Assessment of the Genetic Diversity of Echinococcus multilocularis from Copro-Isolated Eggs
Abstract
:1. Introduction
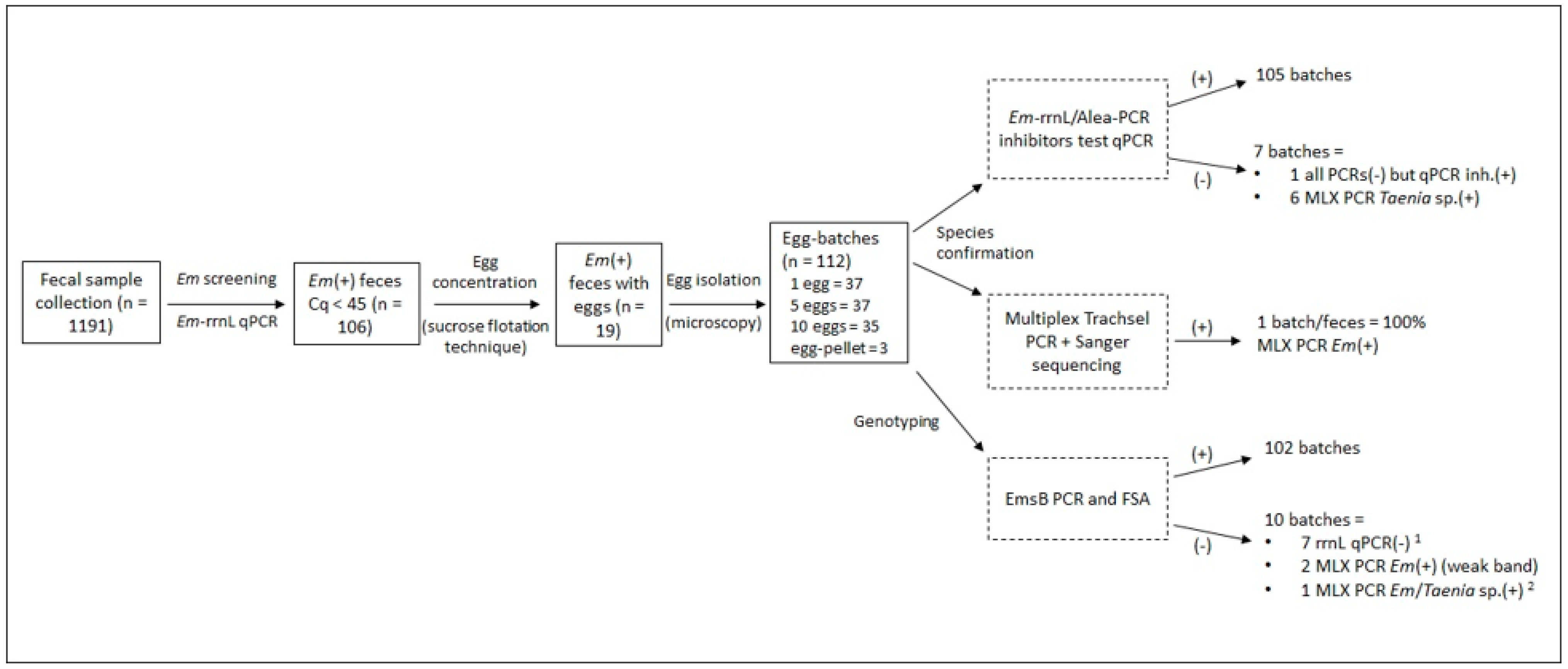
2. Results
2.1. Copro-Sample and Egg Isolation
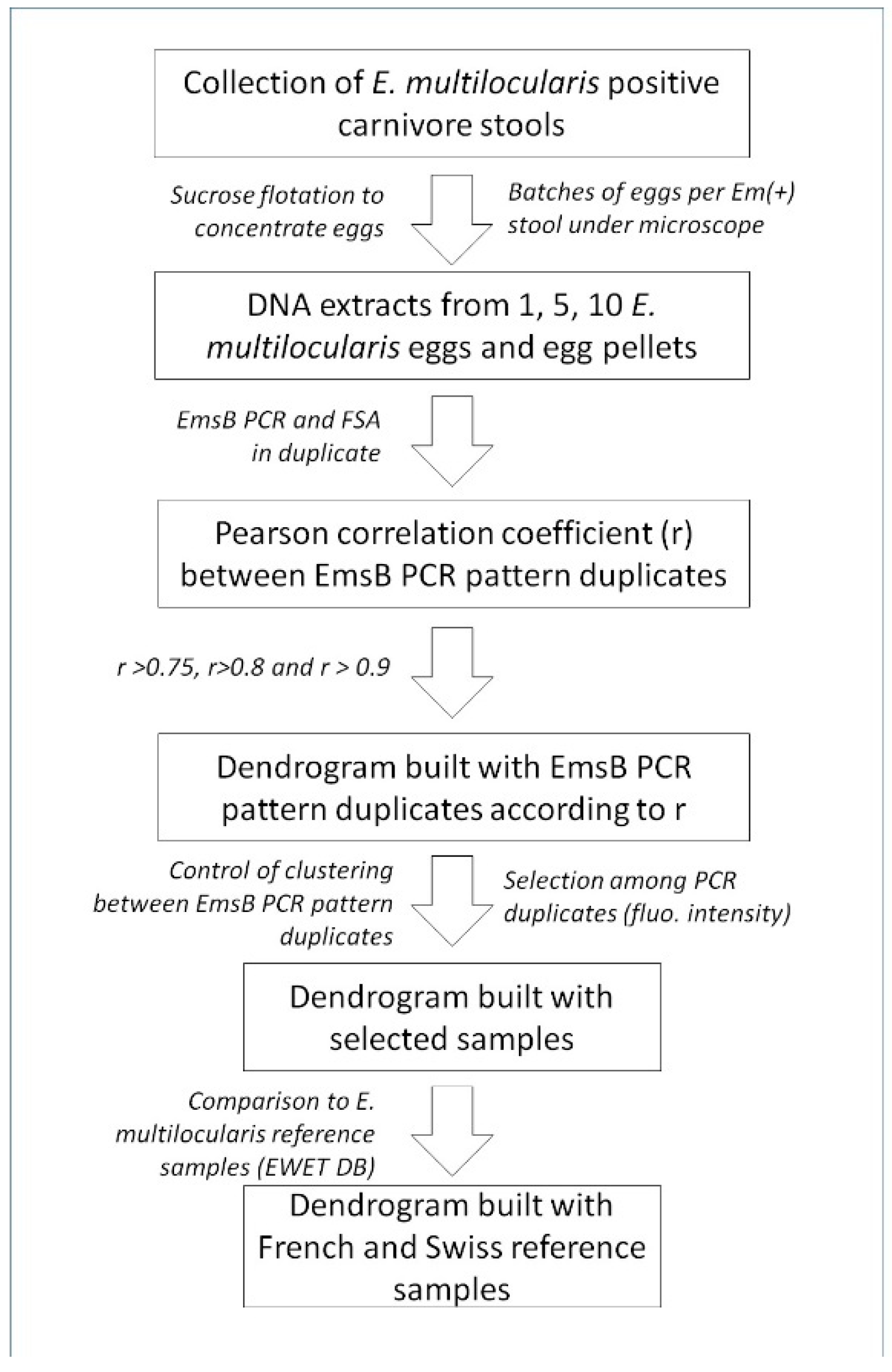
2.2. EmsB Fragment Size Analysis and Comparison of Pearson Coefficient Sorting
2.3. Correlation between EmsB Profiles and Individual Identification of Carnivores
3. Discussion
4. Materials and Methods
4.1. Fecal Samples, Egg Isolation, and DNA Purification
4.2. EmsB Amplification and Fragment Size Analyses
4.3. Hierarchical Clustering Analysis and Pearson Coefficient Sorting
4.4. Correlation between EmsB Profiles and Individual Identification of Carnivores
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vuitton, D.A.; Zhou, H.; Bresson-Hadni, S.; Wang, Q.; Piarroux, M.; Raoul, F.; Giraudoux, P. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology 2003, 127, S87–S107. [Google Scholar] [CrossRef] [PubMed]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.; Wassermann, M.; Takahashi, K.; de la Rue, M. Ecology and life cycle patterns of echinococcus species. Highlighting Oper. Implement. Res. Control. Helminthiasis 2017, 95, 213–314. [Google Scholar] [CrossRef]
- Da Silva, A.M.; Courquet, S.; Raoul, F.; Rieffel, D.; Giraudoux, P.; Millon, L.; Knapp, J. Assessment of the exposure to Echinococcus multilocularis associated with carnivore faeces using real-time quantitative PCR and flotation technique assays. Int. J. Parasitol. 2020, 50, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Combes, B.; Umhang, G.; Aknouche, S.; Millon, L. Could the domestic cat play a significant role in the transmission of Echinococcus multilocularis? A study based on qPCR analysis of cat feces in a rural area in France. Parasite 2016, 23, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beldi, G.; Müller, N.; Gottstein, B. L’échinococcose alvéolaire. Forum Méd. Suisse 2017, 17, 760–766. [Google Scholar] [CrossRef]
- Thompson, R.; McManus, D. Aetiology: Parasites and life-cycles. In Manual WHO/OIE on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; WHO: Geneva, Switzerland, 2002; Volume 1, p. 9. [Google Scholar]
