Gap Analysis of the Habitat Interface of Ticks and Wildlife in Mexico
Abstract
:1. Mexican Ticks: How Many Species and Where to Find Them
2. The Potential of Ticks as Vectors of Pathogens in a Megadiverse Country
3. Diagnostics for the Detection of Tick-Borne Pathogens
3.1. Serological Diagnostics
3.2. Molecular Diagnostics
4. Chronicle of an Announced Zoonosis: The Effect of Global Climate Change on Ticks and Their Associated Pathogens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez, T.M.; Guzmán-Cornejo, C.; Montiel-Parra, G.; Paredes-León, R.; Rivas, G. Biodiversidad de ácaros en México. Rev. Mex. Biodiv. 2014, 85, S399–S407. [Google Scholar] [CrossRef] [Green Version]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Llorente-Bousquets, J.; Ocegueda, S. Estado del Conocimiento de la Biota, en Capital Natural de México, Vol. I: Conocimiento Actual de la Biodiversidad; Conabio: Mexico City, Mexico, 2008.
- Guzman-Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel-Parra, G.; Pérez, T.M. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: Identification keys, distribution and hosts. Zootaxa 2011, 2998, 16–38. [Google Scholar]
- Guzmán-Cornejo, C.; Robbins, R.G. The genus Ixodes (Acari: Ixodidae) in Mexico: Adult identification keys, diagnoses, hosts, and distribution. Rev. Mex. Biodiv. 2010, 81, 289–298. [Google Scholar]
- De León, G.P.P.; García-Prieto, L.; Mendoza-Garfias, B. Describing parasite biodiversity: The case of the helminth fauna of wildlife vertebrates in Mexico. In Changing Diversity in Changing Environment; InTeachOpen: London, UK, 2011; pp. 33–54. [Google Scholar]
- Kolonin, G.V. Mammals as hosts of Ixodid ticks (Acarina, Ixodidae). Entomol. Rev. 2007, 87, 401–412. [Google Scholar] [CrossRef]
- Hernández-Camacho, N.; Jones, R.W.; Pineda-López, R.F.; López-González, C.A. Mexican wild and domestic canids: A potential risk of zoonosis? A review. In Nematodes: Morphology, Functions and Management Strategies; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 229–237. [Google Scholar]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.j6adaq (accessed on 17 August 2021).
- CONABIO. Uso de suelo y vegetación modificado por CONABIO. In Escala 1: 1000000; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2021. [Google Scholar]
- Süss, J.; Klaus, C.; Gerstengarbe, F.W.; Werner, P.C. What makes ticks tick? Climate change, ticks, and tick-borne diseases. J. Travel Med. 2008, 15, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Rzedowki, J. Vegetación de México; 1a. Edición Digital; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2006.
- World Health Organization. Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 17 August 2021).
- Aguilar-Tipacamu, G.; Carvajal-Gamez, B.I.; García-Rejon, J.; Machain-Willians, C.; Mosqueda, J. Immuno-molecular prospecting for vector-borne diseases in central Mexico. Transbound. Emerg. Dis. 2020, 67, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, G.; Candia-Plata, M.d.C.; Delgado-de la Mora, J.; Acuña-Meléndrez, N.H.; Vargas-Ortega, A.P.; Licona-Enríquez, J.D. Fiebre maculosa de las Montañas Rocosas en niños y adolescentes mexicanos: Cuadro clínico y factores de mortalidad. Salud Pública México 2016, 58, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Colunga-Salas, P.; Sánchez-Montes, S.; Volkow, P.; Ruíz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef] [PubMed]
- Grenfell, B.; Dobson, A. Ecology of Infectious Diseases in Natural Populations; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar] [CrossRef]
- Hudson, P.J.; Rizzoli, A.; Grenfell, B.T.; Heesterbeek, J.; Dobson, A.P. Ecology Of Wildlife Diseases; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Betancur, H.O.; Betancourt, E.A.; Giraldo, R.C. Importance of ticks in the transmission of zoonotic agents. Rev. MVZ Córdoba 2015, 20, 5053–5067. [Google Scholar] [CrossRef] [Green Version]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [Green Version]
- Cabello, C.C.; Cabello, C.F. Zoonosis con reservorios silvestres: Amenazas a la salud pública y a la economía. Rev. Méd. Chile 2008, 136, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Oteo, J.A.; Brouqui, P. Ehrlichiosis and human anaplasmosis. Enferm. Infecc. Microbiol. Clin. 2005, 23, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Tinoco-Gracia, L.; Lomelí, M.R.; Hori-Oshima, S.; Stephenson, N.; Foley, J. Molecular confirmation of Rocky mountain spotted fever epidemic agent in Mexicali, Mexico. Emerg. Infect. Dis. 2018, 24, 1723–1725. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 2000, 31, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PAHO. Segunda Reunión para Establecer una Red de Vigilancia para Enfermedades Emergentes y Reemergentes (EER) en la Región Amazónica. Tarapoto, Perú, 14–16 April 1999. Available online: https://www3.paho.org/spanish/ad/dpc/cd/tarapoto.htm (accessed on 17 August 2021).
