Neopterin and CXCL-10 in Cerebrospinal Fluid as Potential Biomarkers of Neuroinvasive Dengue and Chikungunya
Abstract
1. Introduction
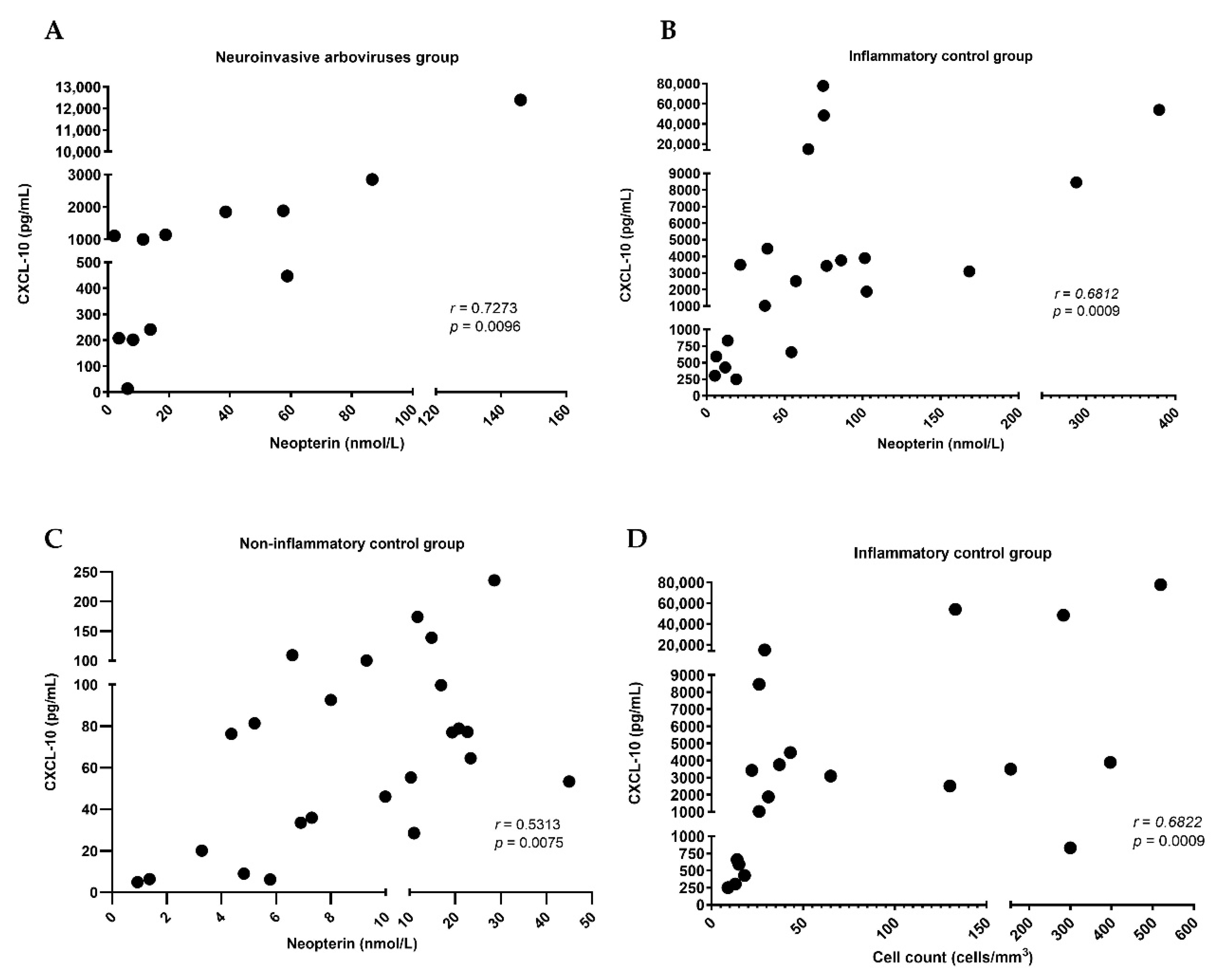
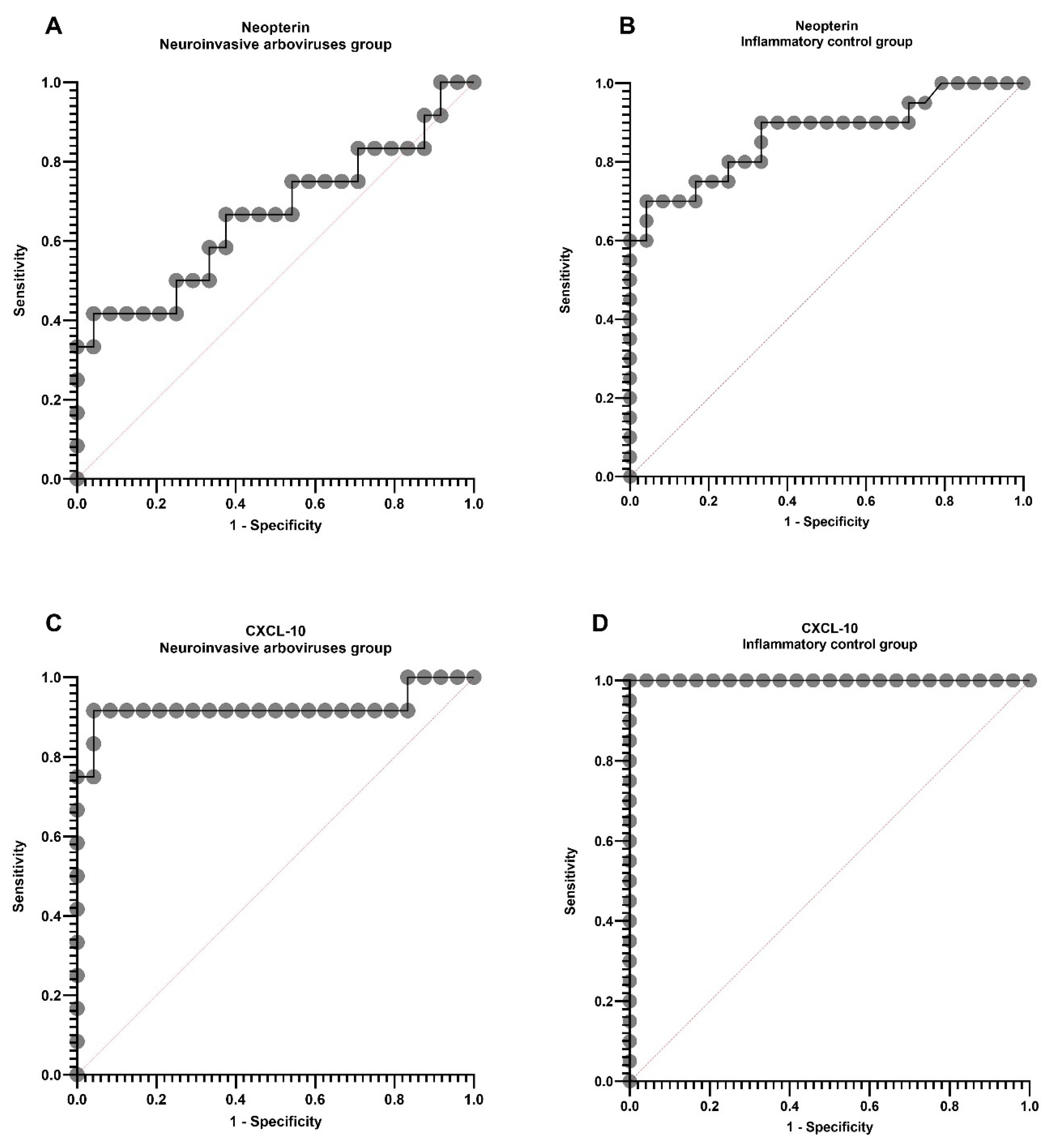
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient’s Samples
4.2. Cerebrospinal Fluid Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministério da Saúde. Dengue. Secretaria de Vigilância em Saúde. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dengue (accessed on 10 September 2021).
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Ministério da Saúde. Chikungunya. Secretaria de Vigilância em Saúde. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/c/chikungunya (accessed on 10 September 2021).
