Assessment of Food and Waterborne Viral Outbreaks by Using Field Epidemiologic, Modern Laboratory and Statistical Methods—Lessons Learnt from Seven Major Norovirus Outbreaks in Finland
Abstract
1. Introduction
2. Results
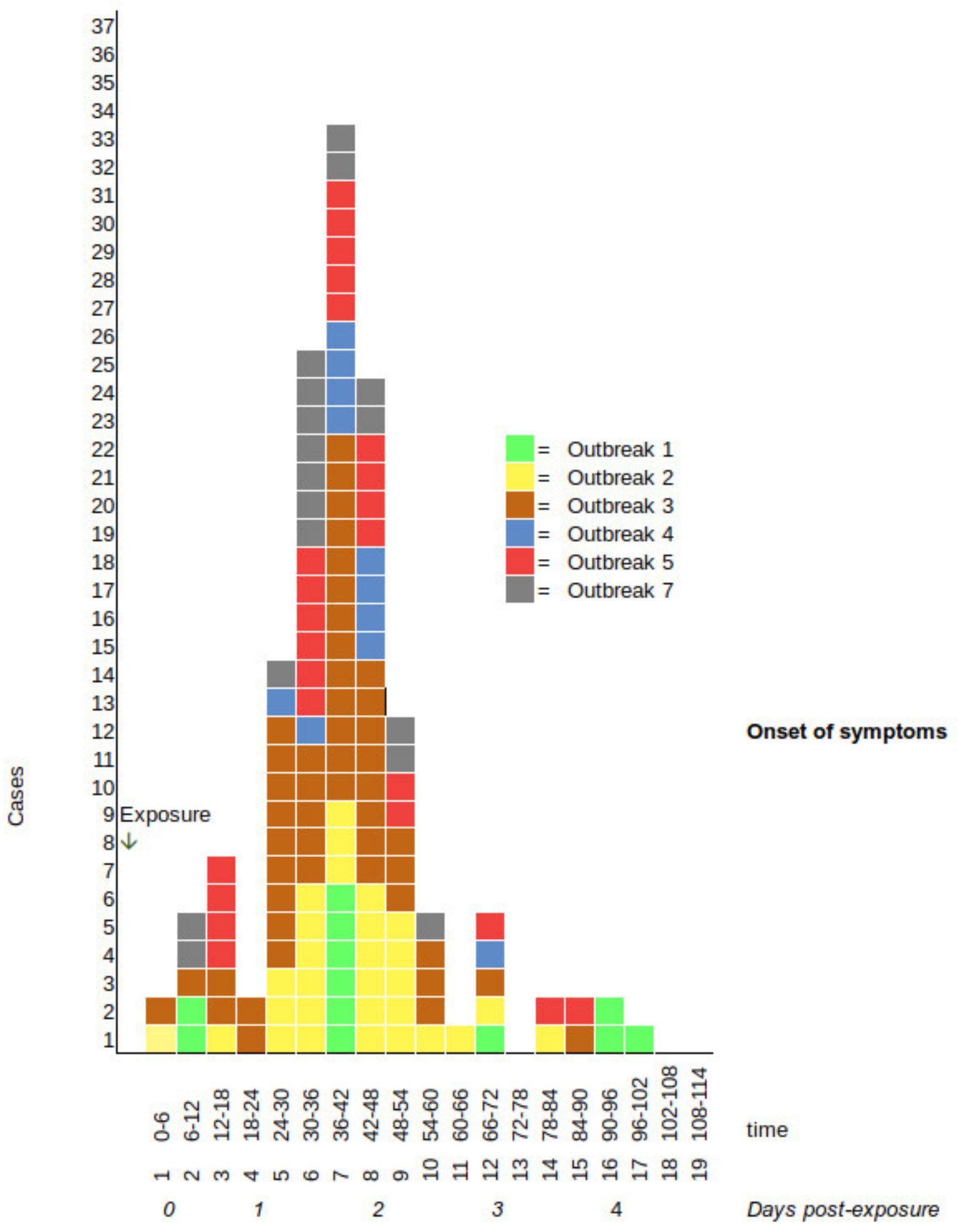
2.1. Descriptive Epidemiology
2.2. Analytical Cohort Studies
2.3. Kitchen Inspections and State of Health of the Kitchen Staff
2.4. Food, Water and Environmental Samples
2.5. Control Measures to Prevent Further Cases
3. Discussion
4. Materials and Methods
4.1. Description of the Events and Patients
4.2. Cohort Studies
4.3. Descriptive and Statistical Analysis
4.4. Kitchen Inspections and Environmental Sampling
4.5. Environmental Microbiology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, D.J.; Adams, N.L.; Aladin, F.; Harris, J.P.; Brown, D.W. Emergence of the GII-4 Norovirus Sydney2012 strain in England, winter 2012–2013. PLoS ONE 2014, 9, e88978. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Jakobsen, L.S.; Ellis-Iversen, J.; Pessoa, J.; Ethelberg, S. Burden of Disease Estimates of Seven Pathogens Commonly Transmitted Through Foods in Denmark, 2017. Foodborne Pathog. Dis. 2020, 17, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Thomson, L.; Mahmoudzadeh, N.; Khaled, A. Estimating deaths from foodborne disease in the UK for 11 key pathogens. BMJ Open Gastroenterol. 2020, 7, e000377. [Google Scholar] [CrossRef] [PubMed]
- Finnish Food Authority Food Borne Outbreaks in Finland. Available online: https://www.ruokavirasto.fi/teemat/zoonoosikeskus/ruokamyrkytykset/ruokamyrkytysepidemiat-suomessa/ruokamyrkytysepidemiat-vuonna-2020 (accessed on 13 December 2021).
- Murphy, H.M.; Thomas, M.K.; Schmidt, P.J.; Medeiros, D.T.; McFADYEN, S.; Pintar, K.D.M. Estimating the burden of acute gastrointestinal illness due to Giardia, Cryptosporidium, Campylobacter, E. coli O157 and norovirus associated with private wells and small water systems in Canada. Epidemiol. Infect. 2016, 144, 1355–1370. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Larose, T.L.; Adak, G.K.; Evans, M.R.; Tam, C.C. Foodborne Disease Attribution Study Group Modelling study to estimate the health burden of foodborne diseases: Cases, general practice consultations and hospitalisations in the UK, 2009. BMJ Open 2016, 6, e011119. [Google Scholar] [CrossRef]
