Affecting the Effectors: Regulation of Legionella pneumophila Effector Function by Metaeffectors
Abstract
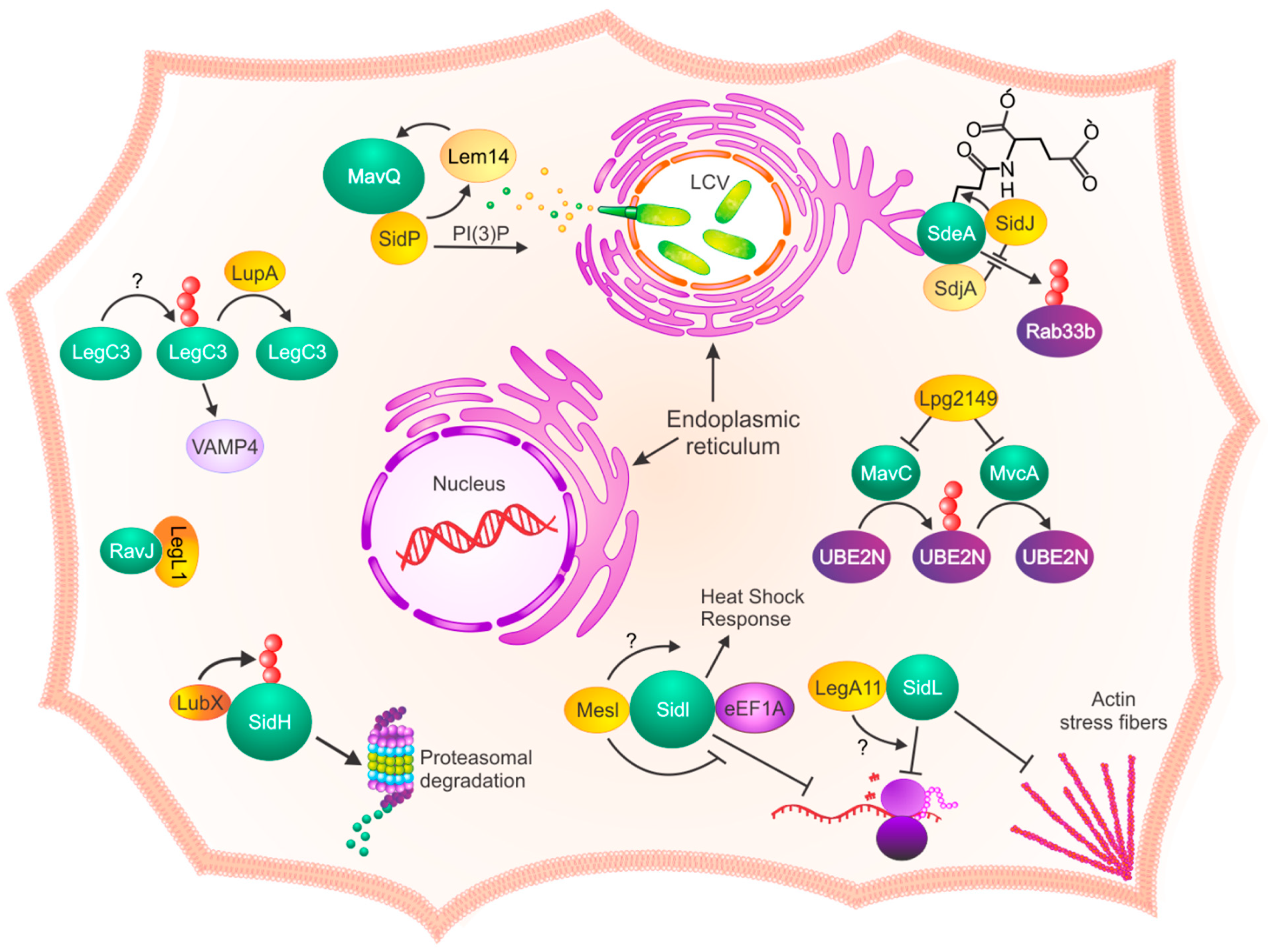
1. Introduction
2. Identification and Function of L. pneumophila Metaeffectors
3. What Makes a Metaeffector?
3.1. Structure
3.2. Proximity
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, E.R.; Mecsas, J. Virulence Mechanisms of Bacterial Pathogens. Microbiol. Spectr. 2019, 213–239. [Google Scholar] [CrossRef]
- Ensminger, A.W. Legionella Pneumophila, Armed to the Hilt: Justifying the Largest Arsenal of Effectors in the Bacterial World. Curr. Opin. Microbio.l 2016, 29, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Best, A.; Kwaik, Y.A. Evolution of the Arsenal of Legionella Pneumophila Effectors to Modulate Protist Hosts. Mbio 2018, 9, e01313-18. [Google Scholar] [CrossRef]
- Kubori, T.; Shinzawa, N.; Kanuka, H.; Nagai, H. Legionella Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector. PLoS Pathog. 2010, 6, e1001216. [Google Scholar] [CrossRef] [PubMed]
- Kubori, T.; Hyakutake, A.; Nagai, H. Legionella Translocates an E3 Ubiquitin Ligase That Has Multiple U-boxes with Distinct Functions. Mol. Microbiol. 2008, 67, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; O’Connor, T.J. Beyond Paralogs: The Multiple Layers of Redundancy in Bacterial Pathogenesis. Front. Cell Infect. Microbiol. 2017, 7, 467. [Google Scholar] [CrossRef] [PubMed]
- Laguna, R.K.; Creasey, E.A.; Li, Z.; Valtz, N.; Isberg, R.R. A Legionella Pneumophila-Translocated Substrate That Is Required for Growth within Macrophages and Protection from Host Cell Death. Proc. Natl. Acad. Sci. USA 2006, 103, 18745–18750. [Google Scholar] [CrossRef]
- Creasey, E.A.; Isberg, R.R. The Protein SdhA Maintains the Integrity of the Legionella-Containing Vacuole. Proc. Natl. Acad. Sci. USA 2012, 109, 3481–3486. [Google Scholar] [CrossRef]
- Havey, J.C.; Roy, C.R. Toxicity and SidJ-Mediated Suppression of Toxicity Require Distinct Regions in the SidE Family of Legionella Pneumophila Effectors. Infect. Immun. 2015, 83, 3506–3514. [Google Scholar] [CrossRef]
- Jeong, K.C.; Sexton, J.A.; Vogel, J.P. Spatiotemporal Regulation of a Legionella Pneumophila T4SS Substrate by the Metaeffector SidJ. PLoS Pathog. 2015, 11, e1004695. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Z.-Q. The Legionella Pneumophila Effector SidJ Is Required for Efficient Recruitment of Endoplasmic Reticulum Proteins to the Bacterial Phagosome. Infect. Immun. 2007, 75, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yu, K.; Fei, X.; Liu, Y.; Nakayasu, E.S.; Piehowski, P.D.; Shaw, J.B.; Puvar, K.; Das, C.; Liu, X.; et al. A Unique Deubiquitinase That Deconjugates Phosphoribosyl-Linked Protein Ubiquitination. Cell Res. 2017, 27, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Luo, Z.