- Veit, P.; Bilger, B.; Schad, V.; Schäfer, J.; Frank, W.; Lucius, R. Influence of environmental factors on the infectivity of Echi-nococcus Multilocularis eggs. Parasitology 1995, 110, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Deplazes, P.; Craig, P.S.; Gemmell, M.A.; Gottstein, B.; Heath, D.; Jenkins, D.J.; Kamiya, M.; Lightowelers, M. Chapitre 3: Echinococcosis in animals: Clinical aspects, diagnosis and treatment. In WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; Eckert, J., Gemmell, M.A., Meslin, F.X., Pawlowski, Z.S., Eds.; WHO: Geneva, Switzerland, 2001; pp. 72–100. ISBN 92-9044-522-X. [Google Scholar]
- Eckert, J.; Conraths, F.; Tackmann, K. Echinococcosis: An emerging or re-emerging zoonosis? Int. J. Parasitol. 2000, 30, 1283–1294. [Google Scholar] [CrossRef]
- Combes, B.; Comte, S.; Raton, V.; Raoul, F.; Boué, F.; Umhang, G.; Favier, S.; Dunoyer, C.; Woronoff, N.; Giraudoux, P. Westward Spread of Echinococcus multilocularisin Foxes, France, 2005–2010. Emerg. Infect. Dis. 2012, 18, 2059–2062. [Google Scholar] [CrossRef]
- Knapp, J.; Gottstein, B.; Saarma, U.; Millon, L. Taxonomy, phylogeny and molecular epidemiology of Echinococcus multilocularis: From fundamental knowledge to health ecology. Vet. Parasitol. 2015, 213, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, K.L.; Zaha, A.; Araújo, A.M.; Gottstein, B. Reduced genetic variability within coding and non-coding regions of the Echinococcus multilocularis genome. Parasitology 1997, 115, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Spotin, A.; Boufana, B.; Ahmadpour, E.; Casulli, A.; Mahami-Oskouei, M.; Rouhani, S.; Javadi-Mamaghani, A.; Shahrivar, F.; Khoshakhlagh, P. Assessment of the global pattern of genetic diversity in Echinococcus multilocularis inferred by mitochondrial DNA sequences. Veter- Parasitol. 2018, 262, 30–41. [Google Scholar] [CrossRef]