- Kauppinen, A.; Pitkänen, T.; Al-Hello, H.; Maunula, L.; Hokajärvi, A.M.; Rimhanen-Finne, R.; Miettinen, I.T. Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland. Int. J. Environ. Res. Public. Health 2019, 16, 4376. [Google Scholar] [CrossRef]
- De Laval, F.; Nivoix, P.; de Santi, V.P.; Caballe, D.; Garnotel, E.; Maslin, J. Severe norovirus outbreak among soldiers in the field: Foodborne followed by person-to-person transmission. Clin. Infect. Dis. 2011, 53, 399–400. [Google Scholar] [CrossRef]
- Petrignani, M.; van Beek, J.; Borsboom, G.; Richardus, J.H.; Koopmans, M. Norovirus introduction routes into nursing homes and risk factors for spread: A systematic review and meta-analysis of observational studies. J. Hosp. Infect. 2015, 89, 163–178. [Google Scholar] [CrossRef]
- Steele, M.K.; Wikswo, M.E.; Hall, A.J.; Koelle, K.; Handel, A.; Levy, K.; Waller, L.A.; Lopman, B.A. Characterizing Norovirus Transmission from Outbreak Data, United States. Emerg. Infect. Dis. 2020, 26, 1818–1825. [Google Scholar] [CrossRef]
- Franck, K.T.; Lisby, M.; Fonager, J.; Schultz, A.C.; Böttiger, B.; Villif, A.; Absalonsen, H.; Ethelberg, S. Sources of Calicivirus contamination in foodborne outbreaks in Denmark, 2005–2011-the role of the asymptomatic food handler. J. Infect. Dis. 2015, 211, 563–570. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodriguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Cook, N.; Williams, L.; D’Agostino, M. Prevalence of Norovirus in produce sold at retail in the United Kingdom. Food Microbiol. 2019, 79, 85–89. [Google Scholar] [CrossRef]
- Saupe, A.A.; Rounds, J.; Sorenson, A.; Hedeen, N.; Bagstad, E.; Reinberg, R.; Wagley, A.G.; Cebelinski, E.; Smith, K. Outbreak of Norovirus Gastroenteritis Associated with Ice Cream Contaminated by Frozen Raspberries from China; Minnesota, USA, 2016. Clin. Infect. Dis. 2020, 73, e3701–e3707. [Google Scholar] [CrossRef]
