Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis
Abstract
:1. Introduction
2. Overview of the Fungal Disease
2.1. Aspergillosis
2.2. Coccidioidomycosis
2.3. Mucormycosis
2.4. Candidiasis (Candida auris)
3. The Current Treatment
3.1. Aspergillosis
3.2. Coccidioidomycosis
3.3. Mucormycosis
3.4. Candidiasis (Candida auris)
| Disease | Current Treatment | References |
|---|---|---|
| Aspergillosis | Amphotericin B, azoles (voriconazole, posaconazole, and itraconazole), and echinocandins. | [60,61] |
| Coccidioidomycosis | Azoles (fluconazole, itraconazole, posaconazole, voriconazole, isavuconazole) and amphotericin B. | [63,74] |
| Mucormycosis | Amphotericin B, posaconazole, and isavuconazole. | [69,70,75] |
| Candidiasis (Candida auris) | Echinocandins (caspofungin, micafungin, and anidulafungin) and isavuconazole | [71,72,73] |
4. Nanotechnology in Antifungal Therapy
4.1. Aspergillosis
4.1.1. Metal Nanoparticles
4.1.2. Organic Materials-Based Nanoparticles
4.1.3. Plant Extracts-Based Nanoparticles
4.1.4. Nanoparticles Obtained from Prokaryotic/Eukaryotic Cultures
4.1.5. Nanoparticles-Based Drug Delivery or Controlled Drug Release Systems
4.2. Coccidioidomycosis
4.3. Mucormycosis
4.4. Candidiasis (Candida auris)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.-M.; Vilgalys, R. Fungal Community Analysis by Large-Scale Sequencing of Environmental Samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Lopez-Ribot, J.L. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-Y. Human fungal pathogens: Why should we learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Santos, M.; Fonseca, A.; Mendonça, P.; Branco, R.; Serra, A.; Morais, P.; Coelho, J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voltan, A.R.; Quindós, G.; Alarcón, K.P.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar] [CrossRef] [Green Version]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Sardi, J.D.C.O.; Pitangui, N.D.S.; Rodríguez-Arellanes, G.; Taylor, M.L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Highlights in pathogenic fungal biofilms. Rev. Iberoam. Micol. 2014, 31, 22–29. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Morones-Ramírez, J.R. Bacterial Exopolysaccharides as Reducing and/or Stabilizing Agents during Synthesis of Metal Nanoparticles with Biomedical Applications. Int. J. Polym. Sci. 2018, 2018, 7045852. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Escárcega-González, C.E.; Barriga Castro, E.D.; Mendiola-Garza, G.; Marichal-Cancino, B.A.; López-Vázquez, M.A.; Morones-Ramirez, J.R. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int. J. Nanomed. 2019, 14, 2557–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Leon-Buitimea, A.; Zárate, X.; Morones-Ramirez, J.R. Antibacterial and antibiofilm activity of biosynthesized silver nanoparticles coated with exopolysaccharides obtained from Rhodotorula mucilaginosa. IEEE Trans. Nanobiosci. 2020, 19, 498–503. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2018, 536, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity Against Gram-Negative Bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [Green Version]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Díaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chávez Morales, R.M.; Regalado-Soto, D.I.; Treviño-González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef] [Green Version]
- Debourgogne, A.; Dorin, J.; Machouart, M. Emerging infections due to filamentous fungi in humans and animals: Only the tip of the iceberg? Environ. Microbiol. Rep. 2016, 8, 332–342. [Google Scholar] [CrossRef]
- Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Luis Hernando, F.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Van der Torre, M.H.; Shen, H.; Rautemaa-Richardson, R.; Richardson, M.D.; Novak-Frazer, L. Molecular Epidemiology of Aspergillus fumigatus in Chronic Pulmonary Aspergillosis Patients. J. Fungi 2021, 7, 152. [Google Scholar] [CrossRef]
