Abstract
Hepatitis E virus (HEV) is a common cause of viral hepatitis in humans. In developing countries, HEV-infections seem to be mainly associated with pigs, but other animal species may be involved in viral transmission. Recently, anti-HEV antibodies were detected in Norwegian wild reindeer. Here, we investigated anti-HEV seroprevalence in Norwegian semi-domesticated reindeer, animals in closer contact with humans than their wild counterparts. Blood samples (n = 516) were obtained from eight reindeer herds during the period 2013–2017 and analysed with a commercial enzyme-linked immunosorbent assay designed for detecting anti-HEV antibodies in livestock. Antibodies were found in all herds and for all sampling seasons. The overall seroprevalence was 15.7% (81/516), with adults showing a slightly higher seroprevalence (18.0%, 46/256) than calves (13.5%, 35/260, p = 0.11). The seroprevalence was not influenced by gender or latitude, and there was no temporal trend (p > 0.15). A positive association between the presence of anti-HEV antibodies and antibodies against alphaherpesvirus and pestivirus, detected in a previous screening, was found (p < 0.05). We conclude that Norwegian semi-domesticated reindeer are exposed to HEV or an antigenically similar virus. Whether the virus is affecting reindeer health or infects humans and poses a threat for human health remains unknown and warrants further investigations.
1. Introduction
Hepatitis E virus (HEV) is a single-stranded positive sense RNA virus that belongs to the virus family Hepeviridae. While the icosahedral virions shed in the faeces are non-enveloped, the virions circulating in the blood are cloaked in host cell membranes and are considered to be “quasi-enveloped“ [1]. The HEV species Orthohepevirus A is divided into eight genotypes (GT), and HEV genomes of GT1-4 and GT7 have been detected in humans [2,3]. HEV GT1 and GT2 seem to be restricted to humans and cause sporadic hepatitis and outbreaks in Africa and Asia due to faecal contamination of drinking water [4]. HEV GT3 and GT4 are mainly found in domesticated pigs and wild boars, but have also been detected in camels, deer, rabbits, mongooses, cattle, goats and yaks [2,3]. In industrialized countries, at least in Europe and America, sporadic human infections by GT3 and, to a lesser extent, GT4 are believed to occur by ingestion of undercooked meat or milk, or through close contact with infected animals [5]. HEV GT7 has been found in camels in the Middle East and is shown to infect humans that consume camel milk and meat [6]. Transmission of HEV can also occur via consumption of contaminated berries, vegetables or shellfish [7,8,9], or via blood transfusions [10] or organ transplantations [11], or by vertical transmission from mother to foetus [12]. Recently, some human cases with HEV of species Orthohepevirus C, which usually infects rats, has been reported [13,14,15].
In most healthy individuals, the acute HEV-infection is either asymptomatic or takes a self-limiting course. For those that develop a systemic infection, a short prodromal phase with unspecific symptoms is followed by itching and liver specific symptoms such as jaundice, uncoloured stools and darkened urine [1,16]. The consequence is influenced by the genotype. Infection with HEV GT1 or GT2 may be aggravated for pregnant women as this increases the risk of preterm delivery, low birth weight, stillbirth and maternal death [4,12]. Infection with HEV GT3 or GT4 may lead to acute-on-chronic liver failure (ACLF) in patients with pre-existing liver disease, and to chronic liver infection in immunocompromised individuals such as transplant recipients, individuals infected by human immunodeficiency virus (HIV) and patients with hematologic malignancy undergoing chemotherapy [17]. Importantly, after only a few years of chronic HEV infection, progressive liver fibrosis and cirrhosis is commonly found [18]. In addition to this, HEV may cause a variety of extrahepatic manifestations such as neurologic diseases, acute pancreatitis, membranous glomerulonephritis, acute thyroiditis or hematologic abnormalities [19,20].
In Europe, anti-HEV seroprevalence rates between 0.035% and 60.9% have been reported in humans [21,22]. Two human seroprevalence studies have been performed in Norway. In the first study, 1200 blood donors and 79 swine farm workers were tested for anti-HEV antibodies, and a seroprevalence of 13.5% and 30.4%, respectively, was found [23]. In a population-based study of 1800 adults in Northern Norway, a seroprevalence of 11.4% was detected [24]. Importantly, a European meta-analysis found the highest seroprevalence in individuals exposed to pigs and/or wild animals [25]. As pig farming is not so common in Norway, especially not in the northernmost part, other animals may be more important for transmission to humans. A seroprevalence study of wild ungulates in a neighbouring country, Sweden, detected anti-HEV antibodies in 27.5% (19/69) of wild moose (Alces alces), 15.1% (21/139) of wild boar (Sus scrofa) and in a few roe deer (2/30, Capreolus capreolus) and red deer (1/15, Cervus elaphus) [26]. A similar study in Norway detected anti-HEV antibodies in 19.5% (32/164) of moose and 23.1% (43/186) of wild reindeer (Rangifer t. tarandus) [27], while a study on semi-domesticated reindeer (Rangifer tarandus) in Russia found anti-HEV IgG in 12.1% (23/191) of examined animals [28].