- Bart, J.; Knapp, J.; Gottstein, B.; El-Garch, F.; Giraudoux, P.; Glowatzki, M.; Berthoud, H.; Maillard, S.; Piarroux, R. EmsB, a tandem repeated multi-loci microsatellite, new tool to investigate the genetic diversity of Echinococcus multilocularis. Infect. Genet. Evol. 2006, 6, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Valot, B.; Knapp, J.; Umhang, G.; Grenouillet, F.; Millon, L. Genomic characterization of EmsB microsatellite loci in Echinococcus multilocularis. Infect. Genet. Evol. 2015, 32, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Afonso, E.; Knapp, J.; Tête, N.; Umhang, G.; Rieffel, D.; van Kesteren, F.; Ziadinov, I.; Craig, P.; Torgerson, P.; Giraudoux, P. Echinococcus multilocularis in Kyrgyzstan: Similarity in the Asian EmsB genotypic profiles from village populations of Eastern mole voles (Ellobius tancrei) and dogs in the Alay valley. J. Helminthol. 2015, 89, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, E.J.; Peregrine, A.S.; Hill, J.E.; Somers, C.; Gesy, K.; Barnes, B.; Gottstein, B.; Polley, L. Detection of European Strain of Echinococcus multilocularisin North America. Emerg. Infect. Dis. 2012, 18, 1010–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, J.; Umhang, G.; Wahlström, H.; Al-Sabi, M.N.S.; Ågren, E.O.; Enemark, H.L. Genetic diversity of Echinococcus multilocularis in red foxes from two Scandinavian countries: Denmark and Sweden. Food Waterborne Parasitol. 2019, 14, e00045. [Google Scholar] [CrossRef]
- Knapp, J.; Staebler, S.; Bart, J.-M.; Stien, A.; Yoccoz, N.; Drögemüller, C.; Gottstein, B.; Deplazes, P. Echinococcus multilocularis in Svalbard, Norway: Microsatellite genotyping to investigate the origin of a highly focal contamination. Infect. Genet. Evol. 2012, 12, 1270–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, J.; Bart, J.-M.; Maillard, S.; Gottstein, B.; Piarroux, R. The genomic Echinococcus microsatellite EmsB sequences: From a molecular marker to the epidemiological tool. Parasitology 2009, 137, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, J.; Guislain, M.-H.; Bart, J.-M.; Raoul, F.; Gottstein, B.; Giraudoux, P.; Piarroux, R. Genetic diversity of Echinococcus multilocularis on a local scale. Infect. Genet. Evol. 2008, 8, 367–373. [Google Scholar] [CrossRef]