- Chatziprodromidou, I.P.; Bellou, M.; Vantarakis, G.; Vantarakis, A. Viral outbreaks linked to fresh produce consumption: A systematic review. J. Appl. Microbiol. 2018, 124, 932–942. [Google Scholar] [CrossRef]
- Vo, T.H.; Okasha, O.; Al-Hello, H.; Polkowska, A.; Räsänen, S.; Bojang, M.; Nuorti, J.P.; Jalava, K. An Outbreak of Norovirus Infections Among Lunch Customers at a Restaurant, Tampere, Finland, 2015. Food Environ. Virol. 2016, 8, 174–179. [Google Scholar] [CrossRef][Green Version]
- Jalava, K.; Kauppinen, A.; Al-Hello, H.; Räsänen, S. An outbreak of norovirus infection caused by ice cubes and a leaking air ventilation valve. Epidemiol. Infect. 2019, 147, 1–6. [Google Scholar] [CrossRef]
- Polkowska, A.; Räsänen, S.; Al-Hello, H.; Bojang, M.; Lyytikäinen, O.; Nuorti, J.P.; Jalava, K. An outbreak of Norovirus infections associated with recreational lake water in Western Finland, 2014. Epidemiol. Infect. 2018, 146, 544–550. [Google Scholar] [CrossRef]
- Ondrikova, N.; Clough, H.E.; Douglas, A.; Iturriza-Gomara, M.; Larkin, L.; Vivancos, R.; Harris, J.P.; Cunliffe, N.A. Differential impact of the COVID-19 pandemic on laboratory reporting of norovirus and Campylobacter in England: A modelling approach. PLoS ONE 2021, 16, e0256638. [Google Scholar] [CrossRef]
- Hotham, M. Potential future implications of the COVID-19 pandemic on Norovirus infections in England. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Bitler, E.J.; Matthews, J.E.; Dickey, B.W.; Eisenberg, J.N.S.; Leon, J.S. Norovirus outbreaks: A systematic review of commonly implicated transmission routes and vehicles. Epidemiol. Infect. 2013, 141, 1563–1571. [Google Scholar] [CrossRef]
- Verhoef, L.; Hewitt, J.; Barclay, L.; Ahmed, S.M.; Lake, R.; Hall, A.J.; Lopman, B.; Kroneman, A.; Vennema, H.; Vinjé, J.; et al. Norovirus genotype profiles associated with foodborne transmission, 1999–2012. Emerg. Infect. Dis. 2015, 21, 592–599. [Google Scholar] [CrossRef]
- CDC Using Clinical and Epidemiologic Criteria for Suspected Norovirus Outbreaks. Available online: https://www.cdc.gov/norovirus/trends-outbreaks/responding.html (accessed on 11 November 2021).