- Thompson, G.; Brown, J.; Benedict, K.; Park, B. Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013, 5, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020, 11, e03364-19. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Auyeung, A.; Casillas-Santana, M.Á.; Martínez-Castañón, G.A.; Slavin, Y.N.; Zhao, W.; Asnis, J.; Häfeli, U.O.; Bach, H. Effective control of molds using a combination of nanoparticles. PLoS ONE 2017, 12, e0169940. [Google Scholar] [CrossRef] [Green Version]
- Tekaia, F.; Latgé, J.P. Aspergillus fumigatus: Saprophyte or pathogen? Curr. Opin. Microbiol. 2005, 8, 385–392. [Google Scholar] [CrossRef]
- Wéry, N. Bioaerosols from composting facilities—A review. Front. Cell. Infect. Microbiol. 2014, 4, 42. [Google Scholar]
- Van De Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkanci, A.; Ozdek, S. Ocular fungal infections. Curr. Eye Res. 2011, 36, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of aspergillosis: Clinical practice guidelines of the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Coccidioides immitis and posadasii: A review of their biology, genomics, pathogenesis, and host immunity. Virulence 2018, 9, 1426–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelthaler, D.M.; Roe, C.C.; Hepp, C.M.; Teixeira, M.; Driebe, E.M.; Schupp, J.M.; Gade, L.; Waddell, V.; Komatsu, K.; Arathoon, E.; et al. Local population structure and patterns of Western Hemisphere dispersal for Coccidioides spp., the fungal cause of valley fever. mBio 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twarog, M.; Thompson, G. Coccidiodomicosis: Recent Update. Semin. Respir. Crit. Care Med. 2015, 36, 746–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.H. Travel-related risk factors for coccidioidomycosis. J. Travel Med. 2018, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Kobayashi, M.; Garg, S.; Chiller, T.; Jackson, B.R. Symptoms in Blastomycosis, Coccidioidomycosis, and Histoplasmosis versus Other Respiratory Illnesses in Commercially Insured Adult Outpatients—United States, 2016–2017. Clin. Infect. Dis. 2020, ciaa1554. [Google Scholar] [CrossRef] [PubMed]
- Kollath, D.R.; Miller, K.J.; Barker, B.M. The mysterious desert dwellers: Coccidioides immitis and Coccidioides posadasii, causative fungal agents of coccidioidomycosis. Virulence 2019, 10, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Alqarihi, A.; Ibrahim, A.S. Mucormycoses. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 600–612. [Google Scholar]
- Hassan, M.I.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med. Mycol. 2019, 57, S245–S256. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.; Pandey, A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus 2020, 12, e10726. [Google Scholar] [CrossRef]
- Sarkar, S.; Gokhale, T.; Choudhury, S.; Deb, A. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021, 69, 1002. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146. [Google Scholar] [CrossRef]
- Revannavar, S.M.; Supriya, P.S.; Samaga, L.; Vineeth, V.K. COVID-19 triggering mucormycosis in a susceptible patient: A new phenomenon in the developing world? BMJ Case Rep. 2021, 14, e241663. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. The use of nanoparticles as alternative therapeutic agents against Candida infections: An up-to-date overview and future perspectives. World J. Microbiol. Biotechnol. 2020, 36, 163. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Hanson, K.E. Candida auris: An Emerging Fungal Pathogen. J. Clin. Microbiol. 2018, 56, e01588-17. [Google Scholar] [CrossRef] [Green Version]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Billamboz, M.; Fatima, Z.; Hameed, S.; Jawhara, S. Promising Drug Candidates and New Strategies for Fighting against the Emerging Superbug Candida auris. Microorganisms 2021, 9, 634. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Jackson, B.R.; Chow, N.; Forsberg, K.; Litvintseva, A.P.; Lockhart, S.R.; Welsh, R.; Vallabhaneni, S.; Chiller, T. On the Origins of a Species: What Might Explain the Rise of Candida auris? J. Fungi 2019, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.; Sherry, L.; Townsend, E.; McKloud, E.; Short, B.; Akinbobola, A.; Mackay, W.G.; Williams, C.; Jones, B.L.; Ramage, G. Surface disinfection challenges for Candida auris: An in-vitro study. J. Hosp. Infect. 2018, 98, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Aigner, M.; Lass-Flörl, C. Treatment of drug-resistant Aspergillus infection. Expert Opin. Pharmacother. 2015, 16, 2267–2270. [Google Scholar] [CrossRef] [Green Version]
- Pascual, A.; Csajka, C.; Buclin, T.; Bolay, S.; Bille, J.; Calandra, T.; Marchetti, O. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: Population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 2012, 55, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Ampel, N.M. Tratamento da coccidioidomicose. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef]
- Thompson, G.R.; Lewis, J.S.; Nix, D.E.; Patterson, T.F. Current Concepts and Future Directions in the Pharmacology and Treatment of Coccidioidomycosis. Med. Mycol. 2019, 57, S76–S84. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, R.; Lash, D.B.; Heidari, A.; Natarajan, P.; Johnson, R.H. Evaluation of Amphotericin B Lipid Formulations for Treatment of Severe Coccidioidomycosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Antoniadou, A.; Dupont, B. Lipid formulations of amphotericin B: Where are we today? J. Mycol. Med. 2005, 15, 230–238. [Google Scholar] [CrossRef]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56, S93–S101. [Google Scholar] [CrossRef] [Green Version]
- Sipsas, N.V.; Gamaletsou, M.N.; Anastasopoulou, A.; Kontoyiannis, D.P. Therapy of Mucormycosis. J. Fungi 2018, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Jenks, J.D.; Salzer, H.J.F.; Prattes, J.; Krause, R.; Buchheidt, D.; Hoenigl, M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: Design, development, and place in therapy. Drug Des. Dev. Ther. 2018, 12, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2014, 41, 285–295. [Google Scholar] [CrossRef]
- Sardi, J.d.C.O.; Silva, D.R.; Mendes-Giannini, M.J.S.; Rosalen, P.L. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilshad, E.; Bibi, M.; Sheikh, N.A.; Tamrin, K.F.; Mansoor, Q.; Maqbool, Q.; Nawaz, M. Synthesis of functional silver nanoparticles and microparticles with modifiers and evaluation of their antimicrobial, anticancer, and antioxidant activity. J. Funct. Biomater. 2020, 11, 76. [Google Scholar] [CrossRef]
- Jung, S.H.; Lim, D.H.; Jung, S.H.; Lee, J.E.; Jeong, K.-S.; Seong, H.; Shin, B.C. Amphotericin B-entrapping lipid nanoparticles and their in vitro and in vivo characteristics. Eur. J. Pharm. Sci. 2009, 37, 313–320. [Google Scholar] [CrossRef]
- Hasan, S. A Review on Nanoparticles: Their Synthesis and Types. Res. J. Recent Sci. Uttar Pradesh 2014, 4, 1–3. [Google Scholar]
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle Characterization: State of the Art, Challenges, and Emerging Technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef]
- Barrak, H.; Saied, T.; Chevallier, P.; Laroche, G.; M’nif, A.; Hamzaoui, A.H. Synthesis, characterization, and functionalization of ZnO nanoparticles by N-(trimethoxysilylpropyl) ethylenediamine triacetic acid (TMSEDTA): Investigation of the interactions between Phloroglucinol and ZnO@TMSEDTA. Arab. J. Chem. 2019, 12, 4340–4347. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yin, L.; Zhong, Z. Nanoparticles. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–483. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. Mater. Sci. 2008, 3, 417–433. [Google Scholar]
