Molecular Detection of Tick-Borne Pathogens in American Bison (Bison bison) at El Uno Ecological Reserve, Janos, Chihuahua, Mexico
Abstract
1. Introduction
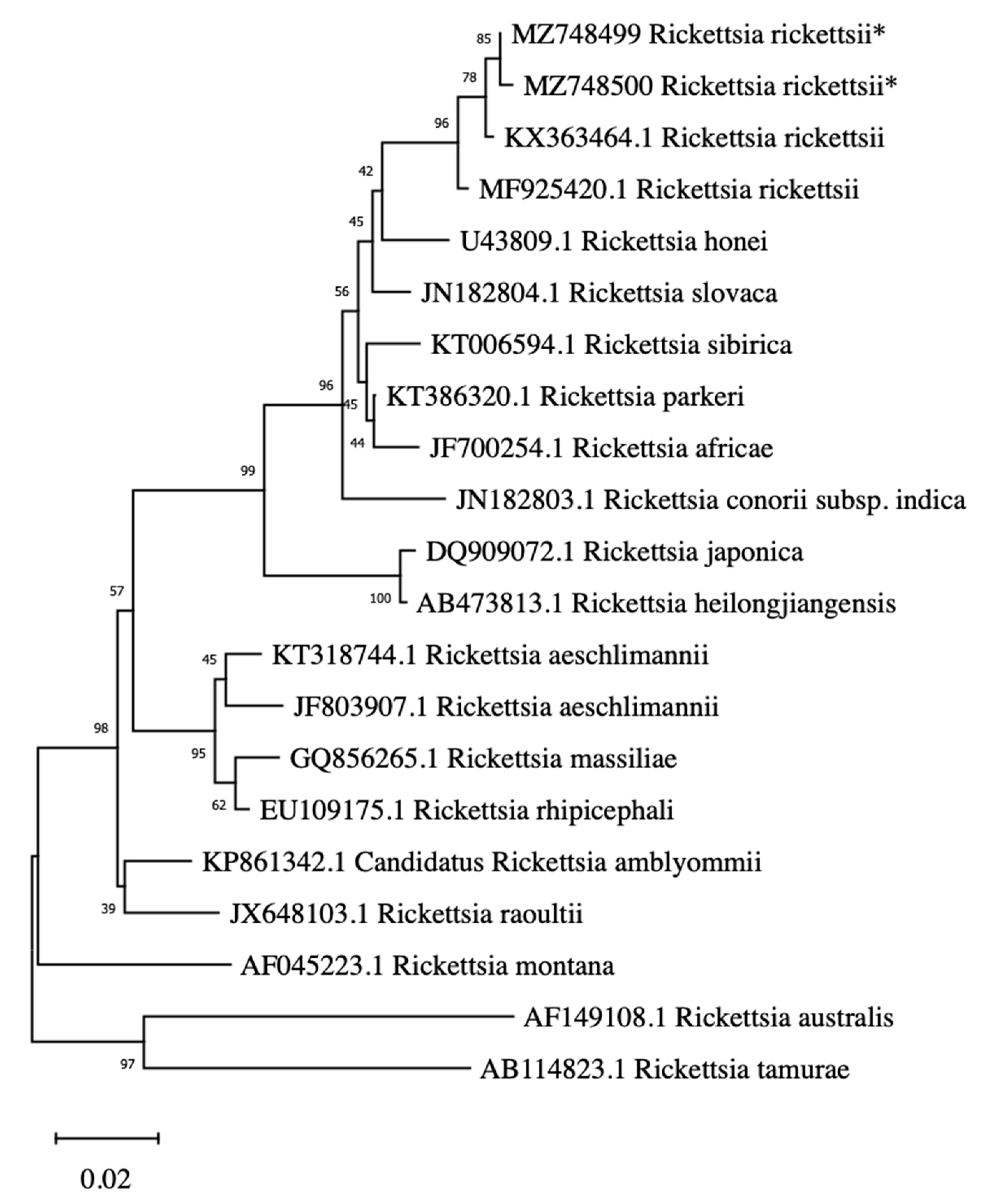
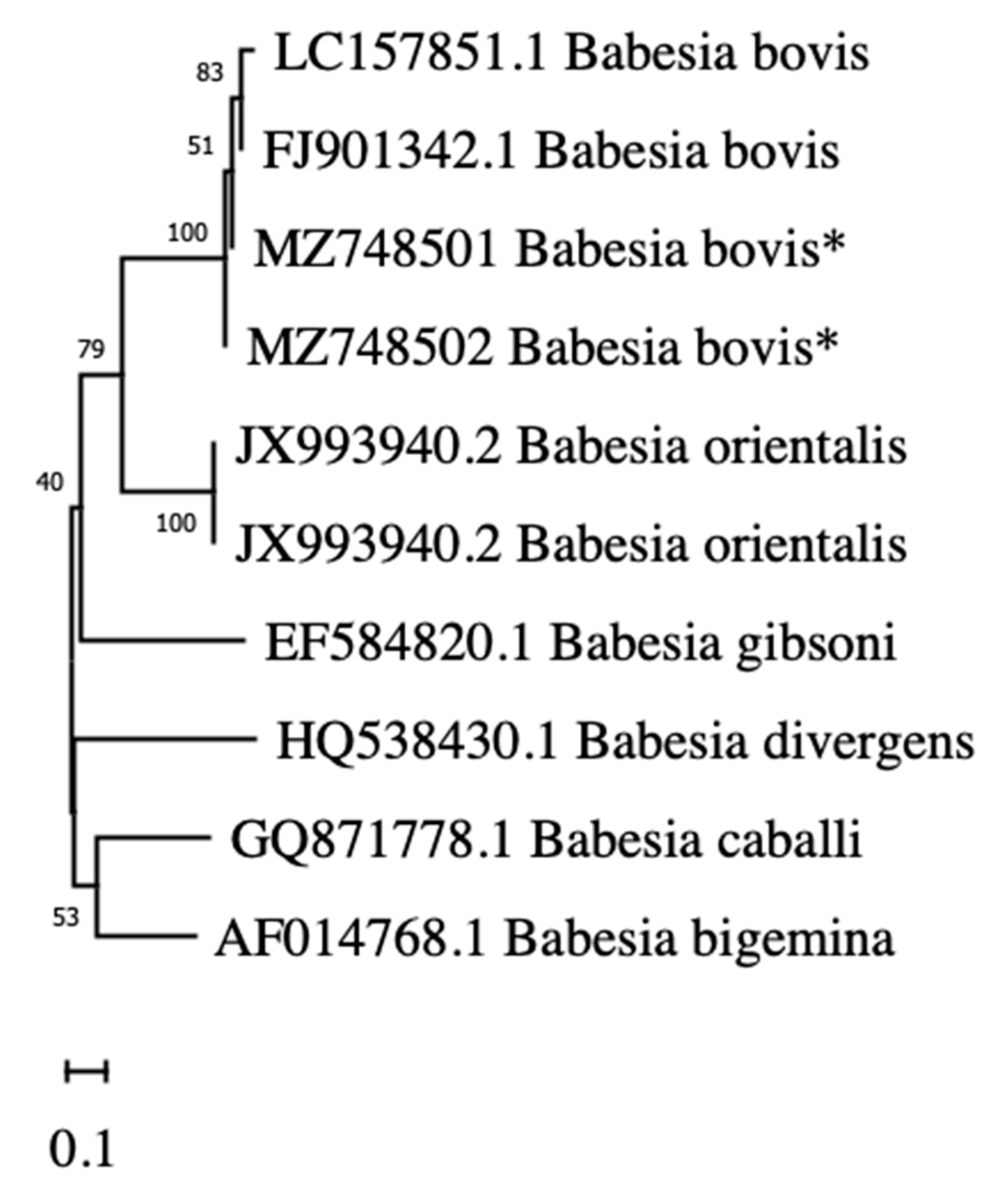
2. Results
3. Discussion
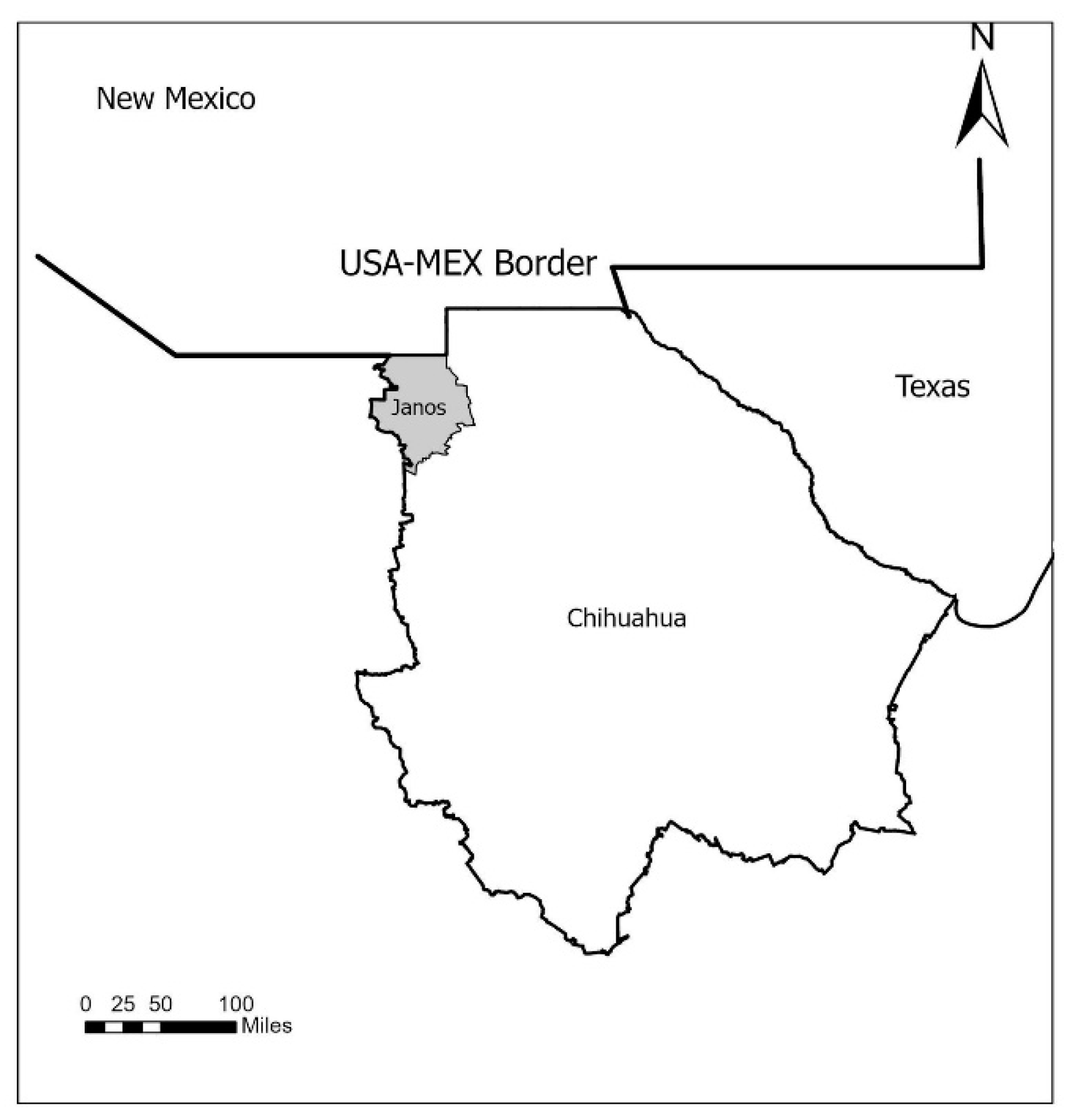
4. Materials and Methods
4.1. Genomic DNA Extraction and Nested PCR
4.2. DNA Sequencing
4.3. Phylogenetic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección Ambiental—Especies Nativas de México de Flora y Fauna Silvestres—Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio—Lista de Especies en Riesgo. Available online: https://dof.gob.mx/nota_detalle_popup.php?codigo=5173091 (accessed on 16 June 2021).