- CDC. Arboviral Diseases, Neuroinvasive and Non-Neuroinvasive: 2015 Case Definition; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2015. Available online: https://ndc.services.cdc.gov/case-definitions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive-2015/ (accessed on 10 September 2021).
- Ministério da Saúde. Manual de Vigilância Sentinela de Doenças Neuroinvasivas por Arbovírus, 1st ed.; Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis: Brasília, Brazil, 2017; 44p.
- Borish, L.C.; Steinke, J.W. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Palomino, D.C.T.; Marti, L.C. Chemokines and immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Liba, Z.; Nohejlova, H.; Capek, V.; Krsek, P.; Sediva, A.; Kayserova, J. Utility of chemokines CCL2, CXCL8, 10 and 13 and interleukin 6 in the pediatric cohort for the recognition of neuroinflammation and in the context of traditional cerebrospinal fluid neuroinflammatory biomarkers. PLoS ONE 2019, 14, e0219987. [Google Scholar] [CrossRef]
- McKimmie, C.S.; Michlmayr, D. Role of CXCL10 in central nervous system inflammation. Int. J. Interferon Cytokine Med. Res. 2014, 6, 1–18. [Google Scholar] [CrossRef]
- Ulker, O.C.; Yucesoy, B.; Durucu, M.; Karakaya, A. Neopterin as a marker for immune system activation in coal workers’ pneumoconiosis. Toxicol. Ind. Health 2007, 23, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Azumagawa, K.; Suzuki, S.; Tanabe, T.; Wakamiya, E.; Kawamura, N.; Tamai, H. Neopterin, biopterin, and nitric oxide concentrations in the cerebrospinal fluid of children with central nervous system infections. Brain Dev. 2003, 25, 200–202. [Google Scholar] [CrossRef]
- Kuehne, L.K.; Reiber, H.; Bechter, K.; Hagberg, L.; Fuchs, D. Cerebrospinal fluid neopterin is brain-derived and not associated with blood-CSF barrier dysfunction in non-inflammatory affective and schizophrenic spectrum disorders. J. Psychiatr. Res. 2013, 47, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Molero-Luis, M.; Casas-Alba, D.; Orellana, G.; Ormazabal, A.; Sierra, C.; Oliva, C.; Valls, A.; Velasco, J.; Launes, C.; Cuadras, D.; et al. Cerebrospinal fluid neopterin as a biomarker of neuroinflammatory diseases. Sci. Rep. 2020, 10, 18291. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, K.; Martins, R.P.; Barbeito, L.; Latini, A. Neopterin as a potential cytoprotective brain molecule. J. Psychiatr. Res. 2015, 71, 134–139. [Google Scholar] [CrossRef]
- Tegeder, I.; Costigan, M.; Griffin, R.S.; Abele, A.; Belfer, I.; Schmidt, H.; Ehnert, C.; Nejim, J.; Marian, C.; Scholz, J.; et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat. Med. 2006, 12, 1269–1277. [Google Scholar] [CrossRef]
- Chan, C.P.; Choi, J.W.; Cao, K.Y.; Wang, M.; Gao, Y.; Zhou, D.H.; Di, B.; Xu, H.F.; Leung, M.F.; Bergmann, A.; et al. Detection of serum neopterin for early assessment of dengue virus infection. J. Infect. 2006, 53, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, A.; Ghorpade, R.P.; Chopra, A. Cytokines in acute chikungunya. PLoS ONE 2014, 9, e111305. [Google Scholar] [CrossRef]
- Soares, C.N.; Faria, L.C.; Peralta, J.M.; de Freitas, M.R.; Puccioni-Sohler, M. Dengue infection: Neurological manifestations and cerebrospinal fluid (CSF) analysis. J. Neurol. Sci. 2006, 249, 19–24. [Google Scholar] [CrossRef]