- Garza-Cervantes, J.A.; Meza-Bustillos, J.F.; Resendiz-Hernandez, H.; Suarez-Cantú, I.A.; Ortega-Rivera, O.A.; Salinas, E.; Escarcega-Gonzalez, C.E.; Morones-Ramirez, J.R. Re-sensitizing ampicillin and kanamycin-resistant E. coli and S. aureus using synergistic metal micronutrients-antibiotic combinations. Front. Bioeng. Biotechnol. 2020, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Vivek, M.; Kumar, P.S.; Steffi, S.; Sudha, S. Biogenic silver nanoparticles by gelidiella acerosa extract and their antifungal effects. Avicenna J. Med. Biotechnol. 2011, 3, 143–148. [Google Scholar]
- Miri, A.; Mahdinejad, N.; Ebrahimy, O.; Khatami, M.; Sarani, M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater. Sci. Eng. C 2019, 104, 109981. [Google Scholar] [CrossRef]
- Quirós, J.; Gonzalo, S.; Jalvo, B.; Boltes, K.; Perdigón-Melón, J.A.; Rosal, R. Electrospun cellulose acetate composites containing supported metal nanoparticles for antifungal membranes. Sci. Total Environ. 2016, 563–564, 912–920. [Google Scholar] [CrossRef]
- Montelongo-Peralta, L.Z.; León-Buitimea, A.; Palma-Nicolás, J.P.; Gonzalez-Christen, J.; Morones-Ramírez, J.R. Antibacterial Activity of combinatorial treatments composed of transition-metal/antibiotics against Mycobacterium tuberculosis. Sci. Rep. 2019, 9, 5471. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521–522, 305–314. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, C.; Li, X.; He, Y.; Zhou, L.; Pang, G.; Sun, S. In vitro antifungal activity of silver nanoparticles against ocular pathogenic filamentous fungi. J. Ocul. Pharmacol. Ther. 2013, 29, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, M.A.; Abdelsalam, H.K.; El-Bassuony, A.A.H. Antimicrobial activity of Novel spinel nanoferrites against pathogenic fungi and bacteria. World J. Microbiol. Biotechnol. 2020, 36, 25. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; De Klerk, C.; Kim, J.; Kang, M.; Fosso-Kankeu, E. Eco friendly approach for synthesis, characterization and biological activities of milk protein stabilized silver nanoparticles. Polymers 2020, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Alagar, M. Analytical detection and biological assay of antileukemic drug 5-fluorouracil using gold nanoparticles as probe. Int. J. Pharm. 2007, 337, 275–281. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Doyle, B.P.; Carleschi, E.; Youmbi Fonkui, T.; Erasmus, R.; Fosso-Kankeu, E.; Pillay, K.; Mbianda, X.Y. Spectroscopic characterization and antimicrobial activity of nanoparticle doped cyclodextrin polyurethane bionanosponge. Mater. Sci. Eng. C 2020, 115, 111092. [Google Scholar] [CrossRef]
- Mane, P.C.; Chaudhari, R.D.; Shinde, M.D.; Kadam, D.D.; Song, C.K.; Amalnerkar, D.P.; Lee, H. Designing Ecofriendly Bionanocomposite Assembly with Improved Antimicrobial and Potent on-site Zika Virus Vector Larvicidal Activities with its Mode of Action. Sci. Rep. 2017, 7, 15531. [Google Scholar] [CrossRef] [Green Version]
- Sabira, S.F.; Kasabe, A.M.; Mane, P.C.; Chaudhari, R.D.; Adhyapak, P.V. Selective antifungal and antibacterial activities of Ag-Cu and Cu-Ag core-shell nanostructures synthesized in-situ PVA. Nanotechnology 2020, 31, 485705. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization, and biological activity of cross-linked chitosan biguanidine loaded with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2016, 27, 1880–1898. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; de Melo, E.M.; Dugmore, T.I.J.; Matharu, A.S.; Morones-Ramirez, J.R. Antimicrobial activity of a silver-microfibrillated cellulose biocomposite against susceptible and resistant bacteria. Sci. Rep. 2020, 10, 7281. [Google Scholar] [CrossRef]