- Cárdenas-Canales, E.M.; Ortega-Santos, J.A.; Campbell, T.A.; García-Vázquez, Z.; Cantú-Covarrubias, A.; Figueroa-Millán, J.V.; DeYoung, R.W.; Hewitt, D.G.; Bryant, F.C. Nilgai antelope in northern Mexico as a possible carrier for cattle fever ticks and Babesia bovis and Babesia bigemina. J. Wildl. Dis. 2011, 47, 777–779. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- De la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis-Hernandez, A.; Rodriguez-Vivas, R.I.; Esteve-Gassent, M.D.; Villegas-Perez, S.L. Prevalence of Borrelia burgdorferi sensu lato in synanthropic rodents in two rural communities of Yucatan, Mexico. Biomedica 2016, 36, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Modarelli, J.; Tomeček, J.M.; French, J.T.; Hilton, C.; Esteve-Gasent, M.D. Prevalence of common tick-borne pathogens in white-tailed deer and coyotes in south Texas. Int. J. Parasitol. Parasites Wildl. 2020, 11, 129–135. [Google Scholar] [CrossRef]
- Ojeda-Chi, M.M.; Rodriguez-Vivas, R.I.; Esteve-Gasent, M.D.; Perez de Leon, A.; Modarelli, J.J.; Villegas-Perez, S. Molecular detection of rickettsial tick-borne agents in white-tailed deer (Odocoileus virginianus yucatanensis), mazama deer (Mazama temama), and the ticks they host in Yucatan, Mexico. Ticks Tick Borne Dis. 2019, 10, 365–370. [Google Scholar] [CrossRef]
- Feria-Arroyo, T.P.; Castro-Arellano, I.; Gordillo-Perez, G.; Cavazos, A.L.; Vargas-Sandoval, M.; Grover, A.; Torres, J.; Medina, R.F.; de León, A.A.; Esteve-Gassent, M.D. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasites Vectors 2014, 7, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán-Cornejo, C.; Sánchez-Montes, S.; Caso, A.; Rendón-Franco, E.; Muñoz-García, C.I. Molecular detection of Rickettsia rickettsii in ticks associated with the bobcat (Lynx rufus) in northeast Mexico. Ticks Tick Borne Dis. 2019, 10, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montes, S.; Fernández-Figueroa, E.; González-Guzmán, S.; Cervantes, V.P.; Ballados-González, G.G.; Rangel-Escareño, C.; Cárdenas-Ovando, R.A.; Becker, I. New records of Haemaphysalis leporispalustris in the Transmexican Volcanic Belt province of Mexico with detection of rickettsial infection. Parasitol. Res. 2020, 119, 1969–1973. [Google Scholar] [CrossRef]
- Panti-May, J.A.; Torres-Castro, M.; Hernández-Betancourt, S.; Dzul-Rosado, K.; Zavala-Castro, J.; López-Avila, K.; Tello-Martín, R. Detection of Rickettsia felis in wild mammals from three municipalities in Yucatan, Mexico. Ecohealth 2015, 12, 523–527. [Google Scholar] [CrossRef]
- López-Pérez, A.M.; Sánchez-Montes, S.; Foley, J.; Guzmán-Cornejo, C.; Colunga-Salas, P.; Pascoe, E.; Becker, I.; Delgado-de la Mora, J.; Licona-Enriquez, J.D.; Suzan, G. Molecular evidence of Borrelia burgdorferi sensu stricto and Rickettsia massiliae in ticks collected from a domestic-wild carnivore interface in Chihuahua, Mexico. Ticks Tick Borne Dis. 2019, 10, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montes, S.; Isaak-Delgado, A.B.; Guzmán-Cornejo, C.; Rendón-Franco, E.; Muñoz-García, C.I.; Bermúdez, S.; Morales-Diaz, J.; Cruz-Romero, A.; Romero-Salas, D.; Dzul-Rosado, K.; et al. Rickettsia species in ticks that parasitize amphibians and reptiles: Novel report from Mexico and review of the worldwide record. Ticks Tick Borne