Fusarium elaeidis Causes Stem and Root Rot on Alocasia longiloba in South China
Abstract
:1. Introduction
2. Results
2.1. Field Symptoms
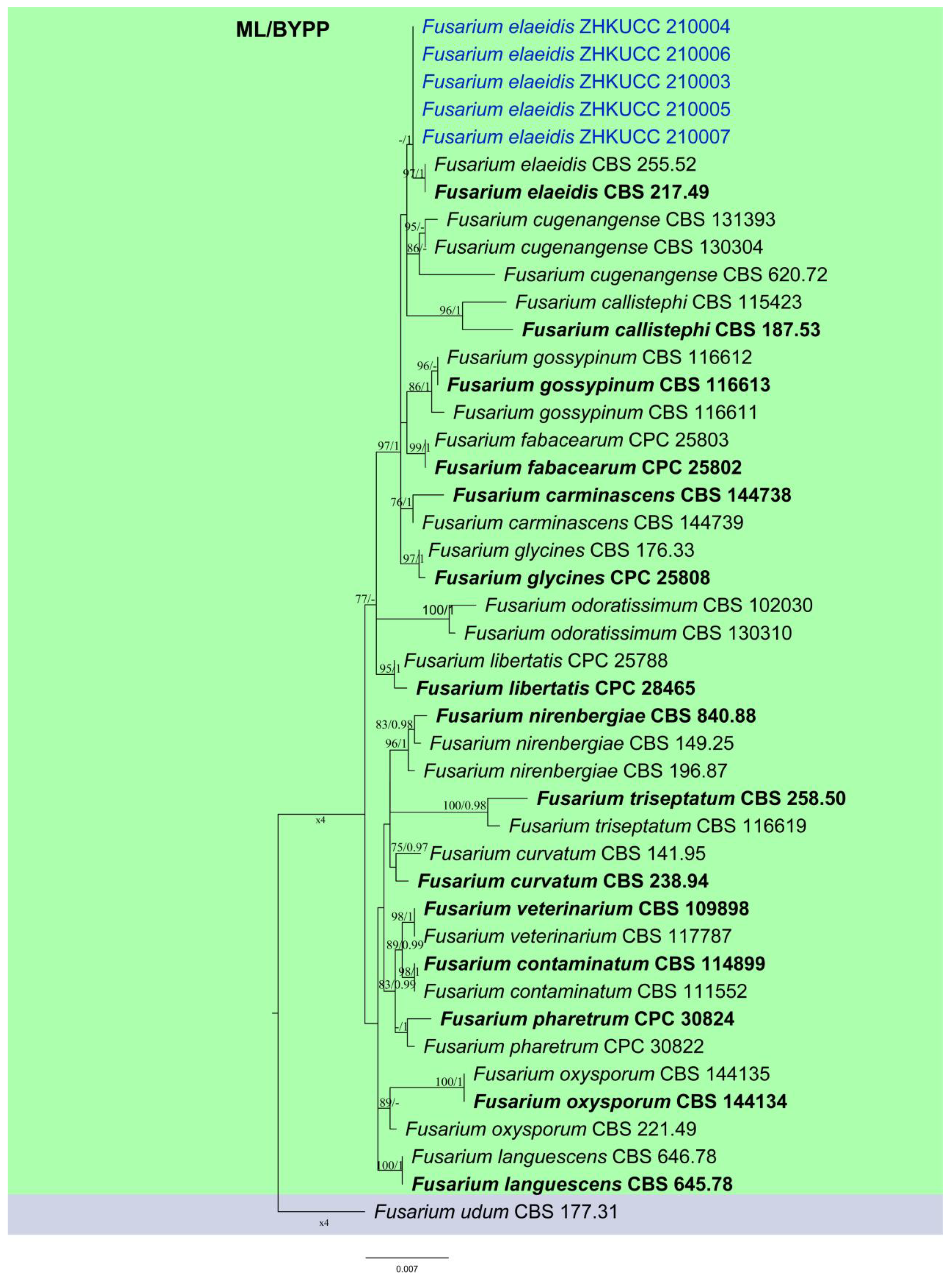
2.2. Phylogenetic Analyses and Species Identification
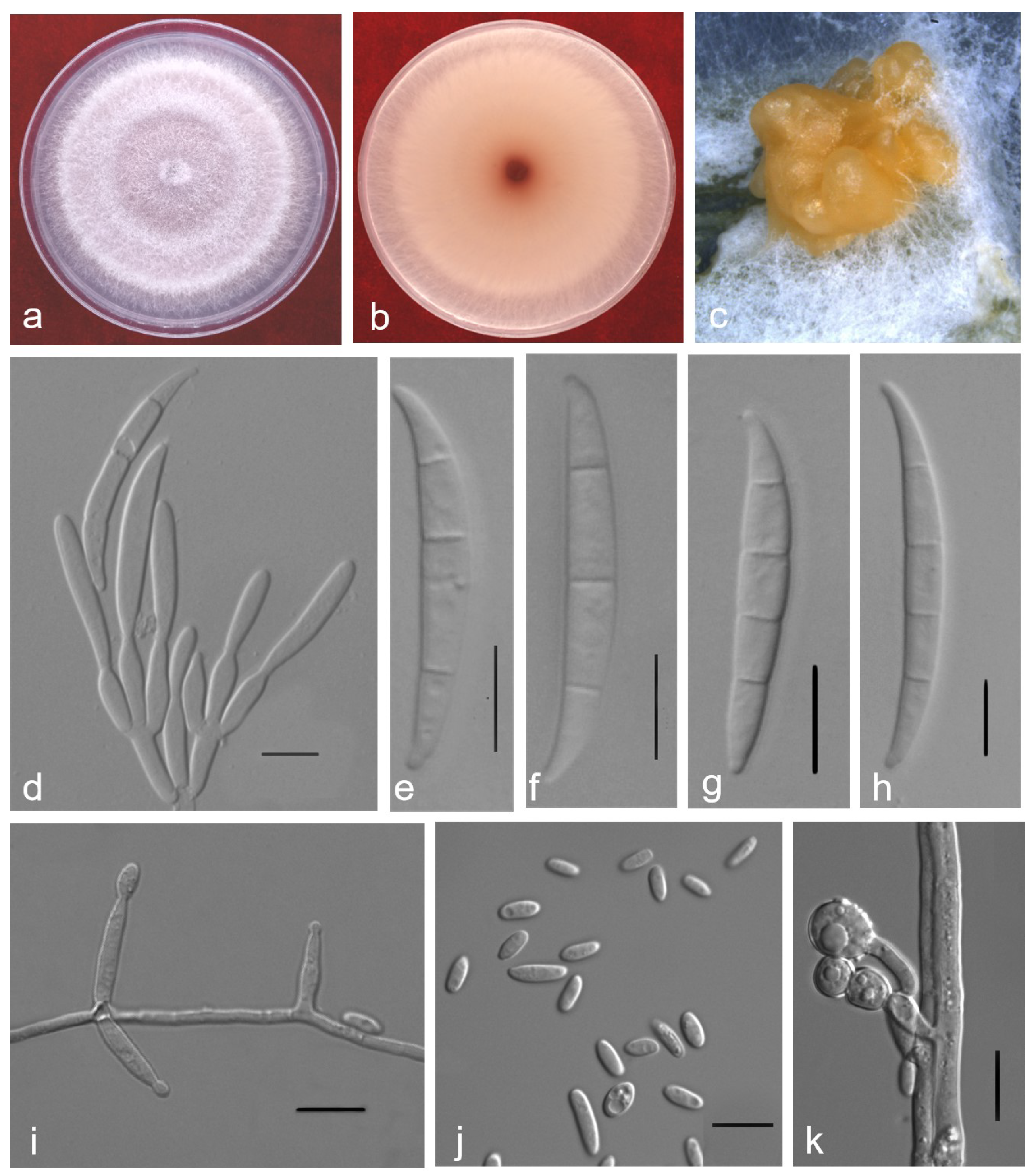
2.3. Taxonomy
2.4. Pathogenicity Test
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Fungal Isolation and Purification
4.3. DNA Extraction, PCR Amplification and Sequencing
4.4. Phylogenetic Analyses
4.5. Morphological Characterisation
4.6. Pathogenicity Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Lecomte, C.; Edel-Hermann, V.; Cannesan, M.A.; Gautheron, N.; Langlois, A.; Alabouvette, C.; Robert, F.; Steinberg, C. Fusarium oxysporum f. sp. cyclaminis: Underestimated genetic diversity. Eur. J. Plant Pathol. 2016, 145, 421–431. [Google Scholar]
- He, Y.; Shu, C.; Chen, J.; Zhou, E. First report of anthracnose of Alocasia macrorrhiza caused by Colletotrichum karstii in Guangdong, China. Plant Dis. 2014, 98, 696. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.Y.; Xu, K.C.; Sun, Y.X.; Huang, Q. First report of Ceratocystis fimbriata causing leaf blight on Alocasia macrorrhiza in China. Plant Dis. 2016, 100, 2172–2173. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhang, J.Z.; Gao, M.X. Taxonomic studies of Alternaria from China III. New species and new record on Anacardiaceae, Araceae and Araliaceae. Mycosystema 1999, 18, 125–129. [Google Scholar]
- Alfieri, S.A., Jr.; Langdon, K.R.; Wehlburg, C.; Kimbrough, J.W. Index of Plant Diseases in Florida, Revised; Florida Dept. Agric. and Consumer Serv.: Tallahassee FL, USA, 1984; pp. 1–389.
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Retrieved 12 July 2021. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 30 July 2021).
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium oxysporum: Genomics, diversity and Plant–Host interaction. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S., Johri, B., Eds.; Springer: Singapore, 2017; pp. 159–199. [Google Scholar]