- Knapp, J.; Bart, J.M.; Glowatzki, M.L.; Ito, A.; Gerard, S.; Maillard, S.; Piarroux, R.; Gottstein, B. Assessment of use of microsatellite polymorphism analysis for improving spatial distribution tracking of Echinococcus multilocularis. J. Clin. Microbiol. 2007, 45, 2943–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umhang, G.; Knapp, J.; Hormaz, V.; Raoul, F.; Boué, F. Using the genetics of Echinococcus multilocularis to trace the history of expansion from an endemic area. Infect. Genet. Evol. 2014, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Meyer, A.; Courquet, S.; Millon, L.; Raoul, F.; Gottstein, B.; Frey, C.F. Echinococcus multilocularis genetic diversity in Swiss domestic pigs assessed by EmsB microsatellite analyzes. Vet. Parasitol. 2021, 293, 109429. [Google Scholar] [CrossRef]
- Knapp, J.; Gottstein, B.; Bretagne, S.; Bart, J.-M.; Umhang, G.; Richou, C.; Bresson-Hadni, S.; Millon, L. Genotyping Echinococcus multilocularis in human alveolar echinococcosis patients: An EmsB microsatellite analysis. Pathogens 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Bart, J.-M.; Giraudoux, P.; Glowatzki, M.-L.; Breyer, I.; Raoul, F.; Deplazes, P.; Duscher, G.; Martinek, K.; Dubinský, P.; et al. Genetic diversity of the cestode Echinococcus multilocularis in red foxes at a continental scale in europe. PLoS Neglected Trop. Dis. 2009, 3, e452. [Google Scholar] [CrossRef] [Green Version]
- Umhang, G.; Woronoff-Rhen, N.; Combes, B.; Boué, F. Segmental Sedimentation and Counting Technique (SSCT): An adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp. Parasitol. 2011, 128, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, D.; Deplazes, P.; Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007, 134, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Murillo, J.; Montoya, A.; Carrillo-Bonilla, L.; Rodriguez, B.; Vélez, I.D.; Robledo, S.M. Verification and monitoring of visceral leishmaniasis in hamsters caused by Leishmania infantum, using non-invasive approaches involving ultrasound imaging and blood gases. Exp. Parasitol. 2019, 201, 78–89. [Google Scholar] [CrossRef]