Dis. 2019, 10, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Barraza-Guerrero, S.I.; Meza-Herrera, C.A.; García-de la Peña, C.; González-Álvarez, V.H.; Vaca-Paniagua, F.; Díaz-Velásquez, C.E.; Sánchez-Tortosa, F.; Ávila-Rodríguez, V.; Valenzuela-Núñez, L.M.; Herrera-Salazar, J.C. General microbiota of the soft tick Ornithodoros turicata parasitizing the bolson tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico. Biology 2020, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Colunga-Salas, P.; Sánchez-Montes, S.; Ochoa-Ochoa, L.M.; Grostieta, E.; Becker, I. Molecular detection of the reptile-associated Borrelia group in Amblyomma dissimile, Mexico. Med. Vet. Entomol. 2021, 35, 202–206. [Google Scholar] [CrossRef]
- Theel, E.S.; Kraft, C.S. The past, present, and (possible) future of serologic testing for lyme disease. J. Clin. Microbiol. 2016, 54, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas-Meléndez, J.A.; Avalos-Ramírez, R.; Riojas-Valdez, V.M.; Martínez-Muñoz, A. Serological survey of canine borreliosis. Rev. Latinoam. Microbiol. 1999, 41, 1–3. [Google Scholar]
- Romero-Salas, D.; Mira, A.; Mosqueda, J.; García-Vázquez, Z.; Hidalgo-Ruiz, M.; Vela, N.A.O.; de León, A.A.P.; Florin-Christensen, M.; Schnittger, L. Molecular and serological detection of Babesia bovis and Babesia bigemina-infection in bovines and water buffaloes raised jointly in an endemic field. Vet. Parasitol. 2016, 217, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarz, R.; Mishra, N.; Tagliafierro, T.; Sameroff, S.; Caciula, A.; Chauhan, L.; Patel, J.; Sullivan, E.; Gucwa, A.; Fallon, B.; et al. A multiplex serologic platform for diagnosis of tick-borne diseases. Sci. Rep. 2018, 8, 3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaulhac, B.; Heller, R.; Limbach, F.X.; Hansmann, Y.; Lipsker, D.; Monteil, H.; Sibilia, J.; Piémont, Y. Direct molecular typing of Borrelia burgdorferi Sensu Lato species in synovial samples from patients with lyme arthritis. J. Clin. Microbiol. 2000, 38, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Zhang, M.Z.; Stich, R.W.; Mitchell, W.J.; Zhang, S. Development of a tick-borne pathogen QPCR panel for detection of Anaplasma, Ehrlichia, Rickettsia, and Lyme disease Borrelia in animals. J. Microbiol. Methods 2018, 151, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hojgaard, A.; Lukacik, G.; Piesman, J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick-Borne Dis. 2014, 5, 349–351. [Google Scholar] [CrossRef]
- Modarelli, J.J.; Ferro, P.J.; de León, A.A.P.; Esteve-Gasent, M.D. TickPath Layerplex: Adaptation of a real-time PCR methodology for the simultaneous detection and molecular surveillance of tick-borne pathogens. Sci. Rep. 2019, 9, 6950. [Google Scholar] [CrossRef] [Green Version]
- Buchan, B.W.; Jobe, D.A.; Mashock, M.; Gerstbrein, D.; Faron, M.L.; Ledeboer, N.A.; Callister, S.M. Evaluation of a novel multiplex high-definition PCR assay for detection of tick-borne pathogens in whole-blood specimens. J. Clin. Microbiol. 2019, 57, e00513–e00519. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Githeko, A.K.; McCarty, J.P.; Hussein, S.; Confalonieri, U.; de Wet, N. Climate Change and Infectious Diseases. Available online: www.who.int/globalchange/publications/cchhbook/en/ (accessed on 17 August 2021).