- Kaplan, J.E.; Gary, G.W.; Baron, R.C.; Singh, N.; Schonberger, L.B.; Feldman, R.; Greenberg, H.B. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann. Intern. Med. 1982, 96, 756–761. [Google Scholar] [CrossRef]
- Van Beek, J.; de Graaf, M.; Al-Hello, H.; Allen, D.J.; Ambert-Balay, K.; Botteldoorn, N.; Brytting, M.; Buesa, J.; Cabrerizo, M.; Chan, M.; et al. NoroNet Molecular surveillance of norovirus, 2005–2016: An epidemiological analysis of data collected from the NoroNet network. Lancet Infect. Dis. 2018, 18, 545–553. [Google Scholar] [CrossRef]
- Turcios, R.M.; Widdowson, M.A.; Sulka, A.C.; Mead, P.S.; Glass, R.I. Reevaluation of epidemiological criteria for identifying outbreaks of acute gastroenteritis due to norovirus: United States, 1998–2000. Clin. Infect. Dis. 2006, 42, 964–969. [Google Scholar] [CrossRef]
- Devasia, T.; Lopman, B.; Leon, J.; Handel, A. Association of host, agent and environment characteristics and the duration of incubation and symptomatic periods of norovirus gastroenteritis. Epidemiol. Infect. 2015, 143, 2308–2314. [Google Scholar] [CrossRef]
- Arias, C.; Sala, M.R.; Dominguez, A.; Torner, N.; Ruiz, L.; Martinez, A.; Bartolome, R.; de Simon, M.; Buesa, J. Epidemiological and clinical features of norovirus gastroenteritis in outbreaks: A population-based study. Clin. Microbiol. Infect. 2010, 16, 39–44. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, Y.H.; Wu, C.Y.; Hung, C.H.; Jiang, D.D.S.; Wu, F.T. A norovirus outbreak in a nursing home: Norovirus shedding time associated with age. J. Clin. Virol. 2013, 56, 96–101. [Google Scholar] [CrossRef]
- Harris, J.P.; Iturriza-Gomara, M.; Allen, D.J.; Kelly, S.; O’Brien, S.J. Norovirus strain types found within the second infectious intestinal diseases (IID2) study an analysis of norovirus circulating in the community. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Lively, J.Y.; Johnson, S.D.; Wikswo, M.; Gu, W.; Leon, J.; Hall, A.J. Clinical and Epidemiologic Profiles for Identifying Norovirus in Acute Gastroenteritis Outbreak Investigations. Open Forum Infect. Dis. 2018, 5, ofy049. [Google Scholar] [CrossRef]
- Olowokure, B.; Clark, L.; Elliot, A.J.; Harding, D.; Fleming, A. Mumps and the media: Changes in the reporting of mumps in response to newspaper coverage. J. Epidemiol. Community Health 2007, 61, 385–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petran, R.L.; White, B.W.; Hedberg, C.W. Health department inspection criteria more likely to be associated with outbreak restaurants in Minnesota. J. Food Prot. 2012, 75, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Duret, S.; Pouillot, R.; Fanaselle, W.; Papafragkou, E.; Liggans, G.; Williams, L.; Van Doren, J.M. Quantitative Risk Assessment of Norovirus Transmission in Food Establishments: Evaluating the Impact of Intervention Strategies and Food Employee Behavior on the Risk Associated with Norovirus in Foods. Risk Anal. 2017, 37, 2080–2106. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Caul, E.O.; White, D.G.; Palmer, S.R. Role of infected food handler in hotel outbreak of Norwalk-like viral gastroenteritis: Implications for control. Lancet 1988, 2, 321–323. [Google Scholar] [CrossRef]
- Rumble, C.; Addiman, S.; Balasegaram, S.; Chima, K.; Ready, D.; Heard, J.; Alexander, E. Role of Food Handlers in Norovirus Outbreaks in London and South East England, 2013 to 2015. J. Food Prot. 2017, 80, 257–264. [Google Scholar] [CrossRef]
- Bartsch, C.; Szabo, K.; Dinh-Thanh, M.; Schrader, C.; Trojnar, E.; Johne, R. Comparison and optimization of detection methods for noroviruses in frozen strawberries containing different amounts of RT-PCR inhibitors. Food Microbiol. 2016, 60, 124–130. [Google Scholar] [CrossRef]
- Sun, B.; Bosch, A.; Myrmel, M. Extended direct lysis method for virus detection on berries including droplet digital RT-PCR or real time RT-PCR with reduced influence from inhibitors. J. Virol. Methods 2019, 271, 113638. [Google Scholar] [CrossRef]