- Boyd, D.P. Conservation of North American Bison: Status and Recommendations. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2003. [Google Scholar]
- Bush-Romero, P. México y África Desde la Mira de mi Rifle, 1950, 1st ed.; Vado, I., Ed.; Grafica Panamericana: Ciudad de Mexico, Mexico, 1958; p. 326. [Google Scholar]
- White, P.; Wallen, R.L.; Geremia, C.; Treanor, J.J.; Blanton, D.W. Management of Yellowstone bison and brucellosis transmission risk—Implications for conservation and restoration. Biol. Conserv. 2011, 144, 1322–1334. [Google Scholar] [CrossRef]
- Newmark, W.D. Isolation of African protected areas. Front. Ecol. Environ. 2008, 6, 321–328. [Google Scholar] [CrossRef]
- Krajewska-Wędzina, M.; Olech, W.; Kozińska, M.; Augustynowicz-Kopeć, E.; Weiner, M.; Szulowski, K. Bovine tuberculosis outbreak in farmed American bison (Bison bison) in Poland. Pol. J. Vet. Sci. 2017, 20, 819–821. [Google Scholar] [PubMed]
- López-Pérez, A.M.; Sánchez-Montes, S.; Foley, J.; Guzmán-Cornejo, C.; Colunga-Salas, P.; Pascoe, E.; Becker, I.; la Mora, J.D.-D.; Licona-Enriquez, J.D.; Suzán, G. Molecular evidence of Borrelia burgdorferi sensu stricto and Rickettsia massiliae in ticks collected from a domestic-wild carnivore interface in Chihuahua, Mexico. Ticks Tick-Borne Dis. 2019, 10, 1118–1123. [Google Scholar] [CrossRef]
- Sánchez-Montes, S.; López-Pérez, A.M.; Guzmán-Cornejo, C.; Colunga-Salas, P.; Becker, I.; Delgado-de la Mora, J.; Licona-Enríquez, J.D.; Delgado-de la Mora, D.; Karpathy, S.E.; Paddock, C.D.; et al. Rickettsia parkeri in Dermacentor parumapertus Ticks, Mexico. Emerg. Infect. Dis. 2018, 24, 1108–1111. [Google Scholar] [CrossRef]
- Colunga-Salas, P.; Sánchez-Montes, S.; Grostieta, E.; Verde-Arregoitia, L.D.; Cabrera-Garrido, M.Y.; Becker, I.; León-Paniagua, L. What do studies in wild mammals tell us about human emerging viral diseases in Mexico? Transbound. Emerg. Dis. 2020, 67, 33–45. [Google Scholar] [CrossRef]
- Rhyan, J.C.; Aune, K.; Roffe, T.; Ewalt, D.; Hennager, S.; Gidlewski, T.; Olsen, S.; Clarke, R. Pathogenesis and epidemiology of brucellosis in yellowstone bison: Serologic and culture results from adult females and their progeny. J. Wildl. Dis. 2009, 45, 729–739. [Google Scholar] [CrossRef][Green Version]
- Mohler, J.R. Report of the Chief of the Bureau of Animal Industry, Pathologic Division; Annual Reports; Department of Agriculture: Washington, DC, USA, 1917.
- Keller, A.; Graefen, A.; Ball, M.; Matzas, M.; Boisguerin, V.; Maixner, F.; Leidinger, P.; Backes, C.; Khairat, R.; Forster, M.; et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat. Commun. 2012, 3, 698. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Gutierrez, C.G.; Vargas-Sandoval, M.; Torres, J.; Gordillo-Pérez, M.G. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J. Vet. Sci. 2016, 17, 353–360. [Google Scholar] [CrossRef]
- Garcia-Vazquez, Z.; Ortega-S, J.A.; Cantu-Covarruvias, A.; Mosqueda, J.; Hewitt, D.G.; Deyoung, R.W.; Campbell, T.A.; Bryant, F.C. Tick-borne Diseases in Syntopic Populations of Fallow Deer (Dama dama) and Axis Deer (Axis axis) in Northern Mexico. J. Wildl. Dis. 2015, 51, 527–529. [Google Scholar] [CrossRef]
- Ojeda-Chi, M.M.; Rodriguez-Vivas, R.I.; Esteve-Gasent, M.D.; de León, A.A.P.; Modarelli, J.J.; Villegas-Perez, S.L. Ehrlichia canis in dogs of Mexico: Prevalence, incidence, co–infection and factors associated. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101351. [Google Scholar] [CrossRef]
- Acosta, R.; Guzmán-Cornejo, C.; Cisneros, F.A.Q.; Quiñonez, A.A.T.; Fernández, J.A. New records of ectoparasites for Mexico and their prevalence in the montane shrew Sorex monticolus (Eulipotyphla: Soricidae) at Cerro del Mohinora, Sierra Madre Occidental of Chihuahua, Mexico. Zootaxa 2020, 4809, 393–396. [Google Scholar] [CrossRef]
- Gulia-Nuss, M.; Nuss, A.; Meyer, J.M.; Sonenshine, D.E.; Roe, R.M.; Waterhouse, R.M.; Sattelle, D.B.; De La Fuente, J.; Ribeiro, J.; Megy, K.; et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016, 7, 10507. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, M.E.; Bosserman, E.A.; Demma, L.J.; Zambrano, M.L.; Blau, D.M.; Dasch, G. Isolation and Identification of Rickettsia massiliae from Rhipicephalus sanguineus Ticks Collected in Arizona. Appl. Environ. Microbiol. 2006, 72, 5569–5577. [Google Scholar] [CrossRef]