- Laurimaa, L.; Süld, K.; Moks, E.; Valdmann, H.; Umhang, G.; Knapp, J.; Saarma, U. First report of the zoonotic tapeworm Echinococcus multilocularis in raccoon dogs in Estonia, and comparisons with other countries in Europe. Vet. Parasitol. 2015, 212, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Casulli, A.; Bart, J.-M.; Knapp, J.; La Rosa, G.; Dusher, G.; Gottstein, B.; Di Cerbo, A.; Manfredi, M.T.; Genchi, C.; Piarroux, R.; et al. Multi-locus microsatellite analysis supports the hypothesis of an autochthonous focus of Echinococcus multilocularis in northern Italy. Int. J. Parasitol. 2009, 39, 837–842. [Google Scholar] [CrossRef]
- Rishi, A.K.; McManus, D.P. Genomic cloning of human Echinococcus granulosus DNA: Isolation of recombinant plasmids and their use as genetic markers in strain characterization. Parasitology 1987, 94, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Umhang, G.; Poulle, M.-L.; Millon, L. Development of a Real-Time PCR for a sensitive one-step coprodiagnosis allowing both the identification of carnivore feces and the detection of Toxocara spp. and Echinococcus multilocularis. Appl. Environ. Microbiol. 2016, 82, 2950–2958. [Google Scholar] [CrossRef] [Green Version]
- Rataj, A.V.; Posedi, J.; Žele, D.; Vengušt, G. Intestinal parasites of the red fox (Vulpes vulpes) in Slovenia. Acta Veter- Hung. 2013, 61, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Escotte-Binet, S.; Da Silva, A.M.; Cancès, B.; Aubert, D.; Dubey, J.; La Carbona, S.; Villena, I.; Poulle, M.-L. A rapid and sensitive method to detect Toxoplasma gondii oocysts in soil samples. Vet. Parasitol. 2019, 274, 108904. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Kamiya, H. Modified sugar centrifugal flotation technique for recovering Echinococcus Multilocularis eggs from soil. J. Parasitol. 2005, 91, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Damy, S.; Brillaud, J.; Tissot, J.-D.; Navion, J.; Mélior, R.; Afonso, E.; Hormaz, V.; Gottstein, B.; Umhang, G.; et al. EWET: Data collection and interface for the genetic analysis of Echinococcus multilocularis based on EmsB microsatellite. PLoS ONE 2017, 12, e0183849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-444-53868-0. [Google Scholar]
- R Studio Team. R Studio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
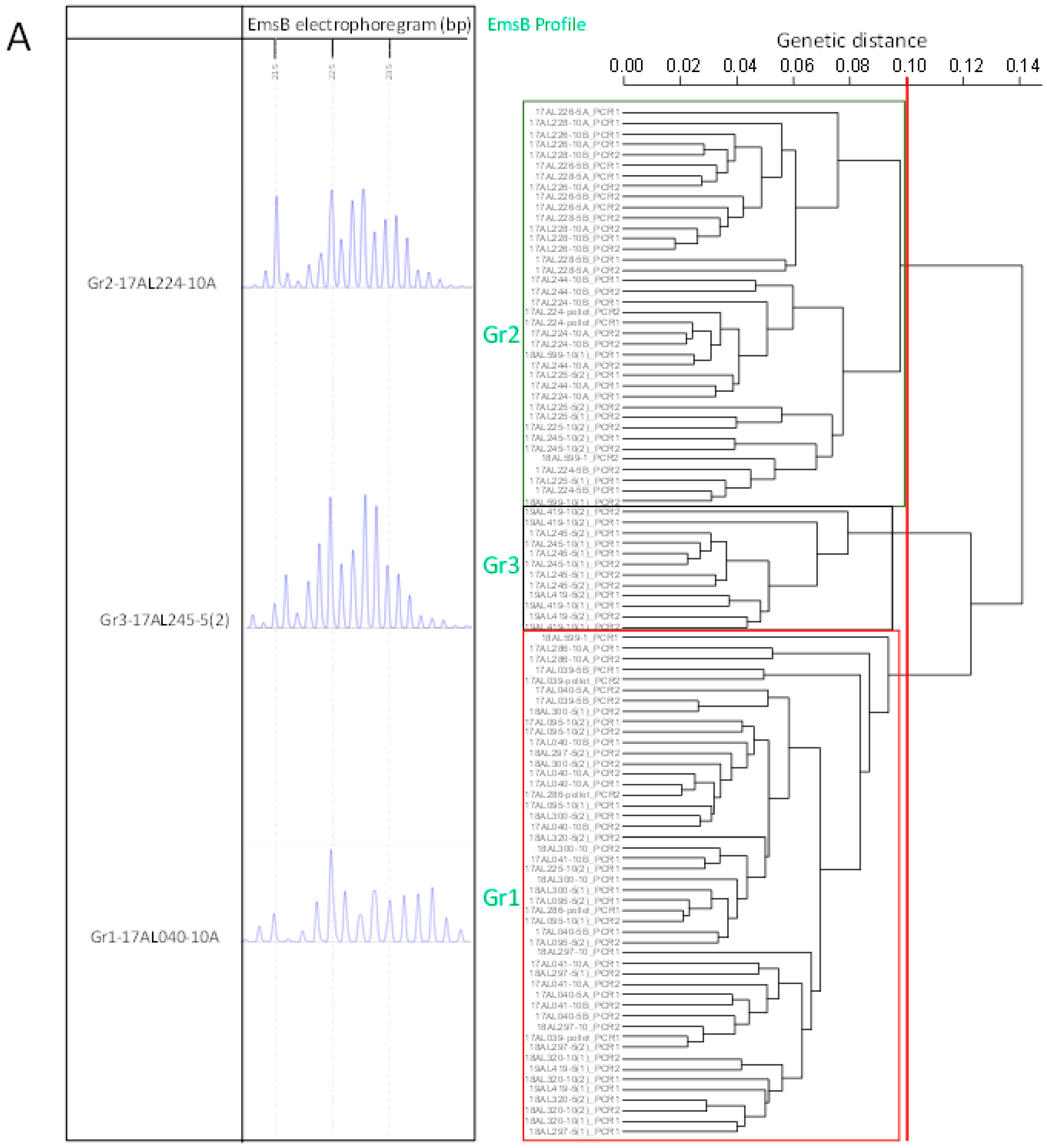
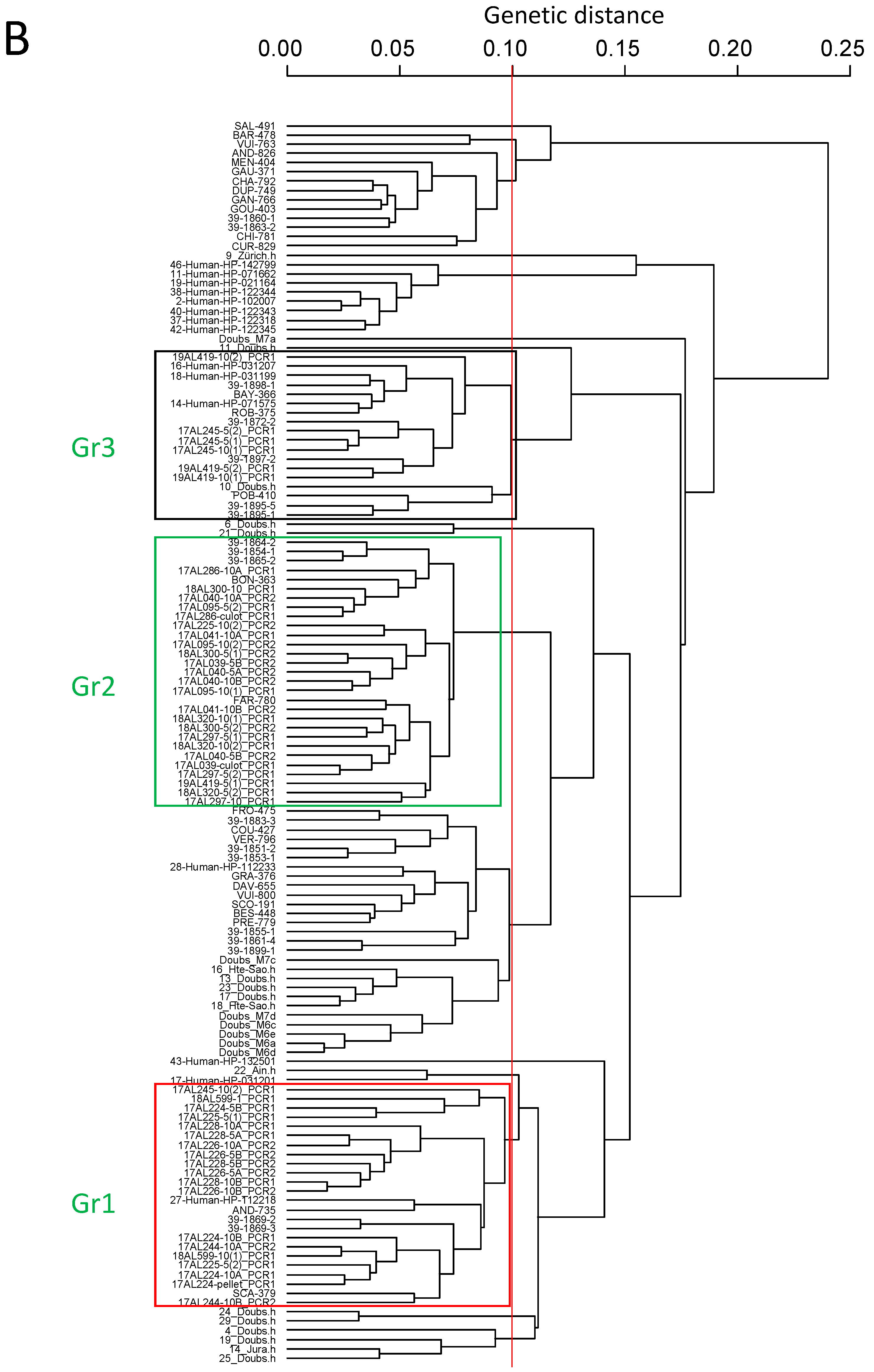