- Morgan, O. How decision makers can use quantitative approaches to guide outbreak responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180365. [Google Scholar] [CrossRef]
- Hatch, E.E.; Hahn, K.A.; Wise, L.A.; Mikkelsen, E.M.; Kumar, R.; Fox, M.P.; Brooks, D.R.; Riis, A.H.; Sorensen, H.T.; Rothman, K.J. Evaluation of Selection Bias in an Internet-based Study of Pregnancy Planners. Epidemiology 2016, 27, 98–104. [Google Scholar] [CrossRef]
- Jalava, K.; Hakkinen, M.; Valkonen, M.; Nakari, U.M.; Palo, T.; Hallanvuo, S.; Ollgren, J.; Siitonen, A.; Nuorti, J.P. An outbreak of gastrointestinal illness and erythema nodosum from grated carrots contaminated with Yersinia pseudotuberculosis. J. Infect. Dis. 2006, 194, 1209–1216. [Google Scholar] [CrossRef]
- Polonsky, J.A.; Baidjoe, A.; Kamvar, Z.N.; Cori, A.; Durski, K.; Edmunds, W.J.; Eggo, R.M.; Funk, S.; Kaiser, L.; Keating, P.; et al. Outbreak analytics: A developing data science for informing the response to emerging pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180276. [Google Scholar] [CrossRef]
- Jore, S.; Braae, U.C.; Trier Møller, F.; Friesema, I.; Paranthaman, K.; Jalava, K.; Jourdan-DaSilva, N.; Löf, E.; Rehn, M.; Ethelberg, S. A common framework for using and reporting consumer purchase data (CPD) in foodborne outbreak investigations in Europe. Infect. Ecol. Epidemiol. 2022, 12, 2007828. [Google Scholar] [CrossRef]
- Bedford, J.; Farrar, J.; Ihekweazu, C.; Kang, G.; Koopmans, M.; Nkengasong, J. A new twenty-first century science for effective epidemic response. Nature 2019, 575, 130–136. [Google Scholar] [CrossRef]
- Fonte, L.; Murtas, R.; Russo, A.G. Comparison between cohort and case-control approaches for health impact assessment on a population exposed to the emissions of an incinerator. Epidemiol. Prev. 2017, 41, 176–183. [Google Scholar]
- Ahn, S.Y.; Park, J.Y.; Lim, I.S.; Chae, S.A.; Yun, S.W.; Lee, N.M.; Kim, S.Y.; Choi, B.S.; Yi, D.Y. Changes in the Occurrence of Gastrointestinal Infections after COVID-19 in Korea. J. Korean Med. Sci. 2021, 36, e180. [Google Scholar] [CrossRef]
- Hanh, T.T.T.; Hanh, M.H. Hygienic Practices and Structural Conditions of the Food Processing Premises Were the Main Drivers of Microbiological Quality of Edible Ice Products in Binh Phuoc Province, Vietnam 2019. Environ. Health Insights 2020, 14, 1178630220929722. [Google Scholar]
- KoBoToolbox KoBoToolbox -Simple, Robust and Powerful Tools for Data Collection. Available online: https://www.kobotoolbox.org (accessed on 5 June 2019).
- Powell, S.C.; Attwell, R. The use of epidemiological data in the control of foodborne viruses. Rev. Environ. Health 1999, 14, 31–37. [Google Scholar] [CrossRef]
- Harrison, S.L.; Nelder, R.; Hayek, L.; Mackenzie, I.F.; Casemore, D.P.; Dance, D. Managing a large outbreak of cryptosporidiosis: How to investigate and when to decide to lift a ‘boil water’ notice. Commun. Dis. Public Health 2002, 5, 230–239. [Google Scholar]
- Jalava, K. First respiratory transmitted food borne outbreak? Int. J. Hyg. Environ. Health 2020, 226, 113490. [Google Scholar] [CrossRef]
- Thompson, R.N.; Jalava, K.; Obolski, U. Sustained transmission of Ebola in new locations: More likely than previously thought. Lancet Infect. Dis. 2019, 19, 1058–1059. [Google Scholar] [CrossRef]
- Thompson, R.N.; Morgan, O.W.; Jalava, K. Rigorous surveillance is necessary for high confidence in end-of-outbreak declarations for Ebola and other infectious diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180431. [Google Scholar] [CrossRef]
- Finnish Food Authority Oiva-In-House Control by Food Sector Operators. Available online: https://www.ruokavirasto.fi/en/companies/food-sector/production/food-categories/foodstuffs-for-particular-nutritional-use/control (accessed on 14 December 2021).
