Sea Turtles in the Cancer Risk Landscape: A Global Meta-Analysis of Fibropapillomatosis Prevalence and Associated Risk Factors
Abstract
1. Introduction
2. Material and Methods
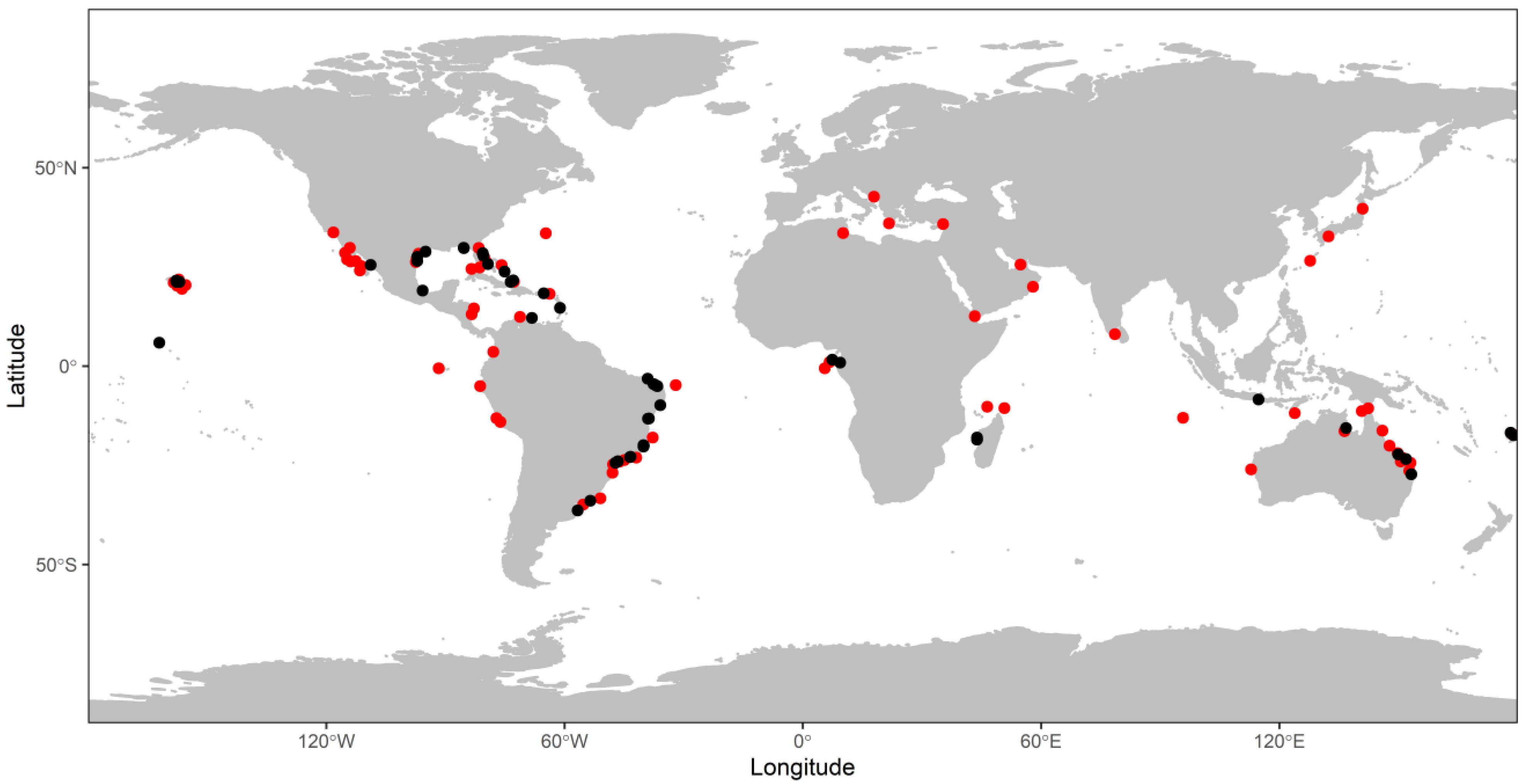
2.1. Literature Review and Data Consolidation
2.2. Identification of Potential Risk Factors
2.2.1. Exposure to the Chelonid Herpesvirus 5 Virus
2.2.2. Ultraviolet Light
2.2.3. Seabed Depth
2.2.4. Eutrophication
2.2.5. Sea Surface Temperature
2.2.6. Potential ChHV5 Vectors
2.3. Data Consolidation and Spatial Scale Selection
2.4. Statistical Analysis
2.4.1. Data Exploration
2.4.2. Quantification of the Effect of Each Risk Factor
3. Results
4. Discussion
4.1. Effect of Eutrophication
4.2. Transmission of the Virus
4.3. Implications for the Conservation of Green Turtles
4.4. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aktipis, C.A.; Boddy, A.M.; Jansen, G.; Hibner, U.; Hochberg, M.E.; Maley, C.C.; Wilkinson, G. Cancer across the tree of life: Cooperation and cheating in multicellularity. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140219. [Google Scholar] [CrossRef]
- Albuquerque, T.A.F.; Val, L.D.D.; Doherty, A.; De Magalhães, J.P. From humans to hydra: Patterns of cancer across the tree of life. Biol. Rev. 2018, 93, 1715–1734. [Google Scholar] [CrossRef]
- Duesberg, P.; Mandrioli, D.; McCormack, A.; Nicholson, J.M. Is carcinogenesis a form of speciation? Cell Cycle 2011, 10, 2100–2114. [Google Scholar] [CrossRef]
- Capp, J.-P.; Thomas, F. A Similar Speciation process relying on cellular stochasticity in microbial and cancer cell populations. iScience 2020, 23, 101531. [Google Scholar] [CrossRef]
- Pienta, K.J.; Hammarlund, E.U.; Axelrod, R.; Amend, S.R.; Brown, J.S. Convergent evolution, evolving evolvability, and the origins of lethal cancer. Mol. Cancer Res. 2020, 18, 801–810. [Google Scholar] [CrossRef] [PubMed]
- McCallum, H.; Jones, M.; Hawkins, C.; Hamede, R.; Lachish, S.; Sinn, D.L.; Beeton, N.; Lazenby, B. Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology 2009, 90, 3379–3392. [Google Scholar] [CrossRef] [PubMed]
- Browning, H.M.; Gulland, F.M.D.; Hammond, J.A.; Colegrove, K.M.; Hall, A.J. Common cancer in a wild animal: The California sea lion (Zalophus californianus) as an emerging model for carcinogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140228. [Google Scholar] [CrossRef] [PubMed]
- Vickers, T.W.; Clifford, D.L.; Garcelon, D.K.; King, J.L.; Duncan, C.L.; Gaffney, P.M.; Boyce, W.M. Pathology and epidemiology of ceruminous gland tumors among endangered Santa Catalina Island foxes (Urocyon littoralis catalinae) in the Channel Islands, USA. PLoS ONE 2015, 10, e0143211. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.P.; Ludwig, A.; Harper, C.; Moodley, Y.; Bertschinger, H.J.; Guthrie, A.J. Comparative genetics of sarcoid tumour-affected and non-affected mountain zebra (Equus zebra) populations. South. Afr. J. Wildl. Res. 2011, 41, 36–49. [Google Scholar] [CrossRef][Green Version]
- Mateo, D.R.; Maccallum, G.S.; Davidson, J. Field and laboratory transmission studies of haemic neoplasia in the soft-shell clam, Mya arenaria, from Atlantic Canada. J. Fish. Dis. 2016, 39, 913–927. [Google Scholar] [CrossRef]