- Montiel-Armendáriz, S.; Verdugo, C.; Juache-Villagrana, A.E.; Jiménez-Vega, F.; Quezada-Casasola, A.; Vital-García, C.; Escárcega-Ávila, A. Molecular identification and morphological variations of Dermacentor albipictus collected from two deer species in northern Mexico. Exp. Appl. Acarol. 2021, 84, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Mutz, I. Las infecciones emergentes transmitidas por garrapatas. Ann. Nestlé 2009, 67, 123–134. [Google Scholar] [CrossRef]
- Matsumoto, K.; Grzeszczuk, A.; Brouqui, P.; Raoult, D. Rickettsia raoultii and Anaplasma phagocytophilum in Dermacentor reticulatus ticks collected from Bialowieza Primeval Forest European bison (Bison bonasus bonasus), Poland. Clin. Microbiol. Infect. 2009, 15, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Biernat, B.; Karbowiak, G.; Stańczak, J.; Masny, A.; Werszko, J. The first detection of the tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks collected from the lowland European bison (Bison bonasus bonasus L.). Acta Parasitol. 2016, 61, 130–135. [Google Scholar] [CrossRef]
- De la Fuente, J.; Thomas, E.J.G.; Bussche, R.A.V.D.; Hamilton, R.G.; Tanaka, E.E.; Druhan, S.E.; Kocan, K.M. Characterization of Anaplasma marginale Isolated from North American Bison. Appl. Environ. Microbiol. 2003, 69, 5001–5005. [Google Scholar] [CrossRef]
- Scoles, G.A.; McElwain, T.F.; Rurangirwa, F.R.; Knowles, D.P.; Lysyk, T.J. A Canadian Bison Isolate of Anaplasma marginale (Rickettsiales: Anaplasmataceae) Is Not Transmissible by Dermacentor andersoni (Acari: Ixodidae), Whereas Ticks from Two Canadian D. andersoni Populations Are Competent Vectors of a U.S. Strain. J. Med. Entomol. 2006, 43, 971–975. [Google Scholar] [CrossRef]
- Grzeszczuk, A.; Ziarko, S.; Prokopowicz, D.; Marek Radziwon, P. Evidence of Anaplasma phagocytophilum infection of European bisons in the Bialowieza Primaveral Forest, Poland. Med. Vet. 2004, 60, 600–601. [Google Scholar]
- Biernat, B.; Karbowiak, G. Study on the occurrence of tick-borne encephalitis virus RNA in European bison (Bison bonasus) eliminated at Białowieza Primeval Forest (north-eastern Poland) in 2005–2009. Ann. Parasitol. 2014, 60, 99–102. [Google Scholar] [PubMed]
- Mooring, M.; Samuel, W. Tick Defense Strategies in Bison: The Role of Grooming and Hair Coat. Behaviour 1998, 135, 693–718. [Google Scholar] [CrossRef]
- Lanier, J.; Grandin, T. The Calming of American Bison (Bison bison) During Routine Handling. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2015. [Google Scholar]
- Duysen, E.; Irvine, K.; Yoder, A.; Topliff, C.; Kelling, C.; Rajaram, S. Assessment of tribal bison worker hazards using trusted research facilitators. J. Agromed. 2017, 22, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and Evaluation of a Seminested PCR for Detection and Differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in Canine Blood Samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef]
- Qurollo, B.A.; Archer, N.R.; Schreeg, M.E.; Marr, H.S.; Birkenheuer, A.J.; Haney, K.N.; Thomas, B.S.; Breitschwerdt, E.B. Improved molecular detection of Babesia infections in animals using a novel quantitative real-time PCR diagnostic assay targeting mitochondrial DNA. Parasites Vectors 2017, 10, 128. [Google Scholar] [CrossRef]
- Tsao, J.I.; Hamer, S.A.; Han, S.; Sidge, J.L.; Hickling, G.J. The Contribution of Wildlife Hosts to the Rise of Ticks and Tick-Borne Diseases in North America. J. Med. Entomol. 2021, 58, 1565–1587. [Google Scholar] [CrossRef]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef]
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Fesler, M.C.; Shah, J.S.; Middelveen, M.J.; Du Cruz, I.; Burrascano, J.J.; Stricker, R.B. Lyme Disease: Diversity of Borrelia Species in California and Mexico Detected Using a Novel Immunoblot Assay. Healthcare 2020, 8, 97. [Google Scholar] [CrossRef]
- Colunga-Salas, P.; Sánchez-Montes, S.; Volkow, P.; Ruíz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Cornejo, C.; Robbins, R.G.; Guglielmone, A.A.; Montiel-Parra, G.; Rivas, G.; Pérez, T.M. The Dermacentor (Acari, Ixodida, Ixodidae) of Mexico: Hosts, geographical distribution and new records. ZooKeys 2016, 569, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Gassent, M.D.; De Leon, A.A.P.; Romero-Salas, D.; Feria-Arroyo, T.P.; Epatino, R.; Ecastro-Arellano, I.; Gordillo-Perez, G.; Eauclair, A.; Egoolsby, J.; Rodriguez-Vivas, R.I.; et al. Pathogenic Landscape of Transboundary Zoonotic Diseases in the Mexico-US Border Along the Rio Grande. Front. Public Health 2014, 2, 177. [Google Scholar] [CrossRef]