- Summa, M.; Maunula, L. Rapid Detection of Human Norovirus in Frozen Raspberries. Food Environ. Virol. 2018, 10, 51–60. [Google Scholar] [CrossRef]
- Loisy, F.; Atmar, R.L.; Guillon, P.; Le Cann, P.; Pommepuy, M.; Le Guyader, F.S. Real-time RT-PCR for norovirus screening in shellfish. J. Virol. Methods 2005, 123, 1–7. [Google Scholar] [CrossRef]

| Outbreak Name | Time | Place | Number of Enrolled (Included in the Cohort Study) | Number of Cases Enrolled in the Cohort Study | Vomiting, % (No./Total) | Mean Length of Incubation Period, hours | Mean Length of Duration of Symptoms, hours | Vehicle (RR, 95% Confidence Intervals) | Evidence | Norovirus Genotype(s) | Infected Food Handler (If not Considered the Source) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outbreak_1 (this study) | 21–23.3.2018 | Birthday party (catering service) | 51 (38) | 12 | 67% (8/12) | 51 | 43 | Mini-burgers, RR = 5.0 (95% CI 1.58–29.81) | Cohort study, Patient samples | GII.P7 | Yes |
| Outbreak_2 (this study) | 7.2.2017 | School, institutional kitchen | 83 (82) | 29 | 66% (19/29) | 41 | 35 | Sliced cucumber (within cheese rolls), RR = 6.12 (95% CI 1.54–103.89) | Cohort study, Patient samples | GII | Yes |
| Outbreak_3 (this study) | 4–5.3.2017 | Catering service, two functions | 72 (72) | 49 | 73% (35/48) | 37 | 49 | Washed, fresh strawberries (cream cake and salad), RR = 2.28 (95% CI 1.14–6.64) | Cohort study, Patient samples, Food samples | GII | No |
| Outbreak_4 [17] | 26.1.2015 | Lunch restaurant | 27 (21) | 11 | 82% (9/11) | 38 | 28 | Salad buffet, RR = 2.33 (95% CI 1.00–8.41) | Cohort study, Patient samples | GI.P7 | (Yes) |
| Outbreak_5 [18] | 9–10.12.2016 | Evening restaurant, two functions | 102 (91) | 24 | 58% (14/24) | 39 | 61 | Ice cubes, air contamination through faulty air ventilation valve, RR = 6.46 (95% CI 1.46–113.97) to RR = 8.17 (95% CI 1.67–145.52) | Cohort study, Patient samples | GI and sapovirus | (Yes) |
| Outbreak_6 [19] | 21–28.7.2014 | Four fresh water lakes | 1656 (1453) | 244 | 85% (193/227) | – | 38 | Contaminated lake water, head under water, RR = 3.24 (95% CI 2.31–4.71) | Cohort study, Patient samples | Several noroviruses: GI, GII, | No |
| Outbreak_7 (this study) | 22.10.2016 | Private christening party (catering service) | 37 (37) (2 children <2 years were excluded from analysis) | 17 | 88% (15/17) | 37 | 66 | Person-to-person transmission, not significant for any exposures | Cohort study, Patient samples | GII.P16-GII.4 | No |
| Clinical Characteristics | Children <18 Years of Age n = 150,% (Exposure/Total) | Adults 18-64 Years of Age n = 207, % (Exposure/Total) | Elderly 265 Years of Age n = 27, % (Exposure/Total) | Comparison of Children to Elderly, 2-Sided Test, p- Value |
|---|---|---|---|---|
| Diarrhoea | 55% (69/125) | 71% (141/199) | 92% (24/26) | 0.004 |
| Vomiting | 96% (143/149) | 69% (132/192) | 64% (16/25) | <0.001 |
| Bloody diarrhoea | 0% (0/7) | 3% (3/96) | 5% (1/21) | 0.56 |
| Nausea | 96% (133/138) | 88% (176/200) | 88% (23/26) | 0.086 |
| Stomach ache) | 90% (120/134) | 80% (156/194) | 69% (18/26) | 0.0059 |
| Fever | 44% (50/113) | 46 % (80/175) | 24% (6/25) | 0.062 |
| Time between exposure and illness onset (mean) | 64 h (n = 7) | 39 h (n = 113) | 36 h (n = 21) | 0.19 |
| Duration of illness (mean) | 38 h (n = 142), in children <5 years 48 h, (n = 27) | 43 h (n = 186) | 63 h (n = 18) | 0.013 |
| Entity | Justification and Reasoning |
|---|---|
| Aim and scope | The immediate aim of the foodborne outbreak investigation is to prevent further illness in the community by withdrawing any suspect food items from the food chain during the initial steps of the outbreak investigation. As a long-term goal, the aim is to determine the complete mechanism by which food became contaminated during primary production and food processing and which factors contributed to the spread of the pathogens to cause human illness. Formulation of food safety standards and recommendations is a priority. |