In Norway, there are approximately 25,000 wild reindeer and 220,000 semi-domesticated reindeer [29]. Reindeer herding is mainly conducted by indigenous Sami herders and is of major importance for their livelihood and culture [30]. Although reindeer husbandry is mostly based on natural pastures, the practice of supplementary feeding is increasing, which may increase nose-to-nose contact between the animals and contact between reindeer and humans. While reindeer meat used to be a traditional food mainly used by Sami people, it is now commonly consumed by many people and could potentially be a source for zoonotic HEV transmission in Norway. In the present study, we investigate, for the first time, anti-HEV seroprevalence in semi-domesticated Norwegian reindeer. The animals examined represent the geographically different reindeer herding regions in Norway. The results showed that all reindeer herds had been exposed to HEV and demonstrated an overall yearly seroprevalence fluctuating between 12.9% and 17.5%.
2. Results
2.1. Anti-HEV Seroprevalence in Semi-domesticated Reindeer
In order to analyse serum samples from Norwegian semi-domesticated reindeer, a commercial HEV ELISA intended for the detection of antibodies to HEV in serum of animals was used. An overall seroprevalence of 15.7% (81/516) was found (Table 1). The seroprevalence was slightly higher in adults than in calves, with antibodies detected in 18.0% (46/256) of the adults and 13.5% (35/260) of the calves, but this difference was not statistically significant (p = 0.11) (Table 1). Similarly, there was no significant difference between genders (p = 0.38), with anti-HEV antibodies detected in 15.7% (53/337) of females and 15.6% (28/179) of males. Anti-HEV antibodies were found in samples from all sampling seasons (Table 2) and from all eight herds (Table 1). The seroprevalence in the different herds varied between 6.7% and 33.8% (Table 1), with a median seroprevalence of 12.5%. There was no clear correlation between seroprevalence and latitude, as the highest seroprevalence was detected in animals from the Tromsø region, and the lowest seroprevalence was found in animals in the nearby Lødingen region (Table 1, Figure 1 and Figure 2A). The anti-HEV seroprevalence for each of the sampling seasons varied from 16.9% in 2013 to 17.5% in 2017, with no clear temporal trend (Table 2, Figure 2B). Of note, the number of samples included per year varied from 177 samples in 2013 to only 40 samples in 2017 (Table 2). We conclude that semi-domesticated reindeer herds representing the different reindeer herding regions of Norway are exposed to HEV.

Table 1.
Anti-HEV seropositive animals in different herds of semi-domesticated reindeer.

Table 2.
Seroprevalence of HEV in semi-domesticated reindeer per year.
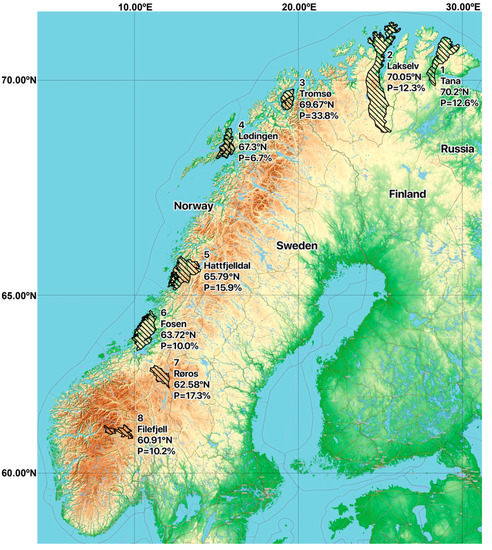
Figure 1.
Map showing the geographical pasture positions of the eight sampled Norwegian herds of semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus) that were screened for the presence of anti-HEV antibodies (n = 516; 2013–2017). The anti-HEV seroprevalence found for each herd is shown. Map created using the Free and Open Source QGIS. Map data: ©OpenStreetMap-Mitwirkende, SRTM | Map position: ©OpenTopoMap (CC-BY-SA). Reindeer pastures data source: NIBIO.