- Halsey, S.J.; Allan, B.F.; Miller, J.R. The role of Ixodes scapularis, Borrelia burgdorferi and wildlife hosts in Lyme disease prevalence: A quantitative review. Ticks Tick-Borne Dis. 2018, 9, 1103–1114. [Google Scholar] [CrossRef]
- Castellaw, A.H.; Chenney, E.F.; Varela-Stokes, A.S. Tick-Borne Disease Agents in Various Wildlife from Mississippi. Vector Borne Zoonotic Dis. 2011, 11, 439–442. [Google Scholar] [CrossRef]
- Yu, S.; Modarelli, J.; Tomeček, J.M.; French, J.T.; Hilton, C.; Esteve-Gasent, M.D. Prevalence of common tick-borne pathogens in white-tailed deer and coyotes in south Texas. Int. J. Parasitol. Parasites Wildl. 2020, 11, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, M.J.; Shock, B.C. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2012, 2, 18–31. [Google Scholar] [CrossRef]
- Adaszek, L.; Dzięgiel, B.; Krzysiak, M.; Skrzypczak, M.; Adaszek, M.; Staniec, M.; Wyłupek, D.; Winiarczyk, S. Detection of Borrelia burgdorferi sensu lato DNA in the blood of wild bison from Białowieza Primeval Forest in eastern Poland. Pol. J. Vet. Sci. 2014, 17, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.A.; Pinter, A.; Medina-Sanchez, A.; Boppana, V.D.; Wikel, S.K.; Saito, T.B.; Shelite, T.; Blanton, L.; Popov, V.; Teel, P.D.; et al. Amblyomma imitator Ticks as Vectors of Rickettsia rickettsii, Mexico. Emerg. Infect. Dis. 2010, 16, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Solís-Hernández, A.; Rodríguez-Vivas, R.I.; Esteve-Gassent, M.D.; Villegas-Pérez, S.L. Detección de Borrelia burgdorferi sensu lato en perros y sus garrapatas en comunidades rurales de Yucatán, México. Rev. Biol. Trop. 2017, 66, 428–437. [Google Scholar] [CrossRef][Green Version]
- Levin, M.L.; Zemtsova, G.E.; Killmaster, L.F.; Snellgrove, A.; Schumacher, L.B. Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick-Borne Dis. 2017, 8, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.L.; Ford, S.L.; Hartzer, K.; Krapiunaya, L.; Stanley, H.; Snellgrove, A.N. Minimal Duration of Tick Attachment Sufficient for Transmission of Infectious Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) by Its Primary Vector Dermacentor variabilis (Acari: Ixodidae): Duration of Rickettsial Reactivation in the Vector Revisited. J. Med. Entomol. 2019, 57, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, M.E.; Zambrano, M.L.; Anaya, L.; Beati, L.; Karpathy, S.E.; Santos-Silva, M.M.; Salceda, B.; Macbeth, D.; Olguin, H.; Dasch, G.; et al. Rickettsia rickettsii in Rhipicephalus Ticks, Mexicali, Mexico. J. Med. Entomol. 2011, 48, 418–421. [Google Scholar] [CrossRef]
- Kobayashi, A.; Iwasaki, H. Human feet bitten by multiple brown dog ticks, Rhipicephalus sanguineus. IDCases 2017, 9, 8. [Google Scholar] [CrossRef]
- Tinoco-Gracia, L.; Rodríguez-Peñuelas, P.; Hori-Oshima, S.; Medina-Basulto, G.E.; López-Valencia, G.; Tamayo-Sosa, A.R.; Barreras-Serrano, A.; Rentería-Evangelista, T.B.; Melgarejo, T.; Field-Cortazares, J. Primera evidencia molecular de borreliosis y leptospirosis en un humano de Sinaloa, México. Rev. Enferm. Infecc. Pediatr. 2020, 33, 1727–1731. [Google Scholar]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Chenery, E.S.; Harms, N.J.; Mandrak, N.E.; Molnár, P.K. First records of Dermacentor albipictus larvae collected by flagging in Yukon, Canada. Parasites Vectors 2020, 13, 565. [Google Scholar] [CrossRef]
- Asman, M.; Nowak, M.; Cuber, P.; Strzelczyk, J.; Szilman, E.; Szilman, P.; Trapp, G.; Siuda, K.; Solarz, K.; Wiczkowski, A. The risk of exposure to Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Babesia sp. and co-infections in Ixodes ricinus ticks on the territory of Niepołomice forest (southern Poland). Ann. Parasitol. 2013, 59, 13–19. [Google Scholar]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef]
- Goodman, J.L.; Jurkovich, P.; Kramber, J.M.; Johnson, R.C. Molecular detection of persistent Borrelia burgdorferi in the urine of patients with active Lyme disease. Infect. Immun. 1991, 59, 269–278. [Google Scholar] [CrossRef]
- Schmidt, B.L. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin. Microbiol. Rev. 1997, 10, 185–201. [Google Scholar] [CrossRef]
- Soares, H.S.; Barbieri, A.R.M.; Martins, T.F.; Minervino, A.; De Lima, J.T.R.; Marcili, A.; Gennari, S.; Labruna, M.B. Ticks and rickettsial infection in the wildlife of two regions of the Brazilian Amazon. Exp. Appl. Acarol. 2014, 65, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Nieri-Bastos, F.A.; Marcili, A.; De Sousa, R.; Paddock, C.D.; Labruna, M.B. Phylogenetic Evidence for the Existence of Multiple Strains of Rickettsia parkeri in the New World. Appl. Environ. Microbiol. 2018, 84, e02872-17. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef]