| Speed | Most of the environmental public health action occurs within the first 24–48 h with successful outbreak investigations. If this important window of opportunity is missed, it may result in increasing number of new cases and potentially excess deaths. Media response also reflects the success and speed. The lead epidemiologist needs to visit quickly all outbreak-affected areas in person, communicate with the press and social media and conduct active case finding. As a rule of thumb, once the environmental health unit is notified of a potential outbreak, the situation is already severe and immediate action needs to be taken (excluding family outbreaks, etc.). |
| Formulation of multidisciplinary outbreak control team (OCT) | OCT needs representatives from public and environmental health, clinical medicine, statistics and media communications. A formal lead to present in the media and an epidemiologist responsible for practical lead of the investigations are needed and preferably should be two separate persons. |
| Rapid food facility assessment | Environmental health will lead a thorough assessment of the implicated facility to formulate hypothesis on the causative agent and mechanism of the outbreak. Often, several visits over the course of the first days of the investigation are needed. |
| Prevention of further spread | Immediate risk assessment; communication with all colleagues with knowledge on the topic. Prevention of possible contaminated food and identification of possible infected food handlers. Downplay of any isolated leading of the incident. Use of Kaplan criteria to assess the possibility of norovirus outbreak. Lead epidemiologist is at the service of others, not vice versa. |
| Collaboration with research institutes | Food and environmental samples present as challenging matrix for microbiological and genetic analysis. Outbreaks present perfect opportunity to develop analytical methods further with research institutes. This also applies to theoretical aspects of epidemiological and statistical methods. |
| Epidemiological and statistical methods | Outbreak response and determination of the implicated vehicle and causative pathogen should be directed by likely probabilities of options based on rapid risk assessments, due to be updated during the course of the investigation. The environmental public health action is prioritised based on mathematical probabilities of events, based on literature and past experience. |
| Technical advances | Novel technologies aid in making outbreak investigations quicker, more reliable and robust. These include, e.g., electronic, online questionnaires and open-source statistical and mathematical programs (R, Python, etc.). In addition, use of consumer purchase data (loyalty cards, etc.) may be useful. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polkowska, A.; Räsänen, S.; Nuorti, P.; Maunula, L.; Jalava, K. Assessment of Food and Waterborne Viral Outbreaks by Using Field Epidemiologic, Modern Laboratory and Statistical Methods—Lessons Learnt from Seven Major Norovirus Outbreaks in Finland. Pathogens 2021, 10, 1624. https://doi.org/10.3390/pathogens10121624
Polkowska A, Räsänen S, Nuorti P, Maunula L, Jalava K. Assessment of Food and Waterborne Viral Outbreaks by Using Field Epidemiologic, Modern Laboratory and Statistical Methods—Lessons Learnt from Seven Major Norovirus Outbreaks in Finland. Pathogens. 2021; 10(12):1624. https://doi.org/10.3390/pathogens10121624
Chicago/Turabian StylePolkowska, Aleksandra, Sirpa Räsänen, Pekka Nuorti, Leena Maunula, and Katri Jalava. 2021. "Assessment of Food and Waterborne Viral Outbreaks by Using Field Epidemiologic, Modern Laboratory and Statistical Methods—Lessons Learnt from Seven Major Norovirus Outbreaks in Finland" Pathogens 10, no. 12: 1624. https://doi.org/10.3390/pathogens10121624
APA StylePolkowska, A., Räsänen, S., Nuorti, P., Maunula, L., & Jalava, K. (2021). Assessment of Food and Waterborne Viral Outbreaks by Using Field Epidemiologic, Modern Laboratory and Statistical Methods—Lessons Learnt from Seven Major Norovirus Outbreaks in Finland. Pathogens, 10(12), 1624. https://doi.org/10.3390/pathogens10121624