| Full Batches | r > 0.75 | r > 0.8 | r > 0.9 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Faeces Code | 1 | 1 | 5 | 5 | 10 | 10 | p | 1 | 1 | 5 | 5 | 10 | 10 | p | 1 | 1 | 5 | 5 | 10 | 10 | p | 1 | 1 | 5 | 5 | 10 | 10 | p |
| 17AL039 | OT | OT | G1 1 | G1 | G1 | G1 | G1 | / | / | / | G1 | G1 | G1 | G1 | / | / | / | G1 | G2 | G3 | G1 | / | / | / | G1 | / | / | G1 |
| 17AL040 | OT | G3 1 | G1 | G1 | G1 | G1 | - | / | / | G1 | G1 | G1 | G1 | - | / | / | G1 | G1 | G1 | G1 | - | / | / | G1 | G1 | G1 | G1 | - |
| 17AL041 | G1 1 | G1 | G1 | G1 | G1 | G1 | - | G1 1 | G1 | G1 | G1 | G1 | G1 | - | G1 1 | / | G1 | G1 | G1 | G1 | - | / | / | / | / | G1 | G1 | - |
| 17AL095 | NE | OT | G1 | G1 | G1 | G1 | - | NE | / | G1 | G1 | G1 | G1 | - | NE | / | G1 | G1 | G1 | G1 | - | NE | / | / | G1 | G1 | G1 | - |
| 17AL224 | G2 1 | G2 1 | G2 1 | G2 | G2 | G2 | G2 | / | G2 1 | G2 1 | G2 | G2 | G2 | G2 | / | G2 1 | G2 1 | G2 | G2 | G2 | G2 | / | / | / | G2 | G2 | G2 | G2 |
| 17AL225 | OT | NE | G2 | G2 | G2 | G1/G2 | - | OT | NE | G2 | G2 | G2 | G1/G2 | - | OT | NE | G2 | G2 | G2 | G1/G2 | - | / | NE | G2 | G2 | / | G1/G2 | - |
| 17AL226 | G2 1 | OT | G2 | G2 | G2 | G2 | - | G2 1 | / | G2 | G2 | G2 | G2 | - | G2 1 | / | G2 | G2 | G2 | G2 | - | / | / | G2 | G2 | G2 | G2 | - |
| 17AL228 | G2 1 | G2 1 | G2 | G2 | G2 | G2 | - | / | G2 1 | G2 | G2 | G2 | G2 | - | / | / | G2 | G2 | G2 | G2 | - | / | / | G2 | G2 | G2 | G2 | - |
| 17AL244 | OT | NE | G2 | G2 | G2 | G2 | - | / | NE | G2 | G2 | G2 | G2 | - | / | NE | G2 | G2 | G2 | G2 | - | / | NE | / | / | G2 | G2 | - |
| 17AL245 | NE | G2 1 | G3 | G3 | G3 | G2 | - | NE | G2 1 | G3 | G3 | G3 | G2 | - | NE | G2 1 | G3 | G3 | G3 | G2 | - | NE | / | G3 | G3 | G3 | G2 | - |
| 17AL286 | G1 1 | NE | OT | G1 1 | G1 | G1 1 | G1 | / | NE | / | / | G1 | OT | G1 | / | NE | / | / | G1 | / | G1 | / | NE | / | / | G1 | / | G1 |
| 18AL297 | G2 | OT | G1 | G1 | G1 | - | - | / | / | G1 | G1 | G1 | - | - | / | / | G1 | G1 | G1 | - | - | / | / | G1 | G1 | G1 | - | - |
| 18AL300 | G1 1 | OT | G1 | G1 | G1 | - | - | / | / | G1 | G1 | G1 | - | - | / | / | G1 | G1 | G1 | - | - | / | / | G1 | G1 | G1 | - | - |