-Q. Hijacking of the Host Ubiquitin Network by Legionella Pneumophila. Front. Cell Infect. Microbiol. 2017, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Zhen, X.; Liu, Y.; Xu, X.; He, C.; Qiu, J.; Liu, Y.; Fujimoto, G.M.; Nakayasu, E.S.; Zhou, B.; et al. Regulation of Phosphoribosyl Ubiquitination by a Calmodulin-Dependent Glutamylase. Nature 2019, 572, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Bhogaraju, S.; Bonn, F.; Mukherjee, R.; Adams, M.; Pfleiderer, M.M.; Galej, W.P.; Matkovic, V.; Lopez-Mosqueda, J.; Kalayil, S.; Shin, D.; et al. Inhibition of Bacterial Ubiquitin Ligases by SidJ–Calmodulin Catalysed Glutamylation. Nature 2019, 572, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Black, M.H.; Osinski, A.; Gradowski, M.; Servage, K.A.; Pawłowski, K.; Tomchick, D.R.; Tagliabracci, V.S. Bacterial Pseudokinase Catalyzes Protein Polyglutamylation to Inhibit the SidE-Family Ubiquitin Ligases. Science 2019, 364, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Sulpizio, A.; Minelli, M.E.; Wan, M.; Burrowes, P.D.; Wu, X.; Sanford, E.J.; Shin, J.-H.; Williams, B.C.; Goldberg, M.L.; Smolka, M.B.; et al. Protein Polyglutamylation Catalyzed by the Bacterial Calmodulin-Dependent Pseudokinase SidJ. Elife 2019, 8, e51162. [Google Scholar] [CrossRef]
- Shames, S.R.; Liu, L.; Havey, J.C.; Schofield, W.B.; Goodman, A.L.; Roy, C.R. Multiple Legionella Pneumophila Effector Virulence Phenotypes Revealed through High-Throughput Analysis of Targeted Mutant Libraries. Proc. Natl. Acad. Sci. USA 2017, 114, E10446–E10454. [Google Scholar] [CrossRef]
- Shen, X.; Banga, S.; Liu, Y.; Xu, L.; Gao, P.; Shamovsky, I.; Nudler, E.; Luo, Z. Targeting EEF1A by a Legionella Pneumophila Effector Leads to Inhibition of Protein Synthesis and Induction of Host Stress Response. Cell Microbiol. 2009, 11, 911–926. [Google Scholar] [CrossRef]
- Joseph, A.M.; Pohl, A.E.; Ball, T.J.; Abram, T.G.; Johnson, D.K.; Geisbrecht, B.V.; Shames, S.R. The Legionella Pneumophila Metaeffector Lpg2505 (MesI) Regulates SidI-Mediated Translation Inhibition and Novel Glycosyl Hydrolase Activity. Infect. Immun. 2020, 88, e00853-19. [Google Scholar] [CrossRef]