- Hollings, T.; Jones, M.; Mooney, N.; Mccallum, H. Trophic cascades following the disease-induced fecline of an apex predator, the Tasmanian devil. Conserv. Biol. 2014, 28, 63–75. [Google Scholar] [CrossRef]
- Hollings, T.; Jones, M.; Mooney, N.; McCallum, H. Disease-induced decline of an apex predator drives invasive dominated states and threatens biodiversity. Ecology 2016, 97, 394–405. [Google Scholar] [CrossRef]
- Cunningham, C.X.; Johnson, C.N.; Barmuta, L.; Hollings, T.; Woehler, E.; Jones, M. Top carnivore decline has cascading effects on scavengers and carrion persistence. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181582. [Google Scholar] [CrossRef]
- Perret, C.; Gidoin, C.; Ujvari, B.; Thomas, F.; Roche, B. Predation shapes the impact of cancer on population dynamics and the evolution of cancer resistance. Evol. Appl. 2020, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boutry, J.; Mistral, J.; Berlioz, L.; Klimovich, A.; Tökölyi, J.; Fontenille, L.; Ujvari, B.; Dujon, A.M.; Giraudeau, M.; Thomas, F. Tumors (re)shape biotic interactions within ecosystems: Experimental evidence from the freshwater cnidarian Hydra. Sci. Total Environ. 2021, 803, 149923. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.; Baars, C.; Hesterman, H.; Hocking, G.; Jones, M.; Lazenby, B.; Mann, D.; Mooney, N.; Pemberton, D.; Pyecroft, S.; et al. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biol. Conserv. 2006, 131, 307–324. [Google Scholar] [CrossRef]
- Dujon, A.M.; Aktipis, A.; Alix-Panabières, C.; Amend, S.R.; Boddy, A.M.; Brown, J.S.; Capp, J.; DeGregori, J.; Ewald, P.; Gatenby, R.; et al. Identifying key questions in the ecology and evolution of cancer. Evol. Appl. 2021, 14, 877–892. [Google Scholar] [CrossRef]
- Vittecoq, M.; Roche, B.; Daoust, S.P.; Ducasse, H.; Missé, D.; Abadie, J.; Labrut, S.; Renaud, F.; Gauthier-Clerc, M.; Thomas, F. Cancer: A missing link in ecosystem functioning? Trends Ecol. Evol. 2013, 28, 628–635. [Google Scholar] [CrossRef]
- Giraudeau, M.; Sepp, T.; Ujvari, B.; Ewald, P.W.; Thomas, F. Human activities might influence oncogenic processes in wild animal populations. Nat. Ecol. Evol. 2018, 2, 1065–1070. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Agnew, D.; Keel, M.K.; Woolard, K.D. Cancer in wildlife: Patterns of emergence. Nat. Rev. Cancer 2018, 18, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.E.; Noble, R. A framework for how environment contributes to cancer risk. Ecol. Lett. 2017, 20, 117–134. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–953. [Google Scholar] [CrossRef]
- Dujon, A.M.; Ujvari, B.; Thomas, F. Cancer risk landscapes: A framework to study cancer in ecosystems. Sci. Total Environ. 2021, 763, 142955. [Google Scholar] [CrossRef]
- Herbst, L.H. Fibropapillomatosis of marine turtles. Annu. Rev. Fish. Dis. 1994, 4, 389–425. [Google Scholar] [CrossRef]