- Lombard, L.; Merwe, N.A.V.D.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Denis, M.; Crous, P.W. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Persoonia 2018, 41, 109–129. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Al-Hatmi, A.M.S.; Aoki, T.; Brankovics, B.; Cano-Lira, J.F.; Coleman, J.J.; Hoog, G.S.D.; Antonio Di Pietro, A.D.; Frandsen, R.J.N.; Geiser, D.M.; et al. No to Neocosmospora: Phylogenomic and Practical Reasons for Continued Inclusion of the Fusarium solani Species Complex in the Genus Fusarium. mSphere 2020, 5, 00810–00820. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Lei, C.; Cock, A.W.A.M.D.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I (2014). Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [Green Version]
- Geiser, D.M.; Jime´nez-Gasco, M.D.M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’donnell, K. Fusarium-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum–clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Ann. Rev. Phyto. 1997, 35, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.; Riley, R.; Zitomer, N.C.; Colyer, P.; Waalwijk, C. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Laurence, M.H.; Summerell, B.A.; Burgess, L.W.; Liew, E.C.Y. Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 2014, 118, 374–384. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Magnon, K.C.; Cox, P.A.; Revankar, S.G.; Sanche, S.; Geiser, D.M.; Juba, J.H.; van Burik, J.-A.H.; et al. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: Evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J. Clin. Microbiol. 2004, 42, 5109–5120. [Google Scholar]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Farias, O.R.; Cruz, J.M.F.L.J.; Veloso, J.S.; Silva, H.A.O.; Oliveira, M.D.M.; Félix, M.R.F.; Varanda, C.M.R.; Materatski, P.; Podestá, G.S.; Nascimento, L.C. First Report of Wilt of Syzygium malaccensis Caused by a Member of the Fusarium oxysporum Species Complex in Brazil. Plant Dis. 2021, 105, 1207. [Google Scholar] [CrossRef]
- Rouxel, F.; Grouet, D. Première observation de la fusariose vasculaire du cyclamen en France. Ann. Phytopathol. 1974, 6, 475–478. [Google Scholar]