- Araya-Anchetta, A.; Busch, J.D.; Scoles, G.A.; Wagner, D.M. Thirty years of tick population genetics: A comprehensive review. Infect. Genet. Evol. 2015, 29, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible effects of climate change on ixodid ticks and the pathogens they transmit: Predictions and observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef]
- Cuervo-Robayo, A.P.; Ureta, C.; Gómez-Albores, M.A.; Meneses-Mosquera, A.K.; Téllez-Valdés, O.; Martínez-Meyer, E. One hundred years of climate change in Mexico. PLoS ONE 2020, 15, e0209808. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray-Tortarolo, G.N. Seven decades of climate change across Mexico. Atmósfera 2021, 34, 217–226. [Google Scholar] [CrossRef]
- Clarke-Crespo, E.; Moreno-Arzate, C.N.; López-González, C.A. Ecological niche models of four hard tick genera (Ixodidae) in Mexico. Animals 2020, 10, 649. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change. The Physical Science Basis; IPCC: Geneva, Switzerland, 2021; p. 3949. [Google Scholar]
- Titcomb, G.; Allan, B.F.; Ainsworth, T.; Henson, L.; Hedlund, T.; Pringle, R.M.; Palmer, T.M.; Njoroge, L.; Campana, M.G.; Fleischer, R.C. Interacting effects of wildlife loss and climate on ticks and tick-borne disease. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170475. [Google Scholar] [CrossRef] [Green Version]
