Waning of Maternal Antibodies against Measles Suggests a Large Window of Susceptibility in Infants in Lao People’s Democratic Republic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Serology
2.3. Statistical Analysis
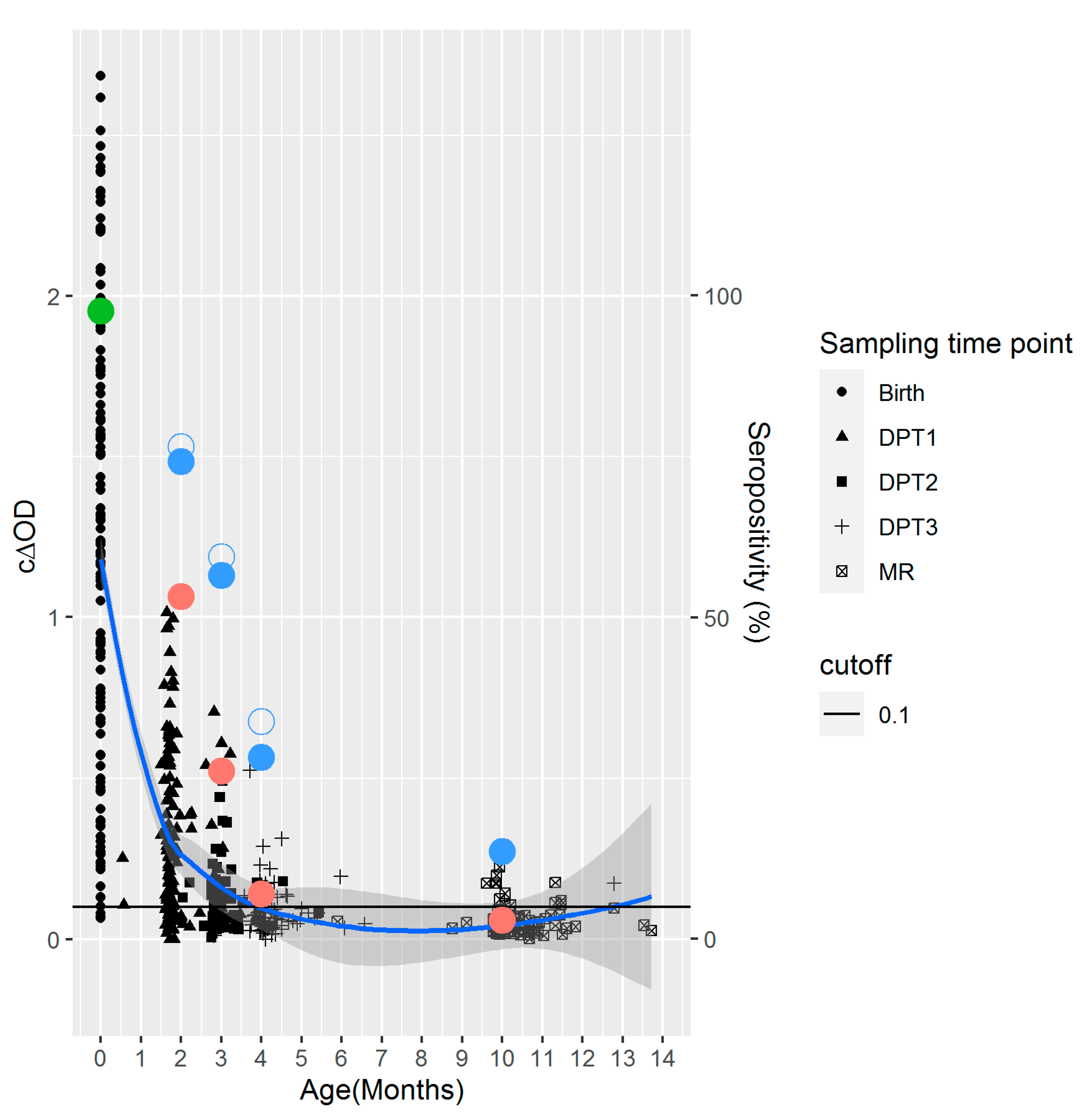
3. Results
3.1. Evaluation of the DBS Elution Protocol
3.2. Statistical Description of the Mother-Infant Pairs
3.3. Serology of the Mothers
3.4. Serology of the Infants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef]
- World Health Organization. Table 2: Summary of WHO Position Papers—Recommended Routine Immunizations for Children (Updated September 2020). 2019. Available online: https://www.who.int/immunization/policy/Immunization_routine_table2.pdf (accessed on 13 August 2021).