- Alfaro-Núñez, A.; Bertelsen, M.F.; Bojesen, A.M.; Rasmussen, I.; Zepeda-Mendoza, L.; Olsen, M.T.; Gilbert, M.T.P. Global distribution of Chelonid fibropapilloma-associated herpesvirus among clinically healthy sea turtles. BMC Evol. Biol. 2014, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in Green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Coates, C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zoologica 1938, 23, 93–98. [Google Scholar] [CrossRef]
- Work, T.M.; Work, T.M.; Balazs, G.H.; Rameyer, R.A.; Morris, R.A. Retrospective pathology survey of green turtles Chelonia mydas with fibropapillomatosis in the Hawaiian Islands, 1993–2003. Dis. Aquat. Organ. 2004, 62, 163–176. [Google Scholar] [CrossRef]
- Herbst, L.H.; Jacobson, E.R.; Moretti, R.; Brown, T.; Sundberg, J.P.; Klein, P.A. Experimental transmission of green turtle fibropapillomatosis using cell-free tumor extracts. Dis. Aquat. Org. 1995, 22, 1–12. [Google Scholar] [CrossRef]
- Patrício, A.R.; Herbst, L.H.; Duarte, A.; Vélez-Zuazo, X.; Loureiro, N.; Pereira, N.; Tavares, L.; Toranzos, G.A. Global phylogeography and evolution of chelonid fibropapilloma-associated herpesvirus. J. Gen. Virol. 2012, 93, 1035–1045. [Google Scholar] [CrossRef]
- Ackermann, M.; Koriabine, M.; Hartmann-Fritsch, F.; De Jong, P.J.; Lewis, T.D.; Schetle, N.; Work, T.M.; Dagenais, J.; Balazs, G.H.; Leong, J.-A.C. The genome of chelonid herpesvirus 5 harbors atypical genes. PLoS ONE 2012, 7, e46623. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Norton, T.M.; Ritchie, B.; Brown, C.; Mancia, C.; Jackwood, M.; Gottdenker, N.L. Quantifying chelonid herpesvirus 5 in symptomatic and asymptomatic rehabilitating green sea turtles. Endanger. Species Res. 2015, 28, 135–146. [Google Scholar] [CrossRef]
- Brill, R.W.; Balazs, G.H.; Holland, K.N.; Chang, R.K.; Sullivan, S.; George, J.C. Daily movements, habitat use, and submergence intervals of normal and tumor-bearing juvenile green turtles (Chelonia mydas L.) within a foraging area in the Hawaiian Islands. J. Exp. Mar. Biol. Ecol. 1995, 185, 203–218. [Google Scholar] [CrossRef]
- Perrault, J.; Levin, M.; Mott, C.; Bovery, C.; Bresette, M.; Chabot, R.; Gregory, C.; Guertin, J.; Hirsch, S.; Ritchie, B.; et al. Insights on immune function in free-ranging green sea turtles (Chelonia mydas) with and without Fibropapillomatosis. Animals 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Balazs, G.H.; Wolcott, M.; Morris, R. Bacteraemia in free-ranging Hawaiian green turtles Chelonia mydas with fibropapillomatosis. Dis. Aquat. Org. 2003, 53, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Page-Karjian, A.; Norton, T.M.; Krimer, P.; Groner, M.; Nelson, S.E.; Gottdenker, N. Factors influencing survivorship of rehabilitating green sea turtles (Chelonia mydas) with fibropapillomatosis. J. Zoo Wildl. Med. 2014, 45, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, S.M.; Gitirana, H.M.; Wanderley, A.V.; Monteiro-Neto, C.; Lobo-Hajdu, G. Evidence of regression of fibropapillomas in juvenile green turtles Chelonia mydas caught in Niterói, southeast Brazil. Dis. Aquat. Org. 2013, 102, 243–247. [Google Scholar] [CrossRef]
- James, A.; Page-Karjian, A.; Charles, K.; Edwards, J.; Gregory, C.; Cheetham, S.; Buter, B.; Marancik, D. Chelonid alphaherpesvirus 5 prevalence and first confirmed case of sea turtle fibropapillomatosis in Grenada, West Indies. Animals 2021, 11, 1490. [Google Scholar] [CrossRef]