- Tadepalli, M.; Vincent, G.; Hii, S.; Watharow, S.; Graves, S.; Stenos, J. Molecular Evidence of Novel Spotted Fever Group Rickettsia Species in Amblyomma albolimbatum Ticks from the Shingleback Skink (Tiliqua rugosa) in Southern Western Australia. Pathogens 2021, 10, 35. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Nava-Reyna, E.; Ávila-Rodríguez, V.; González-Álvarez, V.H.; Castillo-Martínez, A.; Siller, Q.K.; Cabezas-Cruz, A.; Dantas-Torres, F.; Almazán, C. Detection of Rickettsia spp. in Rhipicephalus sanguineus (sensu lato) collected from free-roaming dogs in Coahuila state, northern Mexico. Parasites Vectors 2019, 12, 130. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef]
- León, A.A.P.D.; Strickman, D.A.; Knowles, D.P.; Fish, D.; Thacker, E.; de la Fuente, J.; Krause, P.J.; Wikel, S.K.; Miller, R.S.; Wagner, G.G.; et al. One Health approach to identify research needs in bovine and human babesioses: Workshop report. Parasites Vectors 2010, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- De León, A.A.P.; Teel, P.D.; Auclair, A.N.; Messenger, M.T.; Guerrero, F.D.; Schuster, G.; Miller, R.J. Integrated Strategy for Sustainable Cattle Fever Tick Eradication in USA is Required to Mitigate the Impact of Global Change. Front. Physiol. 2012, 3, 195. [Google Scholar] [CrossRef]
- Thomas, D.B.; Klafke, G.; Busch, J.D.; Olafson, P.U.; Miller, R.A.; Mosqueda, J.; Stone, N.E.; Scoles, G.; Wagner, D.M.; Perez-De-Leon, A. Tracking the Increase of Acaricide Resistance in an Invasive Population of Cattle Fever Ticks (Acari: Ixodidae) and Implementation of Real-Time PCR Assays to Rapidly Genotype Resistance Mutations. Ann. Entomol. Soc. Am. 2020, 113, 298–309. [Google Scholar] [CrossRef]
- USDA APHIS. U.S.—Mexico Binational Committee for Tuberculosis, Brucellosis, and Cattle Fever Tick. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/cattle-disease-information/us-mex-bnc (accessed on 10 May 2021).
- Pet Trade, Parasitic Diseases and Exotic Animal Imports. Available online: https://www.usaha.org/ (accessed on 16 October 2021).
- Bovine Babesiosis. Available online: https://www.iastate.edu/ (accessed on 16 October 2021).
- Salabarria, F.F.; Godshaev, A.; Jimenez, T. Proceso hemolitico agudo en bisontes (Bison bison) causada por Babesia argentina. Rev. Cub. Cienc. Vet. 1981, 12, 165–170. [Google Scholar]
- Zaugg, J.L.; Kuttler, K.L. Experimental infections of Babesia bigemina in American bison. J. Wildl. Dis. 1987, 23, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Findlay, C.; Begg, T. Redwater in American bison caused by Babesia major. Vet. Rec. 1977, 100, 406. [Google Scholar] [CrossRef]
- Paulauskas, A.; Aleksandravičienė, A.; Lipatova, I.; Griciuvienė, L.; Kibiša, A.; Žukauskienė, J.; Radzijevskaja, J. Molecular detection of Babesia spp. in European bison (Bison bonasus) and their ticks. Ticks Tick-Borne Dis. 2021, 12, 101807. [Google Scholar] [CrossRef]
- Lira-Amaya, J.J.; Jesús Polanco-Martínez, D.; Castañeda-Arriola, R.O.; Ramos-Aragón, J.A.; Lara-Hernández, J.d.E.; Preciado-De La Torre, F.; Rojas-Martínez, C.; Antonio Álvarez-Martínez, J.; Ramón Bautista-Garfias, C.; Figueroa-Millan, J.V. Prevalencia serológica y molecular de Babesia bovis, Babesia bigemina y Anaplasma marginale en búfalos de agua mantenidos en zonas de alta incidencia de garrapatas. Entomol. Mex. 2017, 4, 627–632. [Google Scholar]
- Cooper, S.M.; Perotto-Baldivieso, H.; Owens, M.K.; Meek, M.G.; Figueroa-Pagán, M. Distribution and interaction of white-tailed deer and cattle in a semi-arid grazing system. Agric. Ecosyst. Environ. 2008, 127, 85–92. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Barasona, J.A.; Carro, F.; Soriguer, R.; Vicente, J.; Acevedo, P. Spatio-temporal trends in the frequency of interspecific interactions between domestic and wild ungulates from Mediterranean Spain. PLoS ONE 2019, 14, e0211216. [Google Scholar] [CrossRef]
- Obregón, D.; Cabezas-Cruz, A.; Armas, Y.; Silva, J.B.; Fonseca, A.H.; André, M.R.; Alfonso, P.; Oliveira, M.C.; Machado, R.Z.; Corona-González, B. High co-infection rates of Babesia bovis, Babesia bigemina, and Anaplasma marginale in water buffalo in Western Cuba. Parasitol. Res. 2019, 118, 955–967. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Triana-Chávez, O.; Holguín-Rocha, A.; Tobon-Castaño, A.; Mejía-Jaramillo, A.M. Molecular surveillance and phylogenetic traits of Babesia bigemina and Babesia bovis in cattle (Bos taurus) and water buffaloes (Bubalus bubalis) from Colombia. Parasites Vectors 2018, 11, 510. [Google Scholar] [CrossRef]