- Lee, B.D.; Kim, W.G.; Cho, W.D.; Cho, W.D.; Sung, J.M. Occurrence of Dry Rot on Cymbidium Orchids Caused by Fusarium spp. in Korea. Plant Pathol. J. 2002, 18, 156–160. [Google Scholar]
- Zhang, Y.X.; Shi, Z.R.; Huang, J.H.; Xiang, M.M. Identification and Control of Fusarium Wilt of Chinese Orchid. Guangdong Agric. Sci. 2011, 38, 21–22. [Google Scholar]
- Adusei-Fosu, K.; Dickinson, M. Detection of Fusarium oxysporum f. sp. elaeidis Causing Fusarium Wilt of Oil Palm Using Loop-Mediated Isothermal Amplification (LAMP). J. Plant Pathol. Microbiol. 2019, 10, 1–6. [Google Scholar]
- Flood, J. A review of Fusarium wilt of oil palm caused by Fusarium oxysporum f. sp. elaeidis. Phytopathology 2006, 96, 660–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawardena, R.S.; Hyde, K.D.; de Farias, A.R.G.; Bhunjun, C.S.; Ferdinandez, H.S. What is a species in fungal plant pathogens? Fungal Divers. 2021, in press. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Phillips, A.J.L.; Xu, J.; Balasuriya, A.; Hyde, K.D.; Tępień, L.; Harischandra, D.L.; Karunarathna, A.; Yan, J.; Weerasinghe, J.; et al. Defining a species in plant pathology: Beyond the species level. Fungal Divers. 2021, in press. [Google Scholar] [CrossRef]
- O’Donnell, K. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risede, J.; Simoneau, P.; Hywel-Jones, N.L. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004, 50, 415–430. [Google Scholar]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic Relationships among Ascomycetes: Evidence from an RNA Polymerse II Subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, W.J. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenet. Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Michalak, I. RaxmlGUI: A Graphical Front-End for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.A.; Pfeiffer, W.T.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Gateway Computing Environments Workshop (GCE); Institute of Electrical and Electronics, Engineers: New Orleans, LA, USA, 2010. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]