- Jaffri, S.B.; Ahmad, K.S. Neoteric environmental detoxification of organic pollutants and pathogenic microbes via green synthesized ZnO nanoparticles. Environ. Technol. 2019, 40, 3745–3761. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.H.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Safi, S.Z.; Khan, F.U.; Imran, M.; et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J. Photochem. Photobiol. B Biol. 2019, 192, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Akande, M.A.; Ojo, S.A.; Folarin, B.I.; Gueguim-Kana, E.B.; Beukes, L.S. Paper wasp nest-mediated biosynthesis of silver nanoparticles for antimicrobial, catalytic, anticoagulant, and thrombolytic applications. 3 Biotech 2016, 6, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lateef, A.; Folarin, B.I.; Oladejo, S.M.; Akinola, P.O.; Beukes, L.S.; Gueguim-Kana, E.B. Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Prep. Biochem. Biotechnol. 2018, 48, 646–652. [Google Scholar] [CrossRef]
- El Sayed, M.T.; El-Sayed, A.S.A. Biocidal activity of metal nanoparticles synthesized by fusarium solani against multidrug-resistant bacteria and mycotoxigenic fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef]
- Khan, T.; Yasmin, A.; Townley, H.E. An evaluation of the activity of biologically synthesized silver nanoparticles against bacteria, fungi and mammalian cell lines. Colloids Surf. B Biointerfaces 2020, 194, 111156. [Google Scholar] [CrossRef]
- Ojo, S.A.; Lateef, A.; Azeez, M.A.; Oladejo, S.M.; Akinwale, A.S.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Gueguim-Kana, E.B.; et al. Biomedical and Catalytic Applications of Gold and Silver-Gold Alloy Nanoparticles Biosynthesized Using Cell-Free Extract of Bacillus Safensis LAU 13: Antifungal, Dye Degradation, Anti-Coagulant and Thrombolytic Activities. IEEE Trans. Nanobiosci. 2016, 15, 433–442. [Google Scholar] [CrossRef]
- Shakibaie, M.; Mohazab, N.S.; Ayatollahi Mousavi, S.A. Antifungal activity of selenium nanoparticles synthesized by bacillus species Msh-1 against Aspergillus fumigatus and Candida albicans. Jundishapur J. Microbiol. 2015, 8, 26381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenmozhi, M.; Kannabiran, K.; Kumar, R.; Gopiesh Khanna, V. Antifungal activity of Streptomyces sp. VITSTK7 and its synthesized Ag2O/Ag nanoparticles against medically important Aspergillus pathogens. J. Mycol. Med. 2013, 23, 97–103. [Google Scholar] [CrossRef]
- Roy, I.; Thapa, M.; Goswami, A. Nanohexaconazole: Synthesis, characterisation and efficacy of a novel fungicidal nanodispersion. IET Nanobiotechnol. 2018, 12, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, Y.S.; Prasad, Y.D.; Chandasana, H.; Vishvkarma, A.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int. J. Biol. Macromol. 2015, 72, 1451–1458. [Google Scholar] [CrossRef]
- Malhotra, S.; Singh, S.; Rana, N.; Tomar, S.; Bhatnagar, P.; Gupta, M.; Singh, S.K.; Singh, B.K.; Chhillar, A.K.; Prasad, A.K.; et al. Chemoenzymatic synthesis, nanotization, and anti-Aspergillus activity of optically enriched fluconazole analogues. Antimicrob. Agents Chemother. 2017, 61, e00273-17. [Google Scholar] [CrossRef] [Green Version]
- Fukui, H.; Koike, T.; Nakagawa, T.; Saheki, A.; Sonoke, S.; Tomii, Y.; Seki, J. Comparison of LNS-AmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS®), with commercial lipid-based formulations. Int. J. Pharm. 2003, 267, 101–112. [Google Scholar] [CrossRef]
- Van De Ven, H.; Paulussen, C.; Feijens, P.B.; Matheeussen, A.; Rombaut, P.; Kayaert, P.; Van Den Mooter, G.; Weyenberg, W.; Cos, P.; Maes, L.; et al. PLGA nanoparticles and nanosuspensions with amphotericin B: Potent in vitro and in vivo alternatives to Fungizone and AmBisome. J. Control. Release 2012, 161, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Shirkhani, K.; Teo, I.; Armstrong-James, D.; Shaunak, S. Nebulised amphotericin B-polymethacrylic acid nanoparticle prophylaxis prevents invasive aspergillosis. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khames, A.; Khaleel, M.A.; El-Badawy, M.F.; El-Nezhawy, A.O.H. Natamycin solid lipid nanoparticles - sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomed. 2019, 14, 2515–2531. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, P.; Patil, A.; Wu, K.W.; Sweeney, C.; Tripathi, S.; Avula, B.; Taskar, P.; Khan, S.; Majumdar, S. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Int. J. Pharm. 2019, 572, 118771. [Google Scholar] [CrossRef]