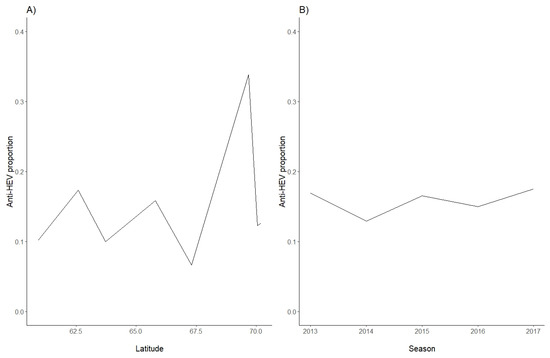
Figure 2.
Seroprevalence proportions in Norwegian semi-domesticated reindeer in herds, sorted by (A) latitude and (B) sampling season (2013–2017).
2.2. Seroprevalence of Previously Examined Viruses
As some of the serum samples had recently been found to contain antibodies against pestivirus, alphaherpesvirus (i.e., Cervid herpesvirus 2; CvHV2) and/or gammaherpes-virus (viruses in the malignant catarrhal fever group; MCFV) [31], we investigated if there was an association between the HEV serostatus of a sample and the presence of antibodies against the previously investigated viruses. Overall, only small differences of 1.2–1.5-fold were detected between HEV-seropositive and HEV-seronegative animals for the three viruses (Table 3). A multiple correspondence analysis showed that the serostatus for pestivirus and HEV was correlated (chi-square test, p = 0.01), as well as for HEV and CvHV2 (chi-squared test, p = 0.05). The presence of gammeherpesvirus-antibodies, on the other hand, was not significantly associated with the presence of HEV-antibodies. We conclude that there was a correlation between being seropositive for HEV and pestivirus, and also between being seropositive for HEV and CvHV2.

Table 3.
Seroprevalence of other viruses in HEV-seronegative and HEV-seropositive semi-domesticated reindeer.
3. Discussion
This is the first investigation of anti-HEV seroprevalence in semi-domesticated reindeer in Fennoscandia. The study revealed that infections by HEV, or an antigenically similar virus, are common in semi-domesticated reindeer living from latitude 60.91° N to 70.2° N in Norway. Anti-HEV antibodies were found in samples from all sampling years and in all herds examined. The total seroprevalence was 15.7%, with only minor yearly variation. This is slightly lower than the 23.1% reported for Norwegian wild reindeer, the latter mainly living around latitude 58.4° N to 62.4° N and sampled during the period 2010–2018 [27]. In contrast, the seroprevalence in semi-domesticated reindeer was slightly higher than the 12.1% reported for semi-domesticated reindeer sampled during the period 2018–2019 in eastern Russia [28]. While the Norwegian study of wild reindeer was based on the same ELISA as used in the present study, an ELISA detecting anti-HEV IgM, IgA and IgG, the Russian study used an ELISA only detecting anti-HEV IgG, which may indicate that these screenings are not necessarily directly comparable. Finally, comparing our results with the results of a recently published study on the same samples, addressing other viruses [31], the serostatus for HEV and pestivirus and HEV and CVHV2 were found to correlate.
The seroprevalence in adults was only slightly higher than in calves, with 18.0% versus 13.5%, respectively. This pattern has also been revealed in humans, where anti-HEV seroprevalence increase with age [24,25]. The results presented herein suggest that the majority of reindeer are infected relatively early in life, similar to what is reported for HEV in pigs. Pigs become infected from about seven weeks of age, when maternal antibody titres are declining, and HEV excretion is highest when they are 3–5 months of age [32]. Our results further suggest that the anti-HEV antibodies in reindeer are rather long-lasting, as we still detect antibodies in adult animals. Alternatively, the antibodies wane over time, but some animals undergo reinfection, as previously described for humans [33]. Another possible explanation for the relatively small difference in seroprevalence among calves and adult reindeer could be that many of the reindeer defined as adults (i.e., >1 year), were quite young. At sampling in October–April, the adults were 17 months or older. Unfortunately, we do not know the exact age as this was not determined. In humans, the persistence and protective role of anti-HEV antibodies is still unclarified. Anti-HEV IgG levels seem to steadily decline, but have in some cases been reported to persist up to 12 years after infection [34,35].
In contrast to humans, ungulates do not actively transfer immunoglobulins from the mother to the developing foetus. Instead, the new-borns acquire maternal immuno-globulins, including IgG, IgM, IgA and IgE, during the first colostrum feeding, and this is freely diffusing from the gastrointestinal tract to the blood stream during the first 1–2 days following birth [36]. Because the calves were already between five to 10 months old at sampling, we can exclude that the measurable amounts of anti-HEV antibodies at sampling was a result from maternal antibody transmission through colostrum.