- Mello, C.D.S.; Cabral-Castro, M.J.; Faria, L.C.S.; Peralta, J.M.; Puccioni-Sohler, M. Use of cerebrospinal fluid for the diagnosis of neuroinvasive Dengue, Zika, and Chikungunya: A 19-year systematic review. Rev. Soc. Bras. Med. Trop. 2021, 54, e0891-2020. [Google Scholar] [CrossRef]
- Puccioni-Sohler, M.; Farias, L.C.; Cabral-Castro, M.J.; Zalis, M.G.; Kalil, R.S.; Salgado, M.C.F. Cerebrospinal Fluid Immunoglobulins as Potential Biomarkers of Chikungunya Encephalitis. Emerg. Infect. Dis. 2018, 24, 939–941. [Google Scholar] [CrossRef]
- Molero-Luis, M.; Fernández-Ureña, S.; Jordán, I.; Serrano, M.; Ormazábal, A.; Garcia-Cazorla, À.; Artuch, R.; Neopterin Working Group. Cerebrospinal fluid neopterin analysis in neuropediatric patients: Establishment of a new cut off-value for the identification of inflammatory-immune mediated processes. PLoS ONE 2013, 8, e83237. [Google Scholar] [CrossRef]
- Kelvin, A.A.; Banner, D.; Silvi, G.; Moro, M.L.; Spataro, N.; Gaibani, P.; Cavrini, F.; Pierro, A.; Rossini, G.; Cameron, M.J.; et al. Inflammatory cytokine expression is associated with Chikungunya virus resolution and symptom severity. PLoS Negl. Trop. Dis. 2011, 5, e1279. [Google Scholar] [CrossRef] [PubMed]
- De-Oliveira-Pinto, L.M.; Gandini, M.; Freitas, L.P.; Siqueira, M.M.; Marinho, C.F.; Setúbal, S.; Kubelka, C.F.; Cruz, O.G.; de Oliveira, S.A. Profile of circulating levels of IL-1Ra, CXCL10/IP-10, CCL4/MIP-1β and CCL2/MCP-1 in dengue fever and parvovirosis. Mem. Inst. Oswaldo Cruz 2012, 107, 48–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antonelli, A.; Ferrari, S.M.; Giuggioli, D.; Ferrannini, E.; Ferri, C.; Fallahi, P. Chemokine (C-X-C motif) ligand (CXCL) 10 in autoimmune diseases. Autoimmun. Rev. 2014, 13, 272–280. [Google Scholar] [CrossRef]
- Almeida, R.S.; Ferreira, M.L.B.; Sonon, P.; Cordeiro, M.T.; Sadissou, I.; Diniz, G.T.N.; Militão-Albuquerque, M.F.P.; Franca, R.F.O.; Donadi, E.A.; Lucena-Silva, N. Cytokines and Soluble HLA-G Levels in the Acute and Recovery Phases of Arbovirus-Infected Brazilian Patients Exhibiting Neurological Complications. Front. Immunol. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Lu, H.L.; Lai, S.L.; Campanella, G.S.; Sung, J.M.; Lu, M.Y.; Wu-Hsieh, B.A.; Lin, Y.L.; Lane, T.E.; Luster, A.D.; et al. Dengue virus induces expression of CXC Chemokine Ligand 10/IFN-γ-Inducible Protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J. Immunol. 2006, 177, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.; Marro, B.S.; Lane, T.E. Chemokine CXCL10 and Coronavirus-induced neurologic disease. Viral Immunol. 2019, 32, 25–37. [Google Scholar] [CrossRef]
- Geisler, S.; Lytton, S.D.; Toan, N.L.; Nghia, T.H.; Nam, N.M.; Hung, H.V.; Son, N.T.; Anh, D.T.; Tuyen, H.T.; Tien, T.V.; et al. Neopterin levels and Kyn/Trp ratios were significantly increased in dengue virus patients and subsequently decreased after recovery. Int. J. Infect. Dis. 2020, 91, 162–168. [Google Scholar] [CrossRef]
- Chandrashekhar, C.; Balaji, K.; Vasudev, P.H.; Panachiyil, G.M.; Babu, T. Estimation of serum neopterinlevel as an early marker for detecting severe dengue infection. Int. J. Pediatr. Adolesc. Med. 2019, 6, 151–154. [Google Scholar] [CrossRef]