- Chellat, F.; Merhi, Y.; Moreau, A.; Yahia, L. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials 2005, 26, 7260–7275. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.P.; Santos, M.d.F.M.A.; Saldanha, C.A.; Iocca, D.C.; Azevedo, R.B. Amphotericin B: An antifungal drug in nanoformulations for the treatment of paracoccidioidomycosis. Rev. Univ. Ind. Santander. Salud 2013, 45, 45–53. [Google Scholar]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Furebring, M.; Öberg, G.; Sjölin, J. Side-effects of Amphotericin B lipid complex (Abelcet) in the Scandinavian population. Bone Marrow Transplant. 2000, 25, 341–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, E.R.; Eldridge, M.L.; McHardy, I.; Cohen, S.H.; Thompson, G.R. Liposomal Amphotericin B as Monotherapy in Relapsed Coccidioidal Meningitis. Mycopathologia 2018, 183, 619–622. [Google Scholar] [CrossRef]
- Antony, S.; Dominguez, D.C.; Sotelo, E. Use of liposomal amphotericin B in the treatment of disseminated coccidioidomycosis. J. Natl. Med. Assoc. 2003, 95, 982–985. [Google Scholar]
- Gómez Rivera, N.; Dorame Castillo, R.; Contreras Soto, J.; Talamante, S. Tratamiento de coccidioidomicosis meníngea con anfotericina liposomal: Presentación de un caso. Boletín Médico Hosp. Infant. México (Ed. Española) 2010, 67, 142–146. [Google Scholar]
- Nakhla, S.G. Complications and Management of a Rare Case of Disseminated Coccidioidomycosis to the Vertebral Spine. Case Rep. Infect. Dis. 2018, 2018, 8954016. [Google Scholar] [CrossRef]
- Clemons, K.V.; Capilla, J.; Sobel, R.A.; Martinez, M.; Tong, A.-J.; Stevens, D.A. Comparative Efficacies of Lipid-Complexed Amphotericin B and Liposomal Amphotericin B against Coccidioidal Meningitis in Rabbits. Antimicrob. Agents Chemother. 2009, 53, 1858–1862. [Google Scholar] [CrossRef] [Green Version]
- González, G.M.; Tijerina, R.; Najvar, L.K.; Bocanegra, R.; Rinaldi, M.G.; Graybill, J.R. Efficacies of Amphotericin B (AMB) Lipid Complex, AMB Colloidal Dispersion, Liposomal AMB, and Conventional AMB in Treatment of Murine Coccidioidomycosis. Antimicrob. Agents Chemother. 2004, 48, 2140–2143. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, P.; Cornely, O.A.; Dannaoui, E. Antifungal combinations in Mucorales: A microbiological perspective. Mycoses 2019, 62, 746–760. [Google Scholar] [CrossRef]
- Dannaoui, E. Antifungal resistance in mucorales. Int. J. Antimicrob. Agents 2017, 50, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20, 5–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, C.; Kuriakose, S.; George, S.; Mathew, T. Antifungal activity of silver nanoparticle-encapsulated β-cyclodextrin against human opportunistic pathogens. Supramol. Chem. 2011, 23, 593–597. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Mohamed, D.Y. Detection the antifungal effect of zirconium oxide nanoparticles on mold which isolated from domestic’s bathroom. Al-Mustansiriyah J. Sci. 2018, 29, 15–22. [Google Scholar] [CrossRef]
- Cao, Z.; Spilker, T.; Fan, Y.; Kalikin, L.M.; Ciotti, S.; LiPuma, J.J.; Makidon, P.E.; Wilkinson, J.E.; Baker, J.R.; Wang, S.H. Nanoemulsion is an effective antimicrobial for methicillin-resistant Staphylococcus aureus in infected wounds. Nanomedicine 2017, 12, 1177–1185. [Google Scholar] [CrossRef]
- Brunet, K.; Rammaert, B. Mucormycosis treatment: Recommendations, latest advances, and perspectives. J. Mycol. Med. 2020, 30, 101007. [Google Scholar] [CrossRef]