- Machtens, D.A.; Willerding, J.M.; Eschenburg, S.; Reubold, T.F. Crystal Structure of the Metaeffector MesI (Lpg2505) from Legionella Pneumophila. Biochem Bioph Res. Commun. 2020, 527, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, M.L.; Quaile, A.T.; Stogios, P.J.; Morar, M.; Rao, C.; Leo, R.D.; Evdokimova, E.; Lam, M.; Oatway, C.; Cuff, M.E.; et al. Diverse Mechanisms of Metaeffector Activity in an Intracellular Bacterial Pathogen, Legionella Pneumophila. Mol. Syst Biol. 2016, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.L.; Kraft, S.M.; Reaves, B.J.; Mima, J.; O’Brien, K.M.; Starai, V.J. LegC3, an Effector Protein from Legionella Pneumophila, Inhibits Homotypic Yeast Vacuole Fusion In Vivo and In Vitro. PLoS ONE 2013, 8, e56798. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Halder, P.; Yavuz, H.; Jahn, R.; Shuman, H.A. Direct Targeting of Membrane Fusion by SNARE Mimicry: Convergent Evolution of Legionella Effectors. Proc. Natl. Acad. Sci. USA 2016, 113, 8807–8812. [Google Scholar] [CrossRef] [PubMed]
- Toulabi, L.; Wu, X.; Cheng, Y.; Mao, Y. Identification and Structural Characterization of a Legionella Phosphoinositide Phosphatase. J. Biol. Chem. 2013, 288, 24518–24527. [Google Scholar] [CrossRef]
- Mosavi, L.K.; Cammett, T.J.; Desrosiers, D.C.; Peng, Z. The Ankyrin Repeat as Molecular Architecture for Protein Recognition. Protein Sci. 2004, 13, 1435–1448. [Google Scholar] [CrossRef]
- De Felipe, K.S.; Pampou, S.; Jovanovic, O.S.; Pericone, C.D.; Ye, S.F.; Kalachikov, S.; Shuman, H.A. Evidence for Acquisition of Legionella Type IV Secretion Substrates via Interdomain Horizontal Gene Transfer. J. Bacteriol. 2005, 187, 7716–7726. [Google Scholar] [CrossRef]
- Fontana, M.F.; Banga, S.; Barry, K.C.; Shen, X.; Tan, Y.; Luo, Z.-Q.; Vance, R.E. Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent Legionella Pneumophila. PLoS Pathog. 2011, 7, e1001289. [Google Scholar] [CrossRef]
- Guo, Z.; Stephenson, R.; Qiu, J.; Zheng, S.; Luo, Z.-Q. A Legionella Effector Modulates Host Cytoskeletal Structure by Inhibiting Actin Polymerization. Microbes Infect. 2014, 16, 225–236. [Google Scholar] [CrossRef]
- Gross, S.R.; Kinzy, T.G. Improper Organization of the Actin Cytoskeleton Affects Protein Synthesis at Initiation. Mol. Cell Biol. 2007, 27, 1974–1989. [Google Scholar] [CrossRef]
- Belyi, Y. Targeting Eukaryotic MRNA Translation by Legionella Pneumophila. Front. Mol. Biosci. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Valleau, D.; Quaile, A.T.; Cui, H.; Xu, X.; Evdokimova, E.; Chang, C.; Cuff, M.E.; Urbanus, M.L.; Houliston, S.; Arrowsmith, C.H.; et al. Discovery of Ubiquitin Deamidases in the Pathogenic Arsenal of Legionella Pneumophila. Cell Rep. 2018, 23, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Guan, H.; Huang, Y.; Yu, T.; Fu, J.; Nakayasu, E.S.; Puvar, K.; Das, C.; Wang, D.; Ouyang, S.; et al. Legionella Pneumophila Regulates the Activity of UBE2N by Deamidase-mediated Deubiquitination. EMBO J. 2020, 39, e102806. [Google Scholar] [CrossRef] [PubMed]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred Interactive Server for Protein Homology Detection and Structure Prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef] [PubMed]