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly. Epidemiol. Rec. 2017, 92, 205–227. [Google Scholar]
- van Boven, M.; Kretzschmar, M.; Wallinga, J.; O’Neill, P.D.; Wichmann, O.; Hahné, S. Estimation of measles vaccine efficacy and critical vaccination coverage in a highly vaccinated population. J. R. Soc. Interface 2010, 7, 1537–1544. [Google Scholar] [CrossRef]
- van den Berg, J.P.; Westerbeek, E.A.; Smits, G.P.; van der Klis, F.R.; Berbers, G.A.; van Elburg, R.M. Lower transplacental antibody transport for measles, mumps, rubella and varicella zoster in very preterm infants. PLoS ONE 2014, 9, e94714. [Google Scholar]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front Immunol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Leuridan, E.; Van Damme, P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 2007, 25, 6296–6304. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Fisker, A.B.; Nebie, E.; Schoeps, A.; Martins, C.; Rodrigues, A.; Zakane, A.; Kagone, M.; Byberg, S.; Thysen, S.M.; Tiendrebeogo, J.; et al. A Two-Center Randomized Trial of an Additional Early Dose of Measles Vaccine: Effects on Mortality and Measles Antibody Levels. Clin. Infect. Dis. 2018, 66, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.L.; Garly, M.-L.; Balé, C.; Rodrigues, A.; Ravn, H.; Whittle, H.C.; Lisse, I.M.; Aaby, P. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: Interim analysis of a randomised clinical trial. BMJ 2008, 337, a661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudenberg, T.; van der Maas, N.A.T.; Knol, M.J.; de Melker, H.; van Binnendijk, R.S.; Hahne, S.J.M. Effectiveness of Early Measles, Mumps, and Rubella Vaccination Among 6–14-Month-Old Infants During an Epidemic in the Netherlands: An Observational Cohort Study. J. Infect. Dis. 2017, 215, 1181–1187. [Google Scholar] [CrossRef] [Green Version]
- Feikin, D.R.; Flannery, B.; Hamel, M.J.; Stack, M.; Hansen, P.M. Vaccines for Children in Low- and Middle-Income Countries. In Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, 3rd ed.; Black, R.E., Laxminarayan, R., Temmerman, M., Walker, N., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 2. [Google Scholar]
- World Health Organization. Laos Introduced the Measles Rubella Second Dose to Protect Children Aged 12 to 18 Months. 2017. Available online: https://www.who.int/laos/news/detail/22-11-2017-laos-introduced-the-measles-rubella-second-dose-to-protect-children-aged-12-to-18-months (accessed on 13 August 2021).
- World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System. 2019 Global Summary. 2019. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=LAO (accessed on 24 June 2020).
- Ministry of Health Lao People’s Democratic Republic. National Strategy and Plan of Action for Measles and Rubella Elimination in Lao PDR, 2018–2022; Lao People’s Democratic Republic, Ministry of Health: Vientiane, Laos, 2018; pp. 1–11.
- Hachiya, M.; Miyano, S.; Mori, Y.; Vynnycky, E.; Keungsaneth, P.; Vongphrachanh, P.; Xeuatvongsa, A.; Sisouk, T.; Som-Oulay, V.; Khamphaphongphane, B.; et al. Evaluation of nationwide supplementary immunization in Lao People’s Democratic Republic: Population-based seroprevalence survey of anti-measles and anti-rubella IgG in children and adults, mathematical modelling and a stability testing of the vaccine. PLoS ONE 2018, 13, e0194931. [Google Scholar] [CrossRef]
- Xeuatvongsa, A.; Hachiya, M.; Miyano, S.; Mizoue, T.; Kitamura, T. Determination of factors affecting the vaccination status of children aged 12–35 months in Lao People’s Democratic Republic. Heliyon 2017, 3, e00265. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Regional Office for the Western Pacific. Measles-Rubella Bulletin. 2020. Available online: https://iris.wpro.who.int/bitstream/handle/10665.1/14490/Measles-Rubella-Bulletin-2020-Vol-14-No-12.pdf (accessed on 13 August 2021).