The seroprevalence of the different herds varied from 6.7% to 33.8%, with a median value of 12.5%. It is not clear why the seroprevalence in Tromsø was much higher than in the other herds, especially because Tromsø had the lowest proportion of adults, only 17.6% (all females), versus 82.4% calves. For the other herds, the proportion of adults varied from 35.4% to 69.4%. A high population density and the gathering of wild ungulates at feeding spots have been suggested as general risk factors for infectious diseases [37]. All reindeer in our study were free ranging, but all herds except the two southernmost herds (Røros and Filefjell) obtained some supplementary feeding, thereby potentially increasing the risk of virus transmission.
In five of the herds (Tana, Tromsø, Lødingen, Hattfjelldal and Røros), the serostatus of the animals for pestivirus and CvHV2 seemed to correlate with the serostatus for HEV. The mechanisms for co-infection or potential facilitation are unclear. Detection of antibodies against different viruses in the same serum sample may be due to co-infections or infections separated in time. Co-infections of HEV and Porcine reproductive and respiratory syndrome virus (PRRSV) and HEV and Porcine circovirus type 2 (PCV2) in pigs have been reported [38]. As both PRRSV and PCV2 are immunomodulating pathogens, the co-infections were associated with a longer period of HEV shedding. If the reindeer had co-infections with HEV and pestivirus or HEV and CvHV2, this may have influenced HEV infection, transmission and possibly pathogenesis. So far, we do not know if reindeer are clinically affected by HEV-infection. Of note, post-mortem examination of experimentally HEV-infected pigs without evident clinical disease, detected multifocal lymphoplasmacytic hepatitis and focal necrotic hepatocytes consistent with mild viral hepatitis, demonstrating HEV-induced pathology [39].
Because the seroprevalence of HEV in reindeer was quite stable over the sampling seasons investigated (2013–2017), HEV seems to be enzootic in reindeer. Originally transmission of HEV to semi-domesticated reindeer may have occurred by indirect or direct contact with other infected animals. Because HEV is non-enveloped outside the body, it is stable and will stay infectious in water and soil for a long time after shedding. Wild boar have been suggested as the most important wildlife animal reservoir [40,41]. Only one to two decades ago there were no wild boar in Norway, but animals have crossed the border from Sweden in the south and today 400–1200 animals are estimated to be living in the south-eastern part of Norway. Some of them are infected by HEV, as shown by the detection of anti-HEV (1/86) in a recent study [42]. Although stray wild boars occasionally may be in the same area as herd 7 (Røros), they have never been observed in the areas close to the other herds and therefore probably played no important role in the transmission of HEV to reindeer. Several studies from continental Europe show that HEV infect rabbits, hares and rats [43,44]. Hares and rats could potentially have been involved in the transmission of HEV to semi-domesticated reindeer.
We cannot exclude the possibility that humans may have infected the semi-domesticated reindeer; however, this is less likely. From studies in pigs, we know that the shedding of HEV in faeces typically lasts 9.7–23.3 days [38], giving infected animals good opportunities to spread the virus to other animals. At this point, we cannot explain why only a relatively small number of animals in each herd appear to become infected.
An important question is whether HEV infecting semi-domesticated reindeer can infect humans, similar to what has been reported for HEV transmission from Sika deer (Cervus nippon) to humans [5]. In order to answer this, it is crucial to isolate HEV from reindeer, sequence the HEV genomes and compare this to HEV genomes detected in humans. In the study of semi-domesticated reindeer in Russia, there were unsuccessful attempts to detect HEV RNA in serum, and no other samples were investigated [28]. However, the authors noted that the seroprevalence in reindeer herders was similar to the seroprevalence of other adults in the area, suggesting that occupational infection did not play a major role. With the exception of reindeer herders, most people are seldom in direct contact with reindeer. In Norway, approximately 1100 tons of reindeer meat is consumed each year. Transmission could possibly occur via consumption of undercooked or raw meat (e.g., roasted, dried, or prepared as carpaccio) or dried/non-heated reindeer liver, the latter being marketed as a food supplement. An internal temperature of 71 °C for 20 minutes is apparently needed to inactivate infectious HEV [45]. In addition to the consumption of meat or liver, handling of the carcass may potentially lead to zoonotic infections, as butchers and slaughterhouse workers are reported to have an increased anti-HEV seroprevalence compared to other people in the same area (reviewed in [46]). In addition, faecal contamination of berries, mushrooms or drinking water could transmit the virus to humans.