- Herbst, L.H.; Klein, A.P. Green turtle fibropapillomatosis: Challenges to assessing the role of environmental cofactors. Environ. Health Perspect. 1995, 103, 27–30. [Google Scholar] [CrossRef]
- Keller, J.M.; Balazs, G.H.; Nilsen, F.; Rice, M.; Work, T.; Jensen, B.A. Investigating the potential role of persistent organic pollutants in Hawaiian green sea turtle fibropapillomatosis. Environ. Sci. Technol. 2014, 48, 7807–7816. [Google Scholar] [CrossRef]
- Gore, M.L.; Wilson, R.; Siemer, W.F.; Hudenko, H.W.; Clarke, C.E.; Hart, P.S.; Maguire, L.A.; Muter, B.A. Application of Risk Concepts to wildlife management: Special issue introduction. Hum. Dimens. Wildl. 2009, 14, 301–313. [Google Scholar] [CrossRef]
- Perez, E.A.; Marco, A.; Martins, S.; Hawkes, L. Is this what a climate change-resilient population of marine turtles looks like? Biol. Conserv. 2016, 193, 124–132. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Lutz, P.L. Marine Turtles as Sentinels of Ecosystem Health: Is Fibropapillomatosis an indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Chaloupka, M.; Balazs, G.H.; Work, T.M. Rise and fall over 26 years of a marine epizootic in Hawaiian green sea turtles. J. Wildl. Dis. 2009, 45, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.M.; Schroeder, B.A.; Redlow, A.E.; Fick-Child, K.J.; Teas, W.G. Fibropapillomatosis in stranded green turtles (Chelonia mydas) from the eastern united states (1980–1998): Trends and associations with environmental factors. J. Wildl. Dis. 2005, 41, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dujon, A.M.; Schofield, G.; Bramwell, G.; Raven, N.; Hamede, R.; Thomas, F.; Ujvari, B. Global meta-analysis of over 50 years of multidisciplinary and international collaborations on transmissible cancers. Evol. Appl. 2020, 13, 1745–1755. [Google Scholar] [CrossRef]
- Dujon, A.; Schofield, G. Importance of machine learning for enhancing ecological studies using information-rich imagery. Endanger. Species Res. 2019, 39, 91–104. [Google Scholar] [CrossRef]
- Nuñez, M.A.; Amano, T. Monolingual searches can limit and bias results in global literature reviews. Nat. Ecol. Evol. 2021, 5, 2021. [Google Scholar] [CrossRef] [PubMed]
- Rodenbusch, C.; Baptistotte, C.; Werneck, M.R.; Pires, T.T.; Melo, M.T.D.; De Ataíde, M.W.; Dos Reis, K.; Testa, P.; Alieve, M.M.; Canal, C. Fibropapillomatosis in green turtles Chelonia mydas in Brazil: Characteristics of tumors and virus. Dis. Aquat. Org. 2014, 111, 207–217. [Google Scholar] [CrossRef]
- Adnyana, W.; Ladds, P.W.; Blair, D. Observations of fibropapillomatosis in green turtles (Chelonia mydas) in Indonesia. Aust. Vet. J. 1997, 75, 737–742. [Google Scholar] [CrossRef]
- Tagliolatto, A.; Guimarães, S.; Lobo-Hajdu, G.; Monteiro-Neto, C. Characterization of fibropapillomatosis in green turtles Chelonia mydas (Cheloniidae) captured in a foraging area in Southeastern Brazil. Dis. Aquat. Org. 2016, 121, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, S.K.K. Hawaiian archipelago fibropapillomatosis data. In Proceedings of the 2015 International Summit on Fibropapillomatosis: Global Status, Trends, and Population Impacts, Honolulu, HI, USA, 11–14 June 2015; pp. 36–56. [Google Scholar]