- Hagan, J.E.; Kriss, J.L.; Takashima, Y.; Mariano, K.M.L.; Pastore, R.; Grabovac, V.; Dabbagh, A.J.; Goodson, J.L. Progress Toward Measles Elimination—Western Pacific Region, 2013–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Nouanthong, P.; Hübschen, J.M.; Billamay, S.; Mongkhoune, S.; Vilivong, K.; Khounvisith, V.; Sinner, R.; Grandadam, M.; Phonekeo, D.; Black, A.P.; et al. Varicella zoster and fever rash surveillance in Lao People’s Democratic Republic. BMC Infect. Dis. 2019, 19, 392. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for the Western Pacific. International Review of the Expanded Programme on Immunization in the Lao People’s Democratic Republic, May 2012. 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/207530/9789290616009_eng.pdf?sequence=1&isAllowed=y (accessed on 13 August 2021).
- Mercader, S.; Featherstone, D.; Bellini, W.J. Comparison of available methods to elute serum from dried blood spot samples for measles serology. J. Virol. Methods 2006, 137, 140–149. [Google Scholar] [CrossRef]
- Riddell, M.A.; Byrnes, G.B.; Leydon, J.A.; Kelly, H.A. Dried venous blood samples for the detection and quantification of measles IgG using a commercial enzyme immunoassay. Bull. World Health Organ 2003, 81, 701–707. [Google Scholar]
- The R Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 13 August 2021).
- Aragon, T.J. EpiTools: R Package for Epidemiologic Data and Graphics. 2012. Available online: http://www2.uaem.mx/r-mirror/web/packages/epitools/index.html (accessed on 13 August 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, Third Edition. 2019. Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 24 June 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. 2016. Available online: https://ggplot2.tidyverse.org/ (accessed on 24 June 2020).
- Cutts, F.T.; Hanson, M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health 2016, 21, 1086–1098. [Google Scholar] [CrossRef]
- Lakshmy, R. Analysis of the use of dried blood spot measurements in disease screening. J. Diabetes Sci. Technol. 2008, 2, 242–243. [Google Scholar] [CrossRef] [Green Version]
- Leuridan, E.; Hens, N.; Hutse, V.; Ieven, M.; Aerts, M.; Van Damme, P. Early waning of maternal measles antibodies in era of measles elimination: Longitudinal study. BMJ 2010, 340, c1626. [Google Scholar] [CrossRef] [Green Version]
- Szenborn, L.; Tischer, A.; Pejcz, J.; Rudkowski, Z.; Wojcik, M. Passive acquired immunity against measles in infants born to naturally infected and vaccinated mothers. Med. Sci. Monit. 2003, 9, CR541-6. [Google Scholar]
- Carazo Perez, S.; De Serres, G.; Bureau, A.; Skowronski, D.M. Reduced Antibody Response to Infant Measles Vaccination: Effects Based on Type and Timing of the First Vaccine Dose Persist After the Second Dose. Clin. Infect. Dis. 2017, 65, 1094–1102. [Google Scholar] [CrossRef]
- Gidding, H.F.; Quinn, H.E.; Hueston, L.; Dwyer, D.E.; McIntyre, P.B. Declining measles antibodies in the era of elimination: Australia’s experience. Vaccine 2018, 36, 507–513. [Google Scholar] [CrossRef]
- Guerra, F.M.; Crowcroft, N.S.; Friedman, L.; Deeks, S.L.; Halperin, S.A.; Severini, A.; Hatchette, T.F.; Bolotin, S. Waning of measles maternal antibody in infants in measles elimination settings—A systematic literature review. Vaccine 2018, 36, 1248–1255. [Google Scholar] [CrossRef]