- Basketter, D.A.; Marriott, M.; Gilmour, N.J.; White, I.R. Strong irritants masquerading as skin allergens: The case of benzalkonium chloride. Contact Dermat. 2004, 50, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Fan, Y.Y.; Vellanki, S.; Huh, E.Y.; Vanegas, D.; Wang, S.H.; Lee, S.C. Nanoemulsion as an Effective Treatment against Human-Pathogenic Fungi. mSphere 2019, 4, e00729-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris Biofilm Formation on Medical and Environmental Surfaces by Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Silver Nanoantibiotics Display Strong Antifungal Activity Against the Emergent Multidrug-Resistant Yeast Candida auris Under Both Planktonic and Biofilm Growing Conditions. Front. Microbiol. 2020, 11, 1673. [Google Scholar] [CrossRef]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.M.; Hakeem, K.R.; Ahmad, A.; Malik, M.A. Facile Bio-Fabrication of Ag-Cu-Co Trimetallic Nanoparticles and Its Fungicidal Activity against Candida auris. J. Fungi 2021, 7, 62. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Bismuth Nanoantibiotics Display Anticandidal Activity and Disrupt the Biofilm and Cell Morphology of the Emergent Pathogenic Yeast Candida auris. Antibiotics 2020, 9, 461. [Google Scholar] [CrossRef]
- Cleare, L.G.; Li, K.L.; Abuzeid, W.M.; Nacharaju, P.; Friedman, J.M.; Nosanchuk, J.D. NO Candida auris: Nitric Oxide in Nanotherapeutics to Combat Emerging Fungal Pathogen Candida auris. J. Fungi 2020, 6, 85. [Google Scholar] [CrossRef] [PubMed]
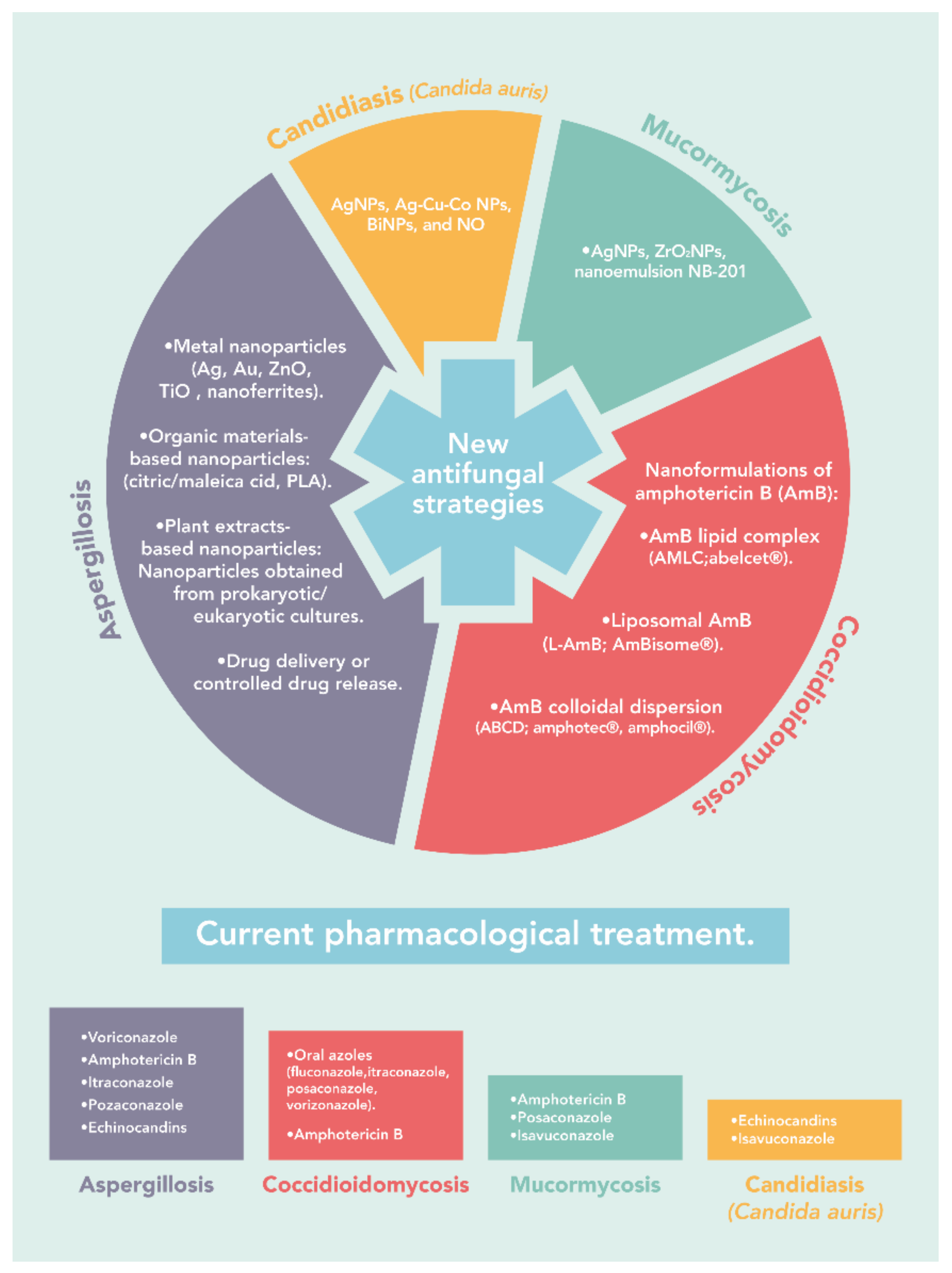
| Filamentous Fungi | Disease | Geographical Distribution (Incidence) | Epidemiological Data | References |
|---|---|---|---|---|
| Aspergillus fumigatus | Aspergillosis | Worldwide distribution | Immunocompromised individuals with altered or weakened immune responses are able to develop aspergillosis. | [25,26] |
| Coccidioides immitis and Coccidioides posadasii | Coccidioidomycosis | Central Valley of California, desert areas of Arizona, Texas, Utah; Mexico; Central (Guatemala and Honduras), and South America (Colombia, Venezuela, Argentina, Paraguay, and Brazil). | Elderly persons, pregnant women, and members of certain ethnic groups are at risk for severe or disseminated coccidioidomycosis. Further, persons with immunodeficiency diseases, diabetes, transplant recipients, and prisoners are particularly vulnerable. | [27,28] |
| Rhizopus, Mucor | Mucormycosis | Europe (34%), Asia (31%), North/South America (28%), Africa (3%), and Australia/New Zealand (3%) | Patients with uncontrolled diabetes mellitus, cancer, solid organ or bone marrow transplantation, hematological malignancy, corticosteroids treatment, and trauma and burns are especially vulnerable to Mucorales infection. | [7,29] |
| Candida auris (non-filamentous fungus) | Candidiasis | Worldwide distribution | Elderly age, diabetes mellitus, recent surgery, the presence of an indwelling medical device, an immunosuppressed state, the use of hemodialysis, a neutropenic state, chronic renal disease, or the use of broad-spectrum antibiotic and/or antifungal drugs are related to C. auris infections. | [30,31] |
| Nanomaterial | Antifungal Effect | Reference |
|---|---|---|
| AgNPs | Growth inhibition at 10 µg/mL | [33] |
| 54% growth inhibition at 100 mg/L | [92] | |
| 75.61% growth inhibition at 150 µg/mL | [105] | |
| Growth inhibition at 150 µg/mL | [106] | |
| Growth inhibition at 40 µg/mL | [107] | |
| 60% growth inhibition at 50 µg/mL | [110] | |
| Marketed AgNPs | 90% growth inhibition at 0.5 µg/mL (clinical isolates) | [93] |
| AgO/Ag NPs | 75.25% growth inhibition at 50 µg/mL | [113] |
| Ag-AuNPs | 90.78% growth inhibition at 200 µg/mL | [111] |
| Ag2Cr2O4 | 3.1 times higher inhibition than fluconazole | [94] |