In most identified human cases of HEV-infection, the source of infection remains unknown. Our finding of anti-HEV antibodies in serum from 15.7% of semi-domesticated reindeer suggest that HEV, or an antigenically similar virus, have infected these animals. So far, we have no indication that HEV is pathogenic for these animals. In order to find out if this virus may infect humans, a molecular characterization of HEV RNA from infected semi-domestic reindeer is urgently needed. Furthermore, a HEV-seroprevalence analysis of reindeer herders is required.
4. Materials and Methods
4.1. Animals and Sampling
Eight different semi-domesticated reindeer herds were sampled during late fall and winter (October–April) in two to five consecutive winter seasons, during the period 2013–2017 (i.e., the winters of 2013–2014, 2014–2015, and so forth). The herds were distributed in Norway from latitude 60.91° N to 70.2° N, with five of the herds (Tana, Lakselv, Tromsø, Lødingen and Hattfjelldal) in Northern Norway, two of the herds (Fosen and Røros) in Central Norway and one herd (Filefjell) in Eastern Norway (Figure 1). Whenever possible, serum samples were obtained from both calves (5–10 months old; both sexes) and adult females (>17 months old) from each of the eight herds, whereas adult bulls were sampled to a lesser extent due to restricted availability. A total of 516 serum samples from 256 adults (49.6%) (220 females and 36 males) and 260 calves (50.4%) (124 female and 136 males) were included (Table 4). The samples were mainly obtained from live animals during routinely herding practices, where the animals were released back into the herd after sampling (444/516; 86.0%), but also from dead animals during slaughter (72/516; 14.0%) (Table 5). Blood samples were collected from the jugular vein in blood tubes (BD Vacutainer®; BD, Plymouth, UK), using a venoject needle (Terumo, Leuven, Belgium) for live animals, or by collecting blood directly into open tubes during bleeding of slaughtered animals. Serum was prepared by centrifugation (10 min, 3000 × g) and stored at –20 °C until further analyses. The serum had been thawed a few times prior to the HEV antibody analysis for the previous seroprevalence study of alphaherpesvirus, gammaherpesvirus, pestivirus, bluetongue virus and Schmallenberg virus [31].

Table 4.
Semi-domesticated reindeer included in the study.

Table 5.
Sampling of semi-domesticated reindeer.
4.2. Serology
Serum samples (n = 516) were analysed for anti-HEV antibodies using a commercial double antigen sandwich enzyme-linked immunosorbent assay (ELISA) (HEV ELISA 4.Ov, MP Diagnostics, Eschwege, Germany), intended for the detection of antibodies to HEV in serum or plasma from swine and other animals such as wild boar, red deer and reindeer [27,47,48]. This ELISA uses a proprietary recombinant antigen, which is highly conserved between different HEV genotypes, to detect specific HEV-antibodies, including IgG, IgM and IgA. Specimens with absorbance values greater than or equal to the cut-off value (0,20 absorbance unit plus the mean absorbance of a negative control) were considered initially reactive but were retested in duplicate before interpretation. Only samples found to be reactive on retesting, were interpreted to contain anti-HEV antibodies.
4.3. Statistical Analyses
Seroprevalence of anti-HEV antibodies were analysed using generalized linear models in R 4.1.0 [49] with a binomial outcome (negative/positive result in the test, coded as 0 or 1, respectively) and a logit link. Each individual’s age group (calf or adult), latitude, sex and sampling season were used as explanatory variables; the significance level was set to p < 0.05 for the explanatory variables. In order to discover a relation between the HEV serostatus and the serostatus of the previously investigated viruses [31], we performed a multiple correspondence analysis [50] using year, latitude, sex, age and the serostatus for these viruses as contrasts. Apparent correlations were further studied with Chi-squared tests to conclude if there was a correlation between the different infections.
Author Contributions
C.H.R., M.T., F.J.A.M. and E.M.B. designed the study. I.H.N., J.S.R. and M.T. obtained reindeer samples. E.M.B. conducted the serology analysis. All authors contributed to the interpretation of results. C.H.R. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by grants from the Norwegian Reindeer Development Fund (RUF; “Climate and reindeer diseases”) and by FRAM–High North Research Centre for Climate and the Environment (The Fram Centre), Effects of climate change on terrestrial ecosystems, landscapes, society, and indigenous peoples (grant number 362256).The Open Access publication charges for this article have been covered by UiT’s Open Access Agreement with academic publishers.