- Mejía-Radillo, R.; Zavala-Norzagaray, A.; Chávez-Medina, J.; Aguirre, A.; Escobedo-Bonilla, C. Presence of chelonid herpesvirus 5 (ChHV5) in sea turtles in northern Sinaloa, Mexico. Dis. Aquat. Org. 2019, 132, 99–108. [Google Scholar] [CrossRef]
- Hirama, S.; Ehrhart, L.M. Description, prevalence and severity of green turtle fibropapillomatosis in three developmental habitats on the East Coast of Florida. Fla. Sci. 2007, 70, 435–448. [Google Scholar]
- Avens, L.; Goshe, L.; Harms, C.; Anderson, E.; Hall, A.G.; Cluse, W.; Godfrey, M.; Braun-McNeill, J.; Stacy, B.; Bailey, R.; et al. Population characteristics, age structure, and growth dynamics of neritic juvenile green turtles in the northeastern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 458, 213–229. [Google Scholar] [CrossRef]
- Campillo, A. Projet Origine, Répartition et Evolution du Fibropapillomas aux îles Barren; Association Caouanne, 2012. [Google Scholar]
- Work, T.M.; Balazs, G.H. Relating Tumor score to hematology in green turtles with fibropapillomatosis in Hawaii. J. Wildl. Dis. 1999, 35, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Sterling, E.J.; McFadden, K.W.; Holmes, K.E.; Vintinner, E.C.; Arengo, F.; Naro-Maciel, E. Ecology and conservation of marine turtles in a central pacific foraging ground. Chelonian Conserv. Biol. 2013, 12, 2–16. [Google Scholar] [CrossRef]
- Loureiro, N.D.S.; Damião, M. Presence of fibropapillomatosis in green turtles Chelonia mydas at Príncipe Island in the Gulf of Guinea. Arquipélago. Life Mar. Sci. 2009, 26, 79–83. [Google Scholar]
- Patrício, A.; Diez, C.; Van Dam, R.; Godley, B. Novel insights into the dynamics of green turtle fibropapillomatosis. Mar. Ecol. Prog. Ser. 2016, 547, 247–255. [Google Scholar] [CrossRef]
- Gillis, J.A. Foraging ecology and diet selection of juvenile green turtles (Chelonia mydas) in the Western Bahamas: Insights from stable isotope analysis and prey mapping. Master’s Thesis, Florida State University, Tallahassee, FL, USA, 2018. [Google Scholar]
- Hamann, M.; Schäuble, C.S.; Simon, T.; Evans, S. Demographic and health parameters of green sea turtles Chelonia mydas foraging in the Gulf of Carpentaria, Australia. Endanger. Species Res. 2006, 2, 81–88. [Google Scholar] [CrossRef]
- Foley, A.M.; Singel, K.E.; Dutton, P.H.; Summers, T.M.; Redlow, A.E.; Lessman, J. Characteristics of a green turtle (Chelonia mydas) assemblage in Northwestern Florida determined during a hypothermic stunning event. Gulf Mex. Sci. 2007, 25, 131–143. [Google Scholar] [CrossRef]
- Stringell, T.B.; Clerveaux, W.V.; Godley, B.J.; Phillips, Q.; Ranger, S.; Richardson, P.B.; Sanghera, A.; Broderick, A.C. Fisher choice may increase prevalence of green turtle fibropapillomatosis disease. Front. Mar. Sci. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Piovano, S.; Lemons, G.; Ciriyawa, A.; Batibasaga, A.; Seminoff, J. Diet and recruitment of green turtles in Fiji, South Pacific, inferred from in-water capture and stable isotope analysis. Mar. Ecol. Prog. Ser. 2020, 640, 201–213. [Google Scholar] [CrossRef]