- Eygelaar, D.; Jori, F.; Mokopasetso, M.; Sibeko, K.P.; Collins, N.E.; Vorster, I.; Troskie, M.; Oosthuizen, M.C. Tick-borne haemoparasites in African buffalo (Syncerus caffer) from two wildlife areas in Northern Botswana. Parasites Vectors 2015, 8, 26. [Google Scholar] [CrossRef]
- Espinaze, M.P.; Hellard, E.; Horak, I.G.; Cumming, G. Domestic mammals facilitate tick-borne pathogen transmission networks in South African wildlife. Biol. Conserv. 2018, 221, 228–236. [Google Scholar] [CrossRef]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef]
- Kocan, K.M.; De La Fuente, J.; Thomas, E.J.G.; Bussche, R.A.; Hamilton, R.G.; Tanaka, E.E.; Druhan, S.E. Recent Studies on the Characterization of Anaplasma marginale Isolated from North American Bison. Ann. N. Y. Acad. Sci. 2004, 1026, 114–117. [Google Scholar] [CrossRef]
- Cossío-Bayúgar, R.; Rodríguez, S.D.; García-Ortiz, M.A.; García-Tapia, D.; Aboytes-Torres, R. Bovine anaplasmosis prevalence in northern Veracruz state, Mexico. Prev. Vet. Med. 1997, 32, 165–170. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.; Mata-Mendez, Y.; Pérez-Gutierrez, E.; Wagner, G. The Effect of Management Factors on the Seroprevalence of Anaplasma marginale in Bos indicus cattle in the Mexican Tropics. Trop. Anim. Health Prod. 2004, 36, 135–143. [Google Scholar] [CrossRef]
- Martínez-Ocampo, F.; Quiroz-Castañeda, R.E.; Amaro-Estrada, I.; Dantán-González, E.; De La Torre, J.F.P.; Rodríguez-Camarillo, S. Whole-Genome Sequencing of Mexican Strains of Anaplasma marginale: An Approach to the Causal Agent of Bovine Anaplasmosis. Int. J. Genom. 2020, 2020, 5902029. [Google Scholar] [CrossRef]
- Rhyan, J.C.; Spraker, T.R.; Kitchen, D.N.; Carlton, W.W.; Tuite, J. Emergence of Diseases From Wildlife Reservoirs. Vet. Pathol. 2010, 47, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, H.Y.; Rojas, M.O.; Wasserman, M. Evaluación de dos métodos para la separación de RNA mensajero de Plasmodium falciparum. Biomédica 1998, 18, 55–65. [Google Scholar] [CrossRef][Green Version]
- .Adelson, M.E.; Rao, R.-V.S.; Tilton, R.C.; Cabets, K.; Eskow, E.; Fein, L.; Occi, J.L.; Mordechai, E. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis Ticks Collected in Northern New Jersey. J. Clin. Microbiol. 2004, 42, 2799–2801. [Google Scholar] [CrossRef]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef]
- Figueroa, J.; Chieves, L.; Johnson, G.; Buening, G. Multiplex polymerase chain reaction based assay for the detection of Babesia bigemina, Babesia bovis and Anaplasma marginale DNA in bovine blood. Vet. Parasitol. 1993, 50, 69–81. [Google Scholar] [CrossRef]
- Torioni De Echaide, S.; Knowles, D.P.; McGuire, T.C.; Palmer, G.H.; Suarez, C.E.; McElwain, T.F. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J. Clin. Microbiol. 1998, 36, 777–782. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Hagimori, T.; Abe, Y.; Date, S.; Miura, K. The First Finding of a Rickettsia Bacterium Associated with Parthenogenesis Induction Among Insects. Curr. Microbiol. 2006, 52, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Izzard, L.; Graves, S.; Cox, E.; Fenwick, S.; Unsworth, N.; Stenos, J. Novel Rickettsia in Ticks, Tasmania, Australia. Emerg. Infect. Dis. 2009, 15, 1654–1656. [Google Scholar] [CrossRef] [PubMed]


| Animal ID | Gene G3PDH | Borrelia burgdorferi s. l. | Rickettsia rickettsii | Babesia bovis | Babesia bigemina | Anaplasma marginale |
|---|---|---|---|---|---|---|
| 2 | + | − | − | + | − | − |
| 3 | + | + | − | + | − | − |
| 4 | + | − | − | − | − | − |
| 5 | + | − | − | − | − | − |
| 8 | + | − | − | − | − | − |
| 9 | + | − | − | − | − | − |
| 10 | + | − | − | − | − | − |
| 12 | + | − | − | − | − | − |
| 14 | + | − | − | − | − | − |
| 16 | + | + | − | − | − | − |
| 18 | + | + | − | + | − | − |
| 20 | + | − | − | − | − | − |
| 22 | + | − | − | + | − | − |
| 24 | + | − | − | − | − | − |
| 25 | + | − | − | − | − | − |
| 27 | + | − | − | − | − | − |
| 30 | + | − | − | − | − | − |
| 31 | + | − | + | − | − | − |
| 32 | + | − | − | + | − | − |
| 34 | + | − | − | − | − | − |
| 38 | + | − | − | − | − | − |
| 40 | + | − | + | − | − | − |
| 44 | + | − | − | − | − | − |
| 45 | + | − | + | − | − | − |
| 47 | + | − | − | − | − | − |
| Pathogen | Oligonucleotide Sequence (5′–3′) | Product Size (bp) | PCR Protocol | References |