- Guglielmone, A.; Robbins, R. Tick Species Found Feeding on Humans: A Global Overview; Springer: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Vislobokova, I.A. Ecological evolution of early Cetartiodactyla and reconstruction of its missing initial link. Paleontol. J. 2013, 47, 533–548. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, B. Prospects of using DNA barcoding for species identification and evaluation of the accuracy of sequence databases for ticks (Acari: Ixodida). Ticks Tick-Borne Dis. 2014, 5, 352–358. [Google Scholar] [CrossRef]
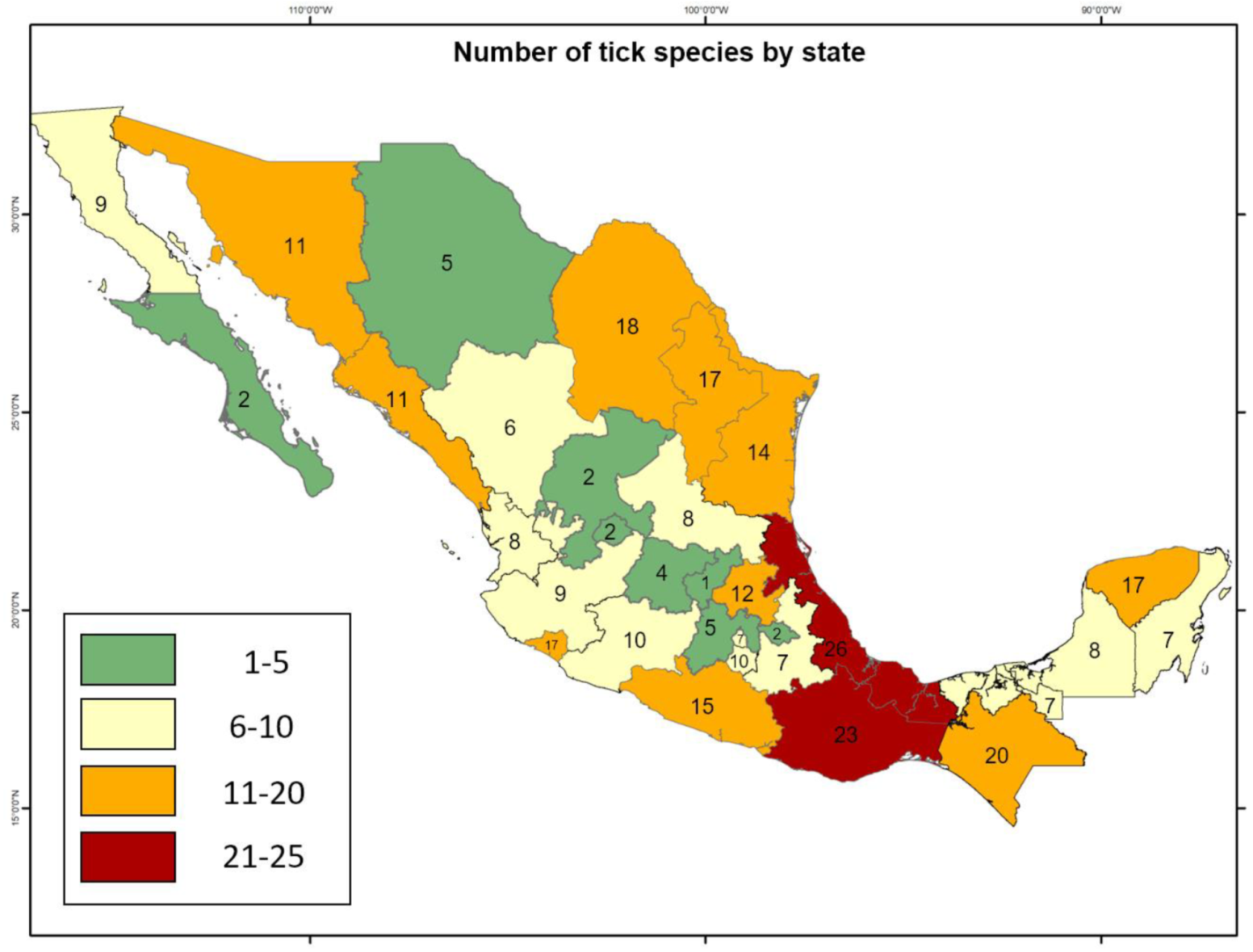
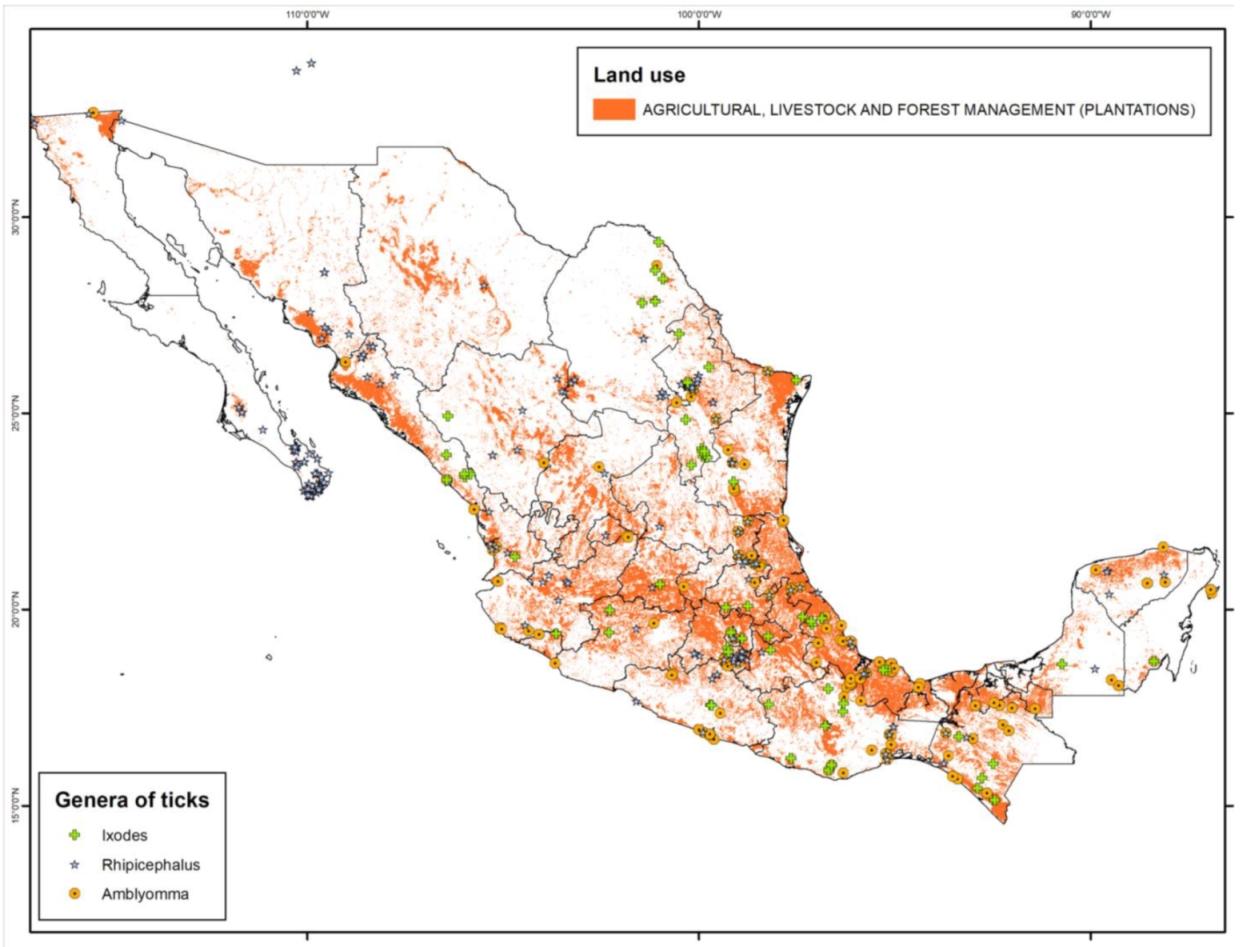
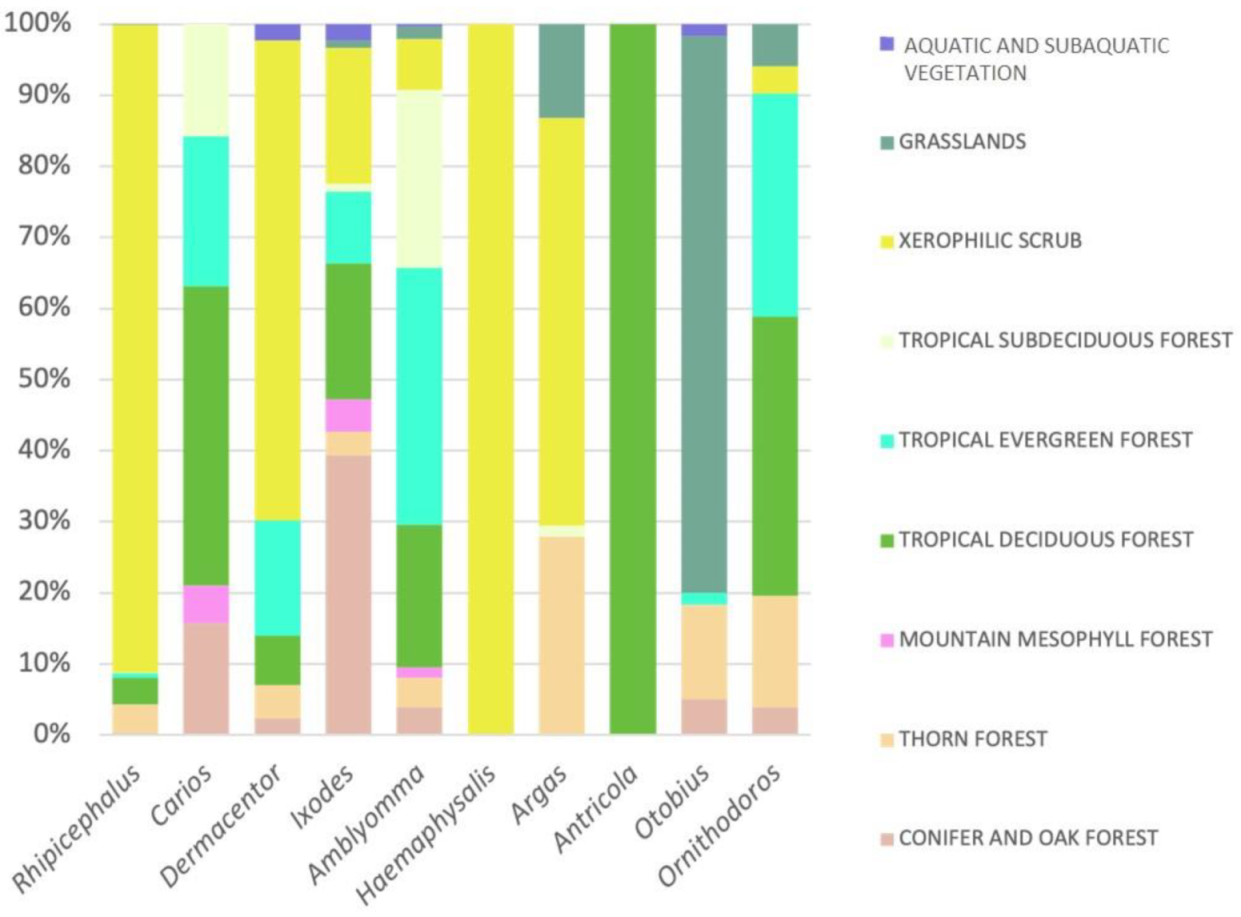
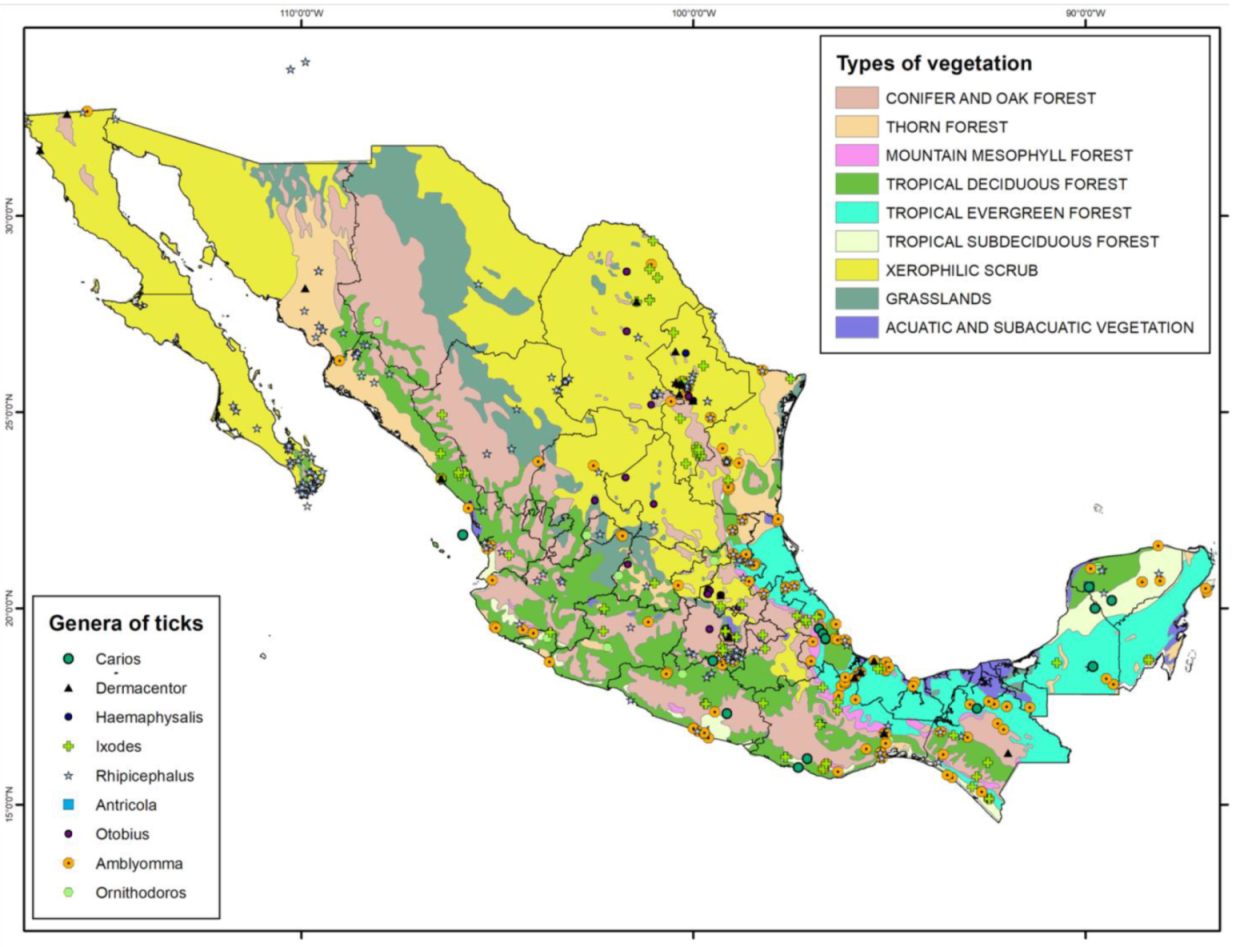
| Pathogen | Pathogen Frequency (%) | Type of Sample | Ticks | Stage | Molecular Detection Technique | Host | State | Reference |
|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi (B. burgdorferi) sensu lato | 40/94 (42.5) | Bladder/ear | - | - | PCR | House mouse (Mus musculus) | Yucatan | [30] |
| B. burgdorferi sensu lato | 5/29 (17.5) | Bladder/ear | - | - | PCR | Rats (Rattus rattus) | ||
| A. phagocytophilum Ehrlichia canis (E. canis) | 48/54 (88.9) 53/59 (89.8) | Serum /blood | - | - | PCR | Mexican deer mouse (Peromyscus mexicanus) | Querétaro | [14] |
| A. phagocytophilum E. canis | 6/54 (11.1) 6/59 (10.1) | Serum /blood | - | Rat (Rattus rattus) | ||||