Institutional Review Board Statement
Ethical review and approval were waived for this study, since samples were obtained from slaughtered/dead animals or during a general health surveillance of the herds when animals were gathered and handled for other herding purposes. The study was therefore not classified as an animal experiment.
Informed Consent Statement
Consent was obtained from the owners for the participation of their animals in this study.
Data Availability Statement
The datasets presented in this article are not readily available because the data on which the article is based on contains personal data on identifiable reindeer herders and their animals. Requests to access the dataset should be directed to the corresponding authors.
Acknowledgments
We thank reindeer herders for their patience and assistance during the sampling of reindeer, and Emily Magnusson, Torill Mørk, Anett K. Larsen, Jörn Klein, Johan Schultze, Mattis Mørk Tryland, Roy Egil Hansen and Inga Bendiksen for assisting in the field.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Prim. 2017, 3, 17086. [Google Scholar] [CrossRef]
- Smith, D.B.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van Der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Nicot, F.; Dimeglio, C.; Migueres, M.; Jeanne, N.; Latour, J.; Abravanel, F.; Ranger, N.; Harter, A.; Dubois, M.; Lameiras, S.; et al. Classification of the Zoonotic Hepatitis E Virus Genotype 3 Into Distinct Subgenotypes. Front. Microbiol. 2020, 11, 634430. [Google Scholar] [CrossRef]
- Horvatits, T.; Schulze zur Wiesch, J.; Lütgehetmann, M.; Lohse, A.W.; Pischke, S. The Clinical Perspective on Hepatitis E. Viruses 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection with Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maunula, L.; Kaupke, A.; Vasickova, P.; Söderberg, K.; Kozyra, I.; Lazic, S.; van der Poel, W.H.; Bouwknegt, M.; Rutjes, S.; Willems, K.A.; et al. Tracing enteric viruses in the European berry fruit supply chain. Int. J. Food Microbiol. 2013, 167, 177–185. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, Z.; Crossan, C.; Craft, J.; Scobie, L. First Report of the Presence of Hepatitis E Virus in Scottish-Harvested Shellfish Purchased at Retail Level. Food Env. Virol. 2018, 10, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terio, V.; Bottaro, M.; Pavoni, E.; Losio, M.N.; Serraino, A.; Giacometti, F.; Martella, V.; Mottola, A.; Di Pinto, A.; Tantillo, G. Occurrence of hepatitis A and E and norovirus GI and GII in ready-to-eat vegetables in Italy. Int. J. Food Microbiol. 2017, 249, 61–65. [Google Scholar] [CrossRef]
- Hewitt, P.E.; Ijaz, S.; Brailsford, S.R.; Brett, R.; Dicks, S.; Haywood, B.; Kennedy, I.; Kitchen, A.; Patel, P.; Poh, J.; et al. Hepatitis E virus in blood components: A prevalence and transmission study in southeast England. Lancet 2014, 384, 1766–1773. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Juarez, A.; Aguado, R.; Lopez-Lopez, P.; Sanchez-Frias, M.; Frias, M.; Briceño, J.; de la Mata, M.; Torre-Cisneros, J.; Rivero, A. Prevalence of hepatitis E virus infection in liver donors in Spain. Clin. Microbiol. Infect. 2018, 24, 1218–1219. [Google Scholar] [CrossRef] [Green Version]
- Bigna, J.J.; Modiyinji, A.F.; Nansseu, J.R.; Amougou, M.A.; Nola, M.; Kenmoe, S.; Temfack, E.; Njouom, R. Burden of hepatitis E virus infection in pregnancy and maternofoetal outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.; Leung, K.H.; Chung, T.W.; Chan, J.F.W.; Chan, W.M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschaumbault, Y.; Murnaghan, K.; Varga, J.; Johnston, B. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Yip, C.C.; Wu, S.; Chew, N.F.; Leung, K.H.; Chan, J.F.; Zhao, P.S.; Chan, W.M.; Poon, R.W.; Tsoi, H.W.; et al. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology 2021, 73, 10–22. [Google Scholar] [CrossRef]