| 18AL320 | G1 1 | NE | G1 1 | G1 | G1 | G1 | - | OT | NE | G1/G3 | G1 | G1 | G1 | - | / | NE | / | G1 | G1 | G1 | - | / | NE | / | G1 | G1 | G1 | - |
| 18AL344 | OT | OT | G2 1 | OT | OT | G1 1 | - | / | / | G1 1 | / | / | / | - | / | / | G1 1 | / | / | / | - | / | / | / | / | / | / | - |
| 18AL460 | NE | - | NE | OT | NE | NE | - | NE | - | NE | / | NE | NE | - | NE | - | NE | / | NE | NE | - | NE | - | NE | / | NE | NE | - |
| 18AL599 | G2 | OT | G2 | G1/G2 | G2 | G2 | - | G2 | / | G2 | G1/G2 | G2 | G2 | - | G2 | / | G2 | G1/G2 | G2 | G2 | - | G1/G2 | / | / | / | G2 | / | - |
| 19AL127 | OT | OT | OT | - | OT | - | - | / | / | / | - | / | - | - | / | / | / | - | / | - | - | / | / | / | - | / | - | - |
| 19AL419 | G1 1 | G3 1 | G1 | G3 | G3 | G3 | - | / | G3 1 | G1 | G3 | G3 | G3 | - | / | G3 1 | G1 | G3 | G3 | G3 | - | / | / | G1 | G3 | G3 | G3 | - |
| qPCR Cq Value 1 | No of Batches Per Egg-Batch Type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feces Code | Host ID | Molecular Host | Sampling Date | Host | Em rrnL | PCR Inhib. Test | Batch Species Typing 2 | 1 | 5 | 10 | Pellet | Total |
| 17AL039 | FOX005 | Vulpes vulpes | July 2017 | 25.85 | 28.66 | 32.92 | E. multilocularis | 2 | 2 | 2 | 1 | 7 |
| 17AL040 | FOX005 | Vulpes vulpes | July 2017 | 22.20 | 31.24 | 33.86 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL041 | FOX005 | Vulpes vulpes | July 2017 | 22.62 | 30.08 | 33.49 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL095 | DOG001 | Canis l. familiaris | March 2017 | 21.31 | 41.04 | 35.63 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL224 | FOX004 | Vulpes vulpes | May 2017 | 24.30 | 33.76 | 34.99 | E. multilocularis | 2 | 2 | 2 | 1 | 7 |
| 17AL225 | FOX020 | Vulpes vulpes | May 2017 | 23.70 | 37.59 | 35.53 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL226 | ND | Vulpes vulpes | May 2017 | 27.53 | 31.44 | 34.55 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL228 | ND | Vulpes vulpes | May 2017 | 32.25 | 33.14 | 35.37 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL244 | FOX004 | Vulpes vulpes | May 2017 | 24.64 | 36.50 | 34.90 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL245 | FOX017 | Vulpes vulpes | May 2017 | 27.91 | 39.58 | 34.81 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 17AL286 | CAT001 | Felis catus | July 2017 | 21.57 | 38.61 | 33.92 | E. multilocularis | 2 | 2 | 2 | 1 | 7 |