- Burstein, D.; Amaro, F.; Zusman, T.; Lifshitz, Z.; Cohen, O.; Gilbert, J.A.; Pupko, T.; Shuman, H.A.; Segal, G. Genomic Analysis of 38 Legionella Species Identifies Large and Diverse Effector Repertoires. Nat. Genet. 2016, 48, 167–175. [Google Scholar] [CrossRef]
| Metaeffector | Gene ID | Activity | Size a | Effector | Gene ID | Activity b | Size a | Refs. |
|---|---|---|---|---|---|---|---|---|
| LegA11/AnkJ | Lpg0436 | Unknown | 269 | SidL/Ceg14 | Lpg0437 | Translation inhibitor | 666 | [23] |
| LegL1 | Lpg0945 | Competitive inhibition | 296 | RavJ | Lpg0944 | Putative transglutaminase | 391 | [23] |
| Lem14 | Lpg1851 | Synergistic with SidP | 220 | MavQ | Lpg2975 | Putative kinase | 871 | [23] |
| Lpg2149 | Lpg2149 | Unknown | 119 | MavC | Lpg2147 | Ubiquitin-ase | 482 | [34] |
| MvcA | Lpg2148 | Deubiquitinase | 426 | |||||
| LubX | Lpg2830 | E3 Ubiquitin Ligase | 246 | SidH | Lpg2829 | SdhA homolog | 2225 | [4] |
| LupA | Lpg1148 | Deubiquitinase | 503 | LegC3 | Lpg1701 | Glutamine (Q)-SNARE-like protein | 506 | [23,25] |
| MavE | Lpg2344 | Unknown | 208 | YlfA/LegC7 | Lpg2298 | SNARE-like Protein | 425 | [23] |
| MesI | Lpg2505 | Unknown | 295 | SidI/Ceg32 | Lpg2504 | Putative mannosyltransferase | 942 | [19,21,22] |
| SdbC | Lpg2391 | Putative Lipase | 434 | SdbB | Lpg2482 | Putative Lipase | 448 | [23] |
| SidJ | Lpg2155 | Calmodulin-dependent transglutamylase | 873 | SidE | Lpg0234 | Ubiquitin Ligases | 1575 | [9,10,11,12,15,16,17,18,23] |
| SdeA | Lpg2157 | 1506 | ||||||
| SdeB | Lpg2156 | 1926 | ||||||
| SdeC | Lpg2153 | 1533 | ||||||
| SidP | Lpg0130 | PI3P Phosphatase | 822 | MavQ | Lpg2975 | Putative PIP Kinase | 871 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, A.M.; Shames, S.R. Affecting the Effectors: Regulation of Legionella pneumophila Effector Function by Metaeffectors. Pathogens 2021, 10, 108. https://doi.org/10.3390/pathogens10020108
Joseph AM, Shames SR. Affecting the Effectors: Regulation of Legionella pneumophila Effector Function by Metaeffectors. Pathogens. 2021; 10(2):108. https://doi.org/10.3390/pathogens10020108
Chicago/Turabian StyleJoseph, Ashley M., and Stephanie R. Shames. 2021. "Affecting the Effectors: Regulation of Legionella pneumophila Effector Function by Metaeffectors" Pathogens 10, no. 2: 108. https://doi.org/10.3390/pathogens10020108
APA StyleJoseph, A. M., & Shames, S. R. (2021). Affecting the Effectors: Regulation of Legionella pneumophila Effector Function by Metaeffectors. Pathogens, 10(2), 108. https://doi.org/10.3390/pathogens10020108