- Hartl, J.; Wehmeyer, M.H.; Pischke, S. Acute Hepatitis E: Two Sides of the Same Coin. Viruses 2016, 8, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamar, N.; Rostaing, L.; Abravanel, F.; Izopet, J. How Should Hepatitis E Virus Infection Be Defined in Organ-Transplant Recipients? Arab. Archaeol. Epigr. 2013, 13, 1935–1936. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; van Eijk, J.J.J.; Cintas, P.; Madden, R.G.; Jones, C.; Webb, G.W.; Norton, B.; Pique, J.; Lutgens, S.; Devooght-Johnson, N.; et al. Hepatitis E virus infection and acute non-traumatic neurological injury: A prospective multicentre study. J. Hepatol. 2017, 67, 925–932. [Google Scholar] [CrossRef]
- Wu, J.; Xiang, Z.; Zhu, C.; Yao, Y.; Bortolanza, M.; Cao, H.; Li, L. Extrahepatic manifestations related to hepatitis E virus infection and their triggering mechanisms. J. Infect. 2021, 83, 298–305. [Google Scholar] [CrossRef]
- Aspinall, E.J.; Couturier, E.; Faber, M.; Said, B.; Ijaz, S.; Tavoschi, L.; Takkinen, J.; Adlhoch, C. On Behalf of the Country Experts. Hepatitis E virus infection in Europe: Surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Euro Surveill. 2017, 22, 30561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bura, M.; Łagiedo-Żelazowska, M.; Michalak, M.; Sikora, J.; Mozer-Lisewska, I. Comparative Seroprevalence of Hepatitis A and E Viruses in Blood Donors from Wielkopolska Region, West-Central Poland. Pol. J. Microbiol. 2018, 67, 113–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, H.; Øverbø, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis E in Norway: Seroprevalence in humans and swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsøy, I.B.; Henriksen, S.; Weissbach, F.H.; Larsen, M.; Borgen, K.; Abravanel, F.; Kamar, N.; Paulssen, E.J.; Hirsch, H.H.; Rinaldo, C.H. Seroprevalence of hepatitis E virus (HEV) in a general adult population in Northern Norway: The Tromsø study. Med. Microbiol. Immunol. 2019, 208, 715–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartl, J.; Otto, B.; Madden, R.G.; Webb, G.; Woolson, K.L.; Kriston, L.; Vettorazzi, E.; Lohse, A.W.; Dalton, H.R.; Pischke, S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses 2016, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.; Lin, J.; Magnius, L.; Karlsson, M.; Belák, S.; Widén, F.; Norder, H. Markers for Ongoing or Previous Hepatitis E Virus Infection Are as Common in Wild Ungulates as in Humans in Sweden. Viruses 2016, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Sacristán, C.; Madslien, K.; Sacristán, I.; Klevar, S.; das Neves, C.G. Seroprevalence of Hepatitis E Virus in Moose (Alces alces), Reindeer (Rangifer tarandus), Red Deer (Cervus elaphus), Roe Deer (Capreolus capreolus), and Muskoxen (Ovibos moschatus) from Norway. Viruses 2021, 13, 224. [Google Scholar] [CrossRef]
- Slukinova, O.S.; Kyuregyan, K.K.; Karlsen, A.A.; Potemkin, I.A.; Kichatova, V.S.; Semenov, S.I.; Stepanov, K.M.; Rumyantseva, T.D.; Mikhailov, M.I. Serological Evidence of Hepatitis E Virus Circulation Among Reindeer and Reindeer Herders. Vector Borne Zoonotic Dis. 2021, 21, 546–551. [Google Scholar] [CrossRef]
- Hætta, L.B. Ressursregnskap for Reindriftsnæringen Reindriftsåret 1. april 2019–31. mars 2020; (Report nr 43/2020 10.12.2020); Retrived from Research on Landbruksdirektoratet.no Website. Available online: https://www.landbruksdirektoratet.no/nb/filarkiv/rapporter/Ressursregnskapet%20for%20reindriftsn%C3%A6ringen_2019-2020_ny%20versjon.pdf/_/attachment/inline/e0377ef7-bc0e-4538-b294-0b238f41a57e:7085e4f9664f4d9b6c4e028cb5c5659581c7c982/Ressursregnskapet%20for%20reindriftsn%C3%A6ringen_2019-2020_ny%20versjon.pdf (accessed on 25 September 2021).
