P.; Gonzalez-Toledo, E.; Ledbetter, C.; Pinskton, J.B.; Nanda, A.; et al. Psammomatous Cavernous Malformation Presenting as Drug Resistant Epilepsy: Case Illustration and Review of Literature. World Neurosurg. 2016, 93, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Puvenna, V.; Engeler, M.; Banjara, M.; Brennan, C.; Schreiber, P.; Dadas, A.; Bahrami, A.; Solanki, J.; Bandyopadhyay, A.; Morris, J.K.; et al. Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res. 2016, 1630, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).