| 18AL297 | ND | Vulpes vulpes | May 2018 | 26.79 | 37.94 | 33.56 | E. multilocularis | 2 | 2 | 1 | 0 | 5 |
| 18AL300 | DOG010 | Canis l. familiaris | July 2018 | 21.75 | 38.52 | 33.71 | E. multilocularis | 2 | 2 | 1 | 0 | 5 |
| 18AL320 | FOX031 | Vulpes vulpes | November 2018 | 23.09 | 38.97 | 33.11 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 18AL344 | FOX031 | Vulpes vulpes | November 2018 | 30.07 | 37.51 | 33.22 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 18AL460 | FOX032 | Vulpes vulpes | September 2018 | 22.82 | 31.64 | 34.59 | Taenia crassiceps | 1 | 2 | 2 | 0 | 5 |
| 18AL599 | FOX039 | Vulpes vulpes | November 2018 | 23.39 | 30.09 | 34.05 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| 19AL127 | FOX030 | Vulpes vulpes | April 2019 | 27.34 | 35.72 | 34.46 | E. multilocularis | 2 | 1 | 1 | 0 | 4 |
| 19AL419 | ND | Canis l. familiaris | November 2019 | 21.06 | 30.59 | 33.41 | E. multilocularis | 2 | 2 | 2 | 0 | 6 |
| Total No of batches | 37 | 37 | 35 | 3 | 112 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapp, J.; Da Silva, A.M.; Courquet, S.; Millon, L. Assessment of the Genetic Diversity of Echinococcus multilocularis from Copro-Isolated Eggs. Pathogens 2021, 10, 1296. https://doi.org/10.3390/pathogens10101296
Knapp J, Da Silva AM, Courquet S, Millon L. Assessment of the Genetic Diversity of Echinococcus multilocularis from Copro-Isolated Eggs. Pathogens. 2021; 10(10):1296. https://doi.org/10.3390/pathogens10101296
Chicago/Turabian StyleKnapp, Jenny, Abdou Malik Da Silva, Sandra Courquet, and Laurence Millon. 2021. "Assessment of the Genetic Diversity of Echinococcus multilocularis from Copro-Isolated Eggs" Pathogens 10, no. 10: 1296. https://doi.org/10.3390/pathogens10101296
APA StyleKnapp, J., Da Silva, A. M., Courquet, S., & Millon, L. (2021). Assessment of the Genetic Diversity of Echinococcus multilocularis from Copro-Isolated Eggs. Pathogens, 10(10), 1296. https://doi.org/10.3390/pathogens10101296