- Science, M.; Savage, R.; Severini, A.; McLachlan, E.; Hughes, S.L.; Arnold, C.; Richardson, S.; Crowcroft, N.; Deeks, S.; Halperin, S.; et al. Measles Antibody Levels in Young Infants. Pediatrics 2019, 144, e20190630. [Google Scholar] [CrossRef]
- Waaijenborg, S.; Hahné, S.J.M.; Mollema, L.; Smits, G.P.; Berbers, G.A.M.; Van Der Klis, F.R.M.; De Melker, H.E.; Wallinga, J. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J. Infect Dis. 2013, 208, 10–16. [Google Scholar] [CrossRef]
- Nic Lochlainn, L.M.; de Gier, B.; van der Maas, N.; Strebel, P.M.; Goodman, T.; van Binnendijk, R.S.; de Melker, H.E.; Hahné, S.J.M. Immunogenicity, effectiveness, and safety of measles vaccination in infants younger than 9 months: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 1235–1245. [Google Scholar] [CrossRef] [Green Version]
- Do, V.A.; Biering-Sorensen, S.; Fisker, A.B.; Bale, C.; Rasmussen, S.M.; Christensen, L.D.; Jensen, K.J.; Martins, C.; Aaby, P.; Benn, C.S. Effect of an Early Dose of Measles Vaccine on Morbidity Between 18 Weeks and 9 Months of Age: A Randomized, Controlled Trial in Guinea-Bissau. J. Infect. Dis. 2017, 215, 1188–1196. [Google Scholar] [CrossRef]
- Gans, H.A.; Yasukawa, L.L.; Sung, P.; Sullivan, B.; DeHovitz, R.; Audet, S.; Beeler, J.; Arvin, A.M. Measles humoral and cell-mediated immunity in children aged 5–10 years after primary measles immunization administered at 6 or 9 months of age. J. Infect. Dis. 2013, 207, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.M.; Biering-Sørensen, S.; Byberg, S.; Andersen, A.; Bjerregaard-Andersen, M.; Rodrigues, A.; Benn, C.S.; Martins, C.L.; Aaby, P. The effect of early measles vaccination at 4.5 months of age on growth at 9 and 24 months of age in a randomized trial in Guinea-Bissau. BMC Pediatr. 2016, 16, 199. [Google Scholar] [CrossRef] [Green Version]
- Woo, E.J.; Winiecki, S.K.; Arya, D.; Beeler, J. Adverse Events after MMR or MMRV Vaccine in Infants Under Nine Months Old. Pediatr. Infect. Dis. J. 2016, 35, e253–e257. [Google Scholar] [CrossRef]
- Nic Lochlainn, L.M.; de Gier, B.; van der Maas, N.; van Binnendijk, R.; Strebel, P.M.; Goodman, T.; de Melker, H.E.; Moss, W.J.; Hahné, S.J.M. Effect of measles vaccination in infants younger than 9 months on the immune response to subsequent measles vaccine doses: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.M. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015, 33, 6469–6472. [Google Scholar] [CrossRef] [Green Version]
- Vono, M.; Eberhardt, C.S.; Auderset, F.; Mastelic-Gavillet, B.; Lemeille, S.; Christensen, D.; Andersen, P.; Lambert, P.-H.; Siegrist, C.-A. Maternal Antibodies Inhibit Neonatal and Infant Responses to Vaccination by Shaping the Early-Life B Cell Repertoire within Germinal Centers. Cell Rep. 2019, 28, 1773–1784.e5. [Google Scholar] [CrossRef] [Green Version]

| Mother-Related Variables | Anti-Measles IgG Seropositivity | IgG Titer (mIU/mL) | ||||
|---|---|---|---|---|---|---|
| n/N (%) | p-Value a | Median (95% CI) | p-Value b | |||
| All time points | Age groups (years) | <20 | 28/32 (87.5) | 0.10 | 2695.8 (998.9–6418.0) | <0.01 |
| 20–24 | 107/112 (95.5) | 3346.6 (1233.4–7203.2) | ||||