| Babesia vogeli (B. vogeli) | 11/22 (9) | Blood | - | - | TickPath Layerplex qPCR | Coyote (Canis latrans) | Texas (USA-Mexico border) | [31] |
| Babesia turicate Ehrlichia chaffensis (E. chaffensis) Theileria cervi (T. cervi) Anaplasma platys (A. platys) | 1/122 (0.8) 4/245 (1.6) 18/245 (7.3) 1/245 (0.4) | Blood | - | - | TickPath Layerplex qPCR | White-tailed deer (Odocoileus virginianus) | ||
| Anaplasma odocoilei (A. odocoilei), A. phagocytophilum and E. chaffensis | 5/25 (20) | Spleen/liver | A. mixtum, Amblyomma parvum A. cf. oblongoguttatum, Ixodes affinis, R. microplus and R. sanguineus sensu lato, and Haemaphysalis juxtakochi. | - | White-tailed deer (Odocoileus virginianus yucatensis) | [32] | ||
| A. odocoilei, A. phagocytophilum and E. chaffensis | 2/4 (50) | TickPath Layerplex qPCR, nested PCR | Mazama deer (Mazama temama) | Yucatan | ||||
| B. burgdorferi | 8/25 (29) | - | I. scapularis | - | Eastern cottontail (Sylvilagus floridanus) | Nuevo Léon | [33] | |
| B. burdorferi | 1/1 (100) | - | I. scapularis | PCR | Jaguar (Panthera onca) | Tamaulipas | ||
| B. burdorferi | 1/6 (0.16) | - | I. scapularis | Painted spiny pocket mice (Liomys pictus) | Nuevo León | |||
| R. rickettsii | 3/60 (5) | - | Dermacentor variabilis (D. variabilis) | PCR | Bobcoat (Lynx rufus) | Tamaulipas | [34] | |