| Species | Culture Accession | GenBank Accession Numbers | ||
|---|---|---|---|---|
| rpb2 | tef1 | tub2 | ||
| Fusarium callistephi | CBS 187.53 T | MH484875 | MH484966 | MH485057 |
| CBS 115423 | MH484905 | MH484996 | MH485087 | |
| F. carminascens | CBS 144739 = CPC 25792 | MH484934 | MH485025 | MH485116 |
| CBS 144738 = CPC 25800 T | MH484937 | MH485028 | MH485119 | |
| F. contaminatum | CBS 111552 | MH484900 | MH484991 | MH485082 |
| CBS 114899 T | MH484901 | MH484992 | MH485083 | |
| F. cugenangense | CBS 620.72 = DSM 11271 = NRRL 36520 | MH484879 | MH484970 | MH485061 |
| CBS 130304 = BBA 69050 = NRRL 25433 | MH484921 | MH485012 | MH485103 | |
| CBS 131393 | MH484928 | MH485019 | MH485110 | |
| F. curvatum | CBS 238.94 = NRRL 26422 = PD 94/184 T | MH484893 | MH484984 | MH485075 |
| CBS 141.95 = NRRL 36251 = PD 94/1518 | MH484894 | MH484985 | MH485076 | |
| F. elaeidis | CBS 217.49 = NRRL 36358 | MH484870 | MH484961 | MH485052 |
| CBS 255.52 = NRRL 36386 | MH484874 | MH484965 | MH485056 | |
| ZHKUCC 21-0003 | MZ439841 | MZ325284 | MZ439836 | |
| ZHKUCC 21-0004 | MZ439842 | MZ325285 | MZ439837 | |
| ZHKUCC 21-0005 | MZ439843 | MZ325286 | MZ439838 | |
| ZHKUCC 21-0006 | MZ439844 | MZ325287 | MZ439839 | |
| ZHKUCC 21-0007 | MZ439845 | MZ325288 | MZ439840 | |
| F. fabacearum | CBS 144743 = CPC 25802 T | MH484939 | MH485030 | MH485121 |
| CBS 144744 = CPC 25803 | MH484940 | MH485031 | MH485122 | |
| F. glycines | CBS 176.33 = NRRL 36286 | MH484868 | MH484959 | MH485050 |
| CBS 144746 = CPC 25808 T | MH484942 | MH485033 | MH485124 | |
| F. gossypinum | CBS 116611 | MH484907 | MH484998 | MH485089 |
| CBS 116612 | MH484908 | MH484999 | MH485090 | |
| CBS 116613 T | MH484909 | MH485000 | MH485091 | |
| F. languescens | CBS 645.78 = NRRL 36531 T | MH484880 | MH484971 | MH485062 |
| CBS 646.78 = NRRL 36532 | MH484881 | MH484972 | MH485063 | |
| F. libertatis | CBS 144747 = CPC 25788 | MH484933 | MH485024 | MH485115 |
| CBS 144749 = CPC 28465 T | MH484944 | MH485035 | MH485126 | |