| 25–29 | 184/188 (97.9) | 3790.8 (1467.5–7988.0) | ||||
| 30–45 | 119/126 (94.4) | 5302.0 (2533.0–10207.0) | ||||
| >45 | 48/50 (96.0) | 4587.8 (2298.2–7215.2) | ||||
| Measles-containing vaccination recalled | No | 141/151 (93.4) | 0.15 | 4174.9 (1880.4-8603.2) | 0.89 | |
| Yes | 345/357 (96.6) | 3937.6 (1499.6–8135.7) | ||||
| Fever rash recalled | No | 339/357 (95.0) | 0.22 | 4030.4 (1717.0–8279.6) | 0.50 | |
| Yes | 130/134 (97.0) | 5332.4 (1444.3–7431.1) | ||||
| unknown | 17 | n.a. | ||||
| Measles infection recalled | No | 395/416 (95.0) | 0.15 | 3878.9 (1563.2–8306.0) | 0.79 | |
| Yes | 91/92 (98.9) | 4166.0 (1754.0–7230.0) | ||||
| Parity number | 1 | 201/208 (96.6) | 0.51 | 3721.0 (1353.9–7657.9) | 0.09 | |
| >1 | 285/300 (95.0) | 4197.0 (1760.3–8652.7) | ||||
| Total | 486/508 (95.7) | n.a. | 4024.2 (1609.0–8157.0) | n.a. | ||
| Child-Related Variables | Anti-measles IgG Seropositivity | IgG Titer (mIU/mL) | ||||
| n/N (%) | p-Value | Median (95% CI) | p-Value | |||
| At birth (cord blood) | Birth weight of child (g) | ≤3000 | 53/54 (98.1) | 1.00 | 4326.9 (1631.9–9682.2) | 0.61 |
| >3000 | 74/76 (97.4) | 3715.4 (1218.6–7314.6) | ||||
| Total | 127/130 (97.7) | n.a. | 4332.1 (1698.8–8309.1) | n.a. | ||
| Other time points (DBS c) | Vaccination schedule | DPT-HepB-Hib 1 | 102/139 (73.4) | <0.01 | 591.3 (295.8–1275.3) | <0.01 |
| DPT-HepB-Hib 2 | 36/63 (57.1) | 246.2 (200.3–361.6) | ||||
| DPT-HepB-Hib 3 | 20/82 (24.4) | 243.9 (208.0–326.9) | ||||
| MCV1 | 13/94 (13.8) | 218.0 (170.2–276.6) | ||||
| Breast feeding | No | 14/51 (27.5) | 0.16 | 360.9 (207.0–647.6) | 0.35 | |
| Yes | 157/327 (48.0) | 358.1 (214.2–831.0) | ||||
| Total | 171/378 (45.2) | n.a. | 358.1 (213.7–808.1) | n.a. | ||
| All time points | Total | 298/508 (58.7) | n.a. | 807.9 (287.7–3369.3) | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khampanisong, P.; Pauly, M.; Nouanthong, P.; Vickers, M.A.; Virachith, S.; Xaydalasouk, K.; Black, A.P.; Muller, C.P.; Hübschen, J.M. Waning of Maternal Antibodies against Measles Suggests a Large Window of Susceptibility in Infants in Lao People’s Democratic Republic. Pathogens 2021, 10, 1316. https://doi.org/10.3390/pathogens10101316
Khampanisong P, Pauly M, Nouanthong P, Vickers MA, Virachith S, Xaydalasouk K, Black AP, Muller CP, Hübschen JM. Waning of Maternal Antibodies against Measles Suggests a Large Window of Susceptibility in Infants in Lao People’s Democratic Republic. Pathogens. 2021; 10(10):1316. https://doi.org/10.3390/pathogens10101316
Chicago/Turabian StyleKhampanisong, Phonepaseuth, Maude Pauly, Phonethipsavanh Nouanthong, Molly A. Vickers, Siriphone Virachith, Kinnaly Xaydalasouk, Antony P. Black, Claude P. Muller, and Judith M. Hübschen. 2021. "Waning of Maternal Antibodies against Measles Suggests a Large Window of Susceptibility in Infants in Lao People’s Democratic Republic" Pathogens 10, no. 10: 1316. https://doi.org/10.3390/pathogens10101316
APA StyleKhampanisong, P., Pauly, M., Nouanthong, P., Vickers, M. A., Virachith, S., Xaydalasouk, K., Black, A. P., Muller, C. P., & Hübschen, J. M. (2021). Waning of Maternal Antibodies against Measles Suggests a Large Window of Susceptibility in Infants in Lao People’s Democratic Republic. Pathogens, 10(10), 1316. https://doi.org/10.3390/pathogens10101316