| Maleic acid capped AgNPs | Growth inhibition | [74] |
| Milk protein synthesized AgNPs | Growth inhibition | [95] |
| Fibroin-AgNPs | Fungicidal activity at 2 µg/mL | [99] |
| Ag-Cu core-shell NPs | Growth inhibition at 0.1 M and fungicidal activity at 15 µg/mL | [100] |
| CChG/AgNPs | Better growth inhibition than AmB at 0.98 µg/mL | [101] |
| CuNPs | Growth inhibition at 31.67 µg/mL | [107] |
| Cu-Ag core-shell NPs | Growth inhibition at 0.1M and fungicidal activity at 25 µg/mL | [100] |
| Au@5FU NPs | Higher inhibition than 5FU | [96] |
| Fibroin-AuNPs | Fungicidal activity at 10 µg/mL | [99] |
| TiO2-PLA NPs | 99.9% growth inhibition at 8 wt% of NPs | [97] |
| pMWCNT-CD/Ag-TiO2 nanosponge | Growth inhibition at 437.5 µg/mL | [98] |
| ZnONPs | Growth inhibition at 20 µg/mL | [33] |
| Higher inhibition zone than AmB (resistant strain) | [103] | |
| 51% growth inhibition at 100 µg/mL | [104] | |
| Growth inhibition at 26.7 µg/mL | [107] | |
| SeNPs | Growth inhibition at 250 µg/mL | [109] |
| Growth inhibition at 100 µg/mL | [112] | |
| N-Hexa | Growth reduction at 10 ppm | [114] |
| Natamicyin encapsulated L/C NPs | Similar growth inhibition than natamycin | [115] |
| AmB encapsulated L/C NPs | Growth inhibition at 0.12 µg/mL | [116] |
| AmB entrapped lipid NPs | Growth inhibition at 0.025 µg/mL | [75] |
| AmB loaded PLGA NPs | 50% growth inhibition at 0.03 µg/mL | [119] |
| AmB-PMA NPs | Growth inhibition with 300 µg of AmB | [120] |
| AmB leaded PEG NLC | Growth inhibition at 1.25 µg/mL | [122] |
| Fluconazole encapsulated O-alkylated dextran | Growth inhibition at 3.16 µg/mL | [117] |
| Natamycin SLNPs | Better inhibition zones than natamycin | [121] |
| Nanomaterial | Antifungal Effect | Reference |
|---|---|---|
| Amphotericin B lipid complex (ABLC, Abelcet®) | Highly effective treatment. | [132,133] |
| Liposomal amphotericin B (L-AmB, AmBisome®) | Successfully used as an alternative and safe option of treatment. | [128,129,130,131,133] |
| Amphotericin B colloidal dispersion (ABCD, Amphotec®/Amphocil®) | Well tolerated and effective treatment | [133] |
| Nanomaterial | Antifungal Effect | Reference |
|---|---|---|
| Nanoemulsions NB-201 | Growth inhibition | [141] |
| Silver nanoparticles (AgNPs), | Growth inhibition | [138] |
| Zirconium oxide nanoparticles (ZrO2NPs) | Growth inhibition | [140] |
| Nanomaterial | Antifungal Effect | Reference |
|---|---|---|
| Silver nanoparticles (AgNPs) | Biofilm formation inhibition, planktonic growth inhibition | [146,148] |
| Trimetallic nanoparticles (Ag-Cu-Co NPs) | Growth reduction, lower viability, cellular arrest, mitochondria membrane damage | [149] |
| Bismuth nanoparticles (BiNPs) | Affect cellular morphology, biofilm formation inhibition | [150] |
| Nitric oxide (NO) | Biofilm formation reduction, planktonic growth inhibition | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Buitimea, A.; Garza-Cervantes, J.A.; Gallegos-Alvarado, D.Y.; Osorio-Concepción, M.; Morones-Ramírez, J.R. Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathogens 2021, 10, 1303. https://doi.org/10.3390/pathogens10101303
León-Buitimea A, Garza-Cervantes JA, Gallegos-Alvarado DY, Osorio-Concepción M, Morones-Ramírez JR. Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathogens. 2021; 10(10):1303. https://doi.org/10.3390/pathogens10101303
Chicago/Turabian StyleLeón-Buitimea, Angel, Javier A. Garza-Cervantes, Diana Y. Gallegos-Alvarado, Macario Osorio-Concepción, and José Ruben Morones-Ramírez. 2021. "Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis" Pathogens 10, no. 10: 1303. https://doi.org/10.3390/pathogens10101303
APA StyleLeón-Buitimea, A., Garza-Cervantes, J. A., Gallegos-Alvarado, D. Y., Osorio-Concepción, M., & Morones-Ramírez, J. R. (2021). Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathogens, 10(10), 1303. https://doi.org/10.3390/pathogens10101303