| Rickettsia bellii | 1/37 (2.7) | - | Haemaphysalis leporispalustris | Adults | PCR | Rabbits (Lepus sp.) | Hidalgo | [35] |
| Rickettsia felis | 10/23 (43.5) 4/7 (57.1) | Spleen | - | - | PCR | Rodents (Mus musculus, Heteromys gaumeri, Sigmodon hispidus, Olgorizomys sp. Peromyscus yucatanis) Virginiana opposum (Didelphis virginiana) | Yucatan | [36] |
| Rickettsia sp. | 2/16 (12.5) | - | R. sanguineus s.1. | Adults | PCR | Coyote (Canis latrans) | Chihuahua | [37] |
| Borrelia sp. | 1/5 (20) 2/31 (6.4) | - | Ixodes kingi R. sanguineus s.1. | Kit fox (Vulpes macrotis) Free roaming dogs (Canis lupus familiaris) | ||||
| Rickettsia parkeri | 6/22 (27.3) | - | A. ovale | Adults | PCR | Owned ad Free roaming dogs (Canis lupus familiaris) | Veracruz | [38] |
| Coxiella burnetti, A. phagocytophilum, Neorerlichia sp. | Tick pool | Ornithodoros turicata | Massive sequencing | Bolson tortoise (Gopherus flavomarginatus) | Chihuahua, Coahuila, Durango | [39] | ||
| Rickettsia monacensis (R. monacensis) | 3/16 (19.7) 2/14 (14.3) | - | Amblyoma dissimile | Nymphs Adults | PCR | Common boa (Boa constrictor) | Veracruz | [38] |
| R. monacensis R. monacensis | 1/9 (11.1) 1/2 (50) | - | Amblyoma dissimileA. mixtum | Adults Adults | Green iguana (Iguana iguana) | |||
| R. monacensis | 2/15 (13.3) 1/16 (6.3) | - | Amblyoma dissimile | Larvae Nymphs | Marine toad (Rinella marina) | Guerrero | ||