| F. nirenbergiae | CBS 840.88 T | MH484887 | MH484978 | MH485069 |
| CBS 149.25 = NRRL 36261 | MH484865 | MH484956 | MH485047 | |
| CBS 196.87 = NRRL 26219 | MH484886 | MH484977 | MH485068 | |
| F. odoratissimum | CBS 102030 | MH484898 | MH484989 | MH485080 |
| CBS 130310 = NRRL 25603 | MH484922 | MH485013 | MH485104 | |
| F. oxysporum | CBS 221.49 = IHEM 4508 = NRRL 22546 | MH484872 | MH484963 | MH485054 |
| CBS 144134 ET | MH484953 | MH485044 | MH485135 | |
| CBS 144135 | MH484954 | MH485045 | MH485136 | |
| F. pharetrum | CBS 144750 = CPC 30822 | MH484951 | MH485042 | MH485133 |
| CBS 144751 = CPC 30824 T | MH484952 | MH485043 | MH485134 | |
| F. triseptatum | CBS 258.50 = NRRL 36389 T | MH484873 | MH484964 | MH485055 |
| CBS 116619 | MH484910 | MH485001 | MH485092 | |
| F. veterinarium | CBS 109898 = NRRL 36153 T | MH484899 | MH484990 | MH485081 |
| CBS 117787 | MH484912 | MH485003 | MH485094 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, C.; Zhao, J.; Chen, C.; Lin, J.; Jayawardena, R.S.; Xiang, M.; Manawasinghe, I.S.; You, C. Fusarium elaeidis Causes Stem and Root Rot on Alocasia longiloba in South China. Pathogens 2021, 10, 1395. https://doi.org/10.3390/pathogens10111395
Zhang Y, Chen C, Zhao J, Chen C, Lin J, Jayawardena RS, Xiang M, Manawasinghe IS, You C. Fusarium elaeidis Causes Stem and Root Rot on Alocasia longiloba in South China. Pathogens. 2021; 10(11):1395. https://doi.org/10.3390/pathogens10111395
Chicago/Turabian StyleZhang, Yunxia, Chao Chen, Jingpeng Zhao, Cantian Chen, Jieying Lin, Ruvishika S. Jayawardena, Meimei Xiang, Ishara S. Manawasinghe, and Chunping You. 2021. "Fusarium elaeidis Causes Stem and Root Rot on Alocasia longiloba in South China" Pathogens 10, no. 11: 1395. https://doi.org/10.3390/pathogens10111395
APA StyleZhang, Y., Chen, C., Zhao, J., Chen, C., Lin, J., Jayawardena, R. S., Xiang, M., Manawasinghe, I. S., & You, C. (2021). Fusarium elaeidis Causes Stem and Root Rot on Alocasia longiloba in South China. Pathogens, 10(11), 1395. https://doi.org/10.3390/pathogens10111395