|---|---|---|---|---|
| Glyceraldehyde-3-Phosphate Dehydrogenase (Housekeeping) | GAPDHF-CCTTCATTGACCTCAACTACAT GAPDHR-CCAAAGTTGTCATGGATGACC | 400 | 94 °C for 5 min initial denaturation, followed by 35 cycles of 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min, then 72 °C for 15 min for the final elongation | [32] |
| Borrelia burgdorferi s. l. | LY2F-GAAATGGCTAAAGTAAGCGGAATTGTAC LY2R-CAGAAATTCTGTAAACTAATCCCACC | 231 | 94 °C for 4 min initial denaturation, followed by 40 cycles of 94 °C for 45 s, 55 °C for 45 s, 72 °C for 45 s, then 72 °C for 7 min for the final elongation | [89] |
| Rickettsia spp. (ompA) | Rr190.70P-ATGGCGAATATTTCTCCAAAA Rr190.701N-GTTCCGTTAATGGCAGCATCT | 631 | 95 °C for 5 min initial denaturation, followed by 35 cycles of 95 °C for 30 s, 58 °C for 30 s, 65 °C for 45 s, then 72 °C for 7 min for the final elongation | [90] |
| Rickettsia rickettsii (ompA) | Rr190.70P-ATGGCGAATATTTCTCCAAAA Rr190.602N-AGTGCAGCATTCGCTCCCCCT | 532 | 96 °C for 30 s initial denaturation, followed by 35 cycles of 94 °C for 30 s, 58 °C for 30 s, 72 °C for 45 s, then 72 °C for 7 min for the final elongation | [90] |
| Babesia bovis (rap-1) | BOF-CGAGGAAGGAACTACCGATG BOR-GGAGCTTCAACGTACGAGGT | 354 | 95 °C for 5 min initial denaturation, followed by 35 cycles of 95 °C for 1 min, 55 °C for 1 min, 73 °C for 1:30 min, then 72 °C for 15 min for the final elongation | [91] |
| Babesia bovis (rap-1) | BOFN-TGGCTACCATGAACTACAAGACTTA BORN-GAGCAGAACCTTCTTCACCAT | 275 | 95 °C for 5 min initial denaturation, followed by 35 cycles of 95 °C for 1 min, 55 °C for 30 s, 73 °C for 1:30 min, then 72 °C for 15 min for the final elongation | [91] |
| Babesia bigemina (SpeI-AvaI) | BIF-CATCTAATTTCTCTCCATACCCC BIR-CCTCGGCTTCAACTCTGATGCC | 278 | 95 °C for 5 min initial denaturation, followed by 35 cycles of 95 °C for 1 min, 65 °C for 1 min, 73 °C for 1:30 min, then 72 °C for 15 min for the final elongation | [91] |
| Babesia bigemina (SpeI-AvaI) | BIFN-CGCAAGCCCAGCACGCCCCGGT BIRN-CCGACCTGGATAGGCTGTGATG | 170 | 95 °C for 5 min initial denaturation, followed by 35 cycles of 95 °C for 1 min, 65 °C for 30 s, 73 °C for 1:30 min, then 72 °C for 15 min for the final elongation | [91] |
| Anaplasma spp. (msp5) | MSP5F-ACCTTCTGCTGTTCGTTGC MSP5R-TGTACCACTGCCATGCTTAAG | 628 | 95 °C for 3 min initial denaturation, followed by 35 cycles of 95 °C for 30 s, 65 °C for 1 min, 72 °C for 1 min, then 72 °C for 10 min for the final elongation | [92] |
| Anaplasma marginale (msp5) | MSP5FN-CATAGCCTCCGCGTCTTT MSP5RN-CTTAAACAGCTCCTCGCCTT | 466 | 95 °C for 3 min initial denaturation, followed by 35 cycles of 95 °C for 30 s, 65 °C for 1 min, 72 °C for 1 min, then 72 °C for 10 min for the final elongation | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beristain-Ruiz, D.M.; Vital-García, C.; Figueroa-Millán, J.V.; Lira-Amaya, J.J.; Garza-Hernández, J.A.; Sánchez-Ayala, J.R.; Flores-Ceballos, S.; Rodríguez-Alarcón, C.A.; Olivas-Sánchez, M.P.; Pons-Monarrez, G. Molecular Detection of Tick-Borne Pathogens in American Bison (Bison bison) at El Uno Ecological Reserve, Janos, Chihuahua, Mexico. Pathogens 2021, 10, 1428. https://doi.org/10.3390/pathogens10111428
Beristain-Ruiz DM, Vital-García C, Figueroa-Millán JV, Lira-Amaya JJ, Garza-Hernández JA, Sánchez-Ayala JR, Flores-Ceballos S, Rodríguez-Alarcón CA, Olivas-Sánchez MP, Pons-Monarrez G. Molecular Detection of Tick-Borne Pathogens in American Bison (Bison bison) at El Uno Ecological Reserve, Janos, Chihuahua, Mexico. Pathogens. 2021; 10(11):1428. https://doi.org/10.3390/pathogens10111428
Chicago/Turabian StyleBeristain-Ruiz, Diana M., Cuauhcihuatl Vital-García, Julio V. Figueroa-Millán, José J. Lira-Amaya, Javier A. Garza-Hernández, Juan R. Sánchez-Ayala, Samuel Flores-Ceballos, Carlos A. Rodríguez-Alarcón, Martha P. Olivas-Sánchez, and Gabriel Pons-Monarrez. 2021. "Molecular Detection of Tick-Borne Pathogens in American Bison (Bison bison) at El Uno Ecological Reserve, Janos, Chihuahua, Mexico" Pathogens 10, no. 11: 1428. https://doi.org/10.3390/pathogens10111428
APA StyleBeristain-Ruiz, D. M., Vital-García, C., Figueroa-Millán, J. V., Lira-Amaya, J. J., Garza-Hernández, J. A., Sánchez-Ayala, J. R., Flores-Ceballos, S., Rodríguez-Alarcón, C. A., Olivas-Sánchez, M. P., & Pons-Monarrez, G. (2021). Molecular Detection of Tick-Borne Pathogens in American Bison (Bison bison) at El Uno Ecological Reserve, Janos, Chihuahua, Mexico. Pathogens, 10(11), 1428. https://doi.org/10.3390/pathogens10111428