- Anfasa, F.; Provacia, L.; GeurtsvanKessel, C.; Wever, R.; Gerstenbluth, I.; Osterhaus, A.D.; Martina, B.E. Hyperferritinemia is a potential marker of chronic chikungunya: A retrospective study on the Island of Curaçao during the 2014–2015 outbreak. J. Clin. Virol. 2017, 86, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Baldoni, N.R.; Cardoso, C.S.; Oliveira, C.D.L. Biomarkers of severity and chronification in chikungunya fever: A systematic review and meta-analysis. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e16. [Google Scholar] [CrossRef] [PubMed]
- Puccioni-Sohler, M.; Ornelas, A.M.M.; de Souza, A.S.; Cabral-Castro, M.J.; Ramos, J.T.M.A.; Rosadas, C.; Salgado, M.C.F.; Castiglione, A.A.; Ferry, F.; Peralta, J.M.; et al. First report of persistent dengue-1-associated autoimmune neurological disturbance: Neuromyelitis optica spectrum disorder. J. Neurovirol. 2017, 5, 768–771. [Google Scholar] [CrossRef] [PubMed]

| Group A, DENV and CHIKV Neuroinvasive (n = 12) | Group B, Inflammatory Control (n = 20) | Group C, Non- Inflammatory Control (n = 24) | p Value | |||
|---|---|---|---|---|---|---|
| A × B | A × C | B × C | ||||
| Sex | ||||||
| Female, n (%) | 8 (66.7%) | 10 (50%) | 18 (75%) | 0.263 | <0.999 | >0.999 |
| Age (years), median (IQR) | 61 (33–64) | 37 (30–53.3) | 48.5 (31.8–72.5) | 0.3485 | 0.5464 | >0.999 |
| Cell count (cells/mm3), median (IQR) | 7.5 (1–60.5) | 34 (19–149.5) | 1 (1–2) | 0.0419 ** | 0.0441 ** | <0.0001 ** |
| Protein (mg/dL), median (IQR) | 63 (43–79) | 86.5 (67–231.3) | 27 (21.25–35.75) | 0.1849 | 0.0045 ** | <0.0001 ** |
| Glucose (mg/dL), median (IQR) | 63 (32.8–80) | 60 (41.5–80.8) | 70.5 (62–87) | >0.999 | 0.4887 | 0.1579 |
| Neopterin (nmol/L), median (IQR) | 16.4 (6.85–58.5) | 61.2 (19.5–97.6) | 9.6 (5.4–18.7) | 0.1912 | 0.3096 | 0.0001 ** |
| CXCL-10 (pg/mL), median (IQR) | 1056 (215.8–1876) | 3266 (702.5–7462) | 70.4 (29.7–98) | 0.3396 | 0.0011 ** | <0.0001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccioni-Sohler, M.; da Silva, S.J.; Faria, L.C.S.; Cabral, D.C.B.I.; Cabral-Castro, M.J. Neopterin and CXCL-10 in Cerebrospinal Fluid as Potential Biomarkers of Neuroinvasive Dengue and Chikungunya. Pathogens 2021, 10, 1626. https://doi.org/10.3390/pathogens10121626
Puccioni-Sohler M, da Silva SJ, Faria LCS, Cabral DCBI, Cabral-Castro MJ. Neopterin and CXCL-10 in Cerebrospinal Fluid as Potential Biomarkers of Neuroinvasive Dengue and Chikungunya. Pathogens. 2021; 10(12):1626. https://doi.org/10.3390/pathogens10121626
Chicago/Turabian StylePuccioni-Sohler, Marzia, Samya J. da Silva, Luiz C. S. Faria, David C. B. I. Cabral, and Mauro J. Cabral-Castro. 2021. "Neopterin and CXCL-10 in Cerebrospinal Fluid as Potential Biomarkers of Neuroinvasive Dengue and Chikungunya" Pathogens 10, no. 12: 1626. https://doi.org/10.3390/pathogens10121626
APA StylePuccioni-Sohler, M., da Silva, S. J., Faria, L. C. S., Cabral, D. C. B. I., & Cabral-Castro, M. J. (2021). Neopterin and CXCL-10 in Cerebrospinal Fluid as Potential Biomarkers of Neuroinvasive Dengue and Chikungunya. Pathogens, 10(12), 1626. https://doi.org/10.3390/pathogens10121626