| R. monacensis | 3/25 (12) | - | Amblyoma dissimile | Larvae | Marine toad (Rinella marina) | Tabasco | ||
| Borrelia sp. | 5/60 (8.3) | - | Amblyoma dissimile | Adults | PCR | Mesoamerican canean toad (Rhinella horribilis) | Veracruz | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López González, C.A.; Hernández-Camacho, N.; Aguilar-Tipacamú, G.; Zamora-Ledesma, S.; Olvera-Ramírez, A.M.; Jones, R.W. Gap Analysis of the Habitat Interface of Ticks and Wildlife in Mexico. Pathogens 2021, 10, 1541. https://doi.org/10.3390/pathogens10121541
López González CA, Hernández-Camacho N, Aguilar-Tipacamú G, Zamora-Ledesma S, Olvera-Ramírez AM, Jones RW. Gap Analysis of the Habitat Interface of Ticks and Wildlife in Mexico. Pathogens. 2021; 10(12):1541. https://doi.org/10.3390/pathogens10121541
Chicago/Turabian StyleLópez González, Carlos A., Norma Hernández-Camacho, Gabriela Aguilar-Tipacamú, Salvador Zamora-Ledesma, Andrea M. Olvera-Ramírez, and Robert W. Jones. 2021. "Gap Analysis of the Habitat Interface of Ticks and Wildlife in Mexico" Pathogens 10, no. 12: 1541. https://doi.org/10.3390/pathogens10121541
APA StyleLópez González, C. A., Hernández-Camacho, N., Aguilar-Tipacamú, G., Zamora-Ledesma, S., Olvera-Ramírez, A. M., & Jones, R. W. (2021). Gap Analysis of the Habitat Interface of Ticks and Wildlife in Mexico. Pathogens, 10(12), 1541. https://doi.org/10.3390/pathogens10121541