- Riseth, J.Å.; Tømmervik, H.; Forbes, B.C. Sustainable and Resilient Reindeer Herding. In Reindeer and Caribou—Health and Disease; Tryland, M., Kutz, S., Eds.; CRC Press—Taylor & Francis: Boca Raton, FL, USA, 2019; pp. 23–43. [Google Scholar]
- Tryland, M.; Romano, J.S.; Nymo, I.H.; Breines, E.M.; Murguzur, F.J.A.; Kjenstad, O.C.; Li, H.; Cunha, C.W. A Screening for Virus Infections in Eight Herds of Semi-domesticated Eurasian Tundra Reindeer (Rangifer tarandus tarandus) in Norway, 2013–2018. Front. Veter Sci. 2021, 8, 707787. [Google Scholar] [CrossRef]
- Casas, M.; Cortés, R.; Pina, S.; Peralta, B.; Allepuz, A.; Cortey, M.; Casal, J.; Martín, M. Longitudinal study of hepatitis E virus infection in Spanish farrow-to-finish swine herds. Vet Microbiol. 2011, 148, 27–34. [Google Scholar] [CrossRef]
- Lin, X.-N.; Lin, Q.-X.; Li, S.-M.; Xie, K.-P.; Hou, J.; Chen, R. Hepatitis E virus re-infection accelerates hepatocellular carcinoma development and relapse in a patient with liver cirrhosis: A case report and review of literature. World J. Hepatol. 2020, 12, 1358–1366. [Google Scholar] [CrossRef]
- Chadha, M.S.; Walimbe, A.M.; Arankalle, V.A. Retrospective serological analysis of hepatitis E patients: A long-term follow-up study. J. Viral Hepatol. 1999, 6, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Krain, L.J.; Nelson, K.E.; Labrique, A.B. Host Immune Status and Response to Hepatitis E Virus Infection. Clin. Microbiol. Rev. 2014, 27, 139–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolles, A.E.; Beechler, B.R.; Dolan, B.P. Beyond mice and men: Environmental change, immunity and infections in wild ungulates. Parasite Immunol. 2015, 37, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Fons, F.; Vidal, D.; Vicente, J.; Acevedo, P.; Fernández-de-Mera, I.G.; Montoro, V.; Gortázar, C. Epidemiological risk factors of Aujeszk™ s disease in wild boars (Sus scrofa) and domestic pigs in Spain. Eur. J. Wildl. Res. 2008, 54, 549–555. [Google Scholar] [CrossRef]
- Meester, M.; Tobias, T.J.; Bouwknegt, M.; Kusters, N.E.; Stegeman, J.A.; van der Poel, W.H.M. Infection dynamics and persistence of hepatitis E virus on pig farms—A review. Porc. Health Manag. 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative Pathogenesis of Infection of Pigs with Hepatitis E Viruses Recovered from a Pig and a Human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anheyer-Behmenburg, H.E.; Szabo, K.; Schotte, U.; Binder, A.; Klein, G.; Johne, R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect. Dis. 2017, 23, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Boadella, M. Hepatitis E in wild ungulates: A review. Small Rumin. Res. 2015, 128, 64–71. [Google Scholar] [CrossRef]
- Grøntvedt, C.A.; Madslien, K.; Nordstoga, A.; Hamnes, I.S.; Bergsjø, B.; Urdahl, A.M.; Slettemeås, J.S.; Norström, M.; Danielsen, A.V.; Welde, H.; et al. Surveillance of Wild Boar Health in Norway—Results from 2018 and 2019; Contract No.: Report 14–2020; Norwegian Veterinary Institute: Oslo, Norway, 2020. [Google Scholar]
- Niendorf, S.; Harms, D.; Hellendahl, K.; Heuser, E.; Böttcher, S.; Jacobsen, S.; Bock, C.-T.; Ulrich, R. Presence and Diversity of Different Enteric Viruses in Wild Norway Rats (Rattus norvegicus). Viruses 2021, 13, 992. [Google Scholar] [CrossRef]
- Corman, V.M.; Hilgensloh, L.; Voigt, U.; Marklewitz, M.; Siebert, U.; Drosten, C.; Drexler, J.F. Hepatitis E Virus Infection in European Brown Hares, Germany, 2007–2014. Emerg. Infect. Dis. 2019, 25, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal Inactivation of Infectious Hepatitis E Virus in Experimentally Contaminated Food. Appl. Env. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrzljak, A.; Balen, I.; Barbic, L.; Ilic, M.; Vilibic-Cavlek, T. Hepatitis E virus in professionally exposed: A reason for concern? World J. Hepatol. 2021, 13, 723–730. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Lodder-Verschoor, F.; Lodder, W.J.; van der Giessen, J.; Reesink, H.; Bouwknegt, M.; de Roda Husman, A.M. Seroprevalence and molecular detection of hepatitis E virus in wild boar and red deer in The Netherlands. J. Virol Methods. 2010, 168, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound. Emerg. Dis. 2017, 64, 764–773. [Google Scholar] [CrossRef]
- RCoreTeam. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria, 2018. Available online: https://www.R-project.org/ (accessed on 25 September 2021).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).