- Liebart, M. Photo-Identification des Tortues Vertes (Chelonia mydas) et Son Application Dans L’indice D’abondance ou de Fidélité aux Sites D’alimentation en Martinique; Ecole Nationale Vétérinaire de Toulouse: Toulouse, France, 2019. [Google Scholar]
- Patricio, A.R.; Centre, E.S. Fibropapillomatosis in marine turtles of the caribbean region: The case study of Puerto Rico. In Proceedings of the International Summit on Fibropapillomatosis: Global Status, Trends, and Population Impacts, Honolulu, HI, USA, 11–14 June 2016. [Google Scholar]
- Van Mil, C. Fibropapillomatosis Affecting Green Turtles (Chelonia mydas); Sea Turtle Conservation Bonaire: Gainesville, FL, USA, 2014. [Google Scholar]
- Albareda, D.A.; Garne, M.; Prosdocimi, L.; Rodriguez, H.S.; Di, P.J.L.; Loureiro, J. Pathological studies in green sea turtles (Chelonia mydas) and loggerhead sea turtles (Caretta caretta) from the northern coastal area of Buenos Aires, Argentina. In Proceedings of the Twenty-Seventh Annual Symposium on Sea Turtle Biology and Conservation, Myrtle Beach, SC, USA, 22–28 February 2008. [Google Scholar]
- Da Silva-Júnior, E.S.; De Farias, D.S.D.; Bomfim, A.D.C.; Freire, A.C.D.B.; Revorêdo, R.Â.; Rossi, S.; Matushima, E.R.; Grisi-Filho, J.H.H.; Silva, F.J.D.L.; Gavilan, S.A. Stranded marine turtles in Northeastern Brazil: Incidence and spatial–temporal distribution of fibropapillomatosis. Chelonian Conserv. Biol. 2019, 18, 249. [Google Scholar] [CrossRef]
- Shaver, D.J.; Walker, J.S.; Backof, T.F. Fibropapillomatosis prevalence and distribution in green turtles Chelonia mydas in Texas (USA). Dis. Aquat. Org. 2019, 136, 175–182. [Google Scholar] [CrossRef]
- López-Mendilaharsu, M.; Vélez-Rubio, G.M.; Lezama, C.; Aisenberg, A.; Bauzá, A.; Berrondo, L.; Calvo, V.; Caraccio, N.; Estrades, A.; Hernández, M.; et al. Demographic and tumour prevalence data for juvenile green turtles at the Coastal-Marine Protected Area of Cerro Verde, Uruguay. Mar. Biol. Res. 2016, 12, 541–550. [Google Scholar] [CrossRef]
- Bjorndal, K.; Bolten, A.; Chaloupka, M.Y. Evaluating trends in abundance of immature green turtles, Chelonia mydas, in the greater caribbean. Ecol. Appl. 2005, 15, 304–314. [Google Scholar] [CrossRef]
- Formia, A.; Deem, S.; Billes, A.; Ngouessono, S.; Parnell, R.; Collinis, T.; Sounguet, G.P.; Gibudi, A.; Villarubia, A.; Balazs, G.H.; et al. Fibropapillomatosis confirmed in Chelonia mydas in the Gulf of Guinea, West Africa. Mar. Turt. Newsl. 2007, 116, 20–22. [Google Scholar]
- Baptistotte, C.; Sclfoni, J.T.; Gallo, B.M.G.; dos Santos, A.S.; de Castilhos, J.C.; Lima, E.H.S.M.; Bellini, C.; Barata, P.C.R. Prevalence of sea turtle fribropapillomatosis in Brazil. In Proceedings of the 21st Annual Symposium on Sea Turtle Biology and Conservation, Philadelphia, PA, USA, 24–28 February 2001. [Google Scholar]
- Jones, K. Environmental Influences on the Epidemiology of Fibropapillomatosis in Green Turtles (Chelonia mydas) and Consequences for Management of Inshore Areas of the Great Barrier Reef; James Cook University: Townsville, Australia, 2019. [Google Scholar]
- Jones, K.; Burgess, G.; Budd, A.M.; Huerlimann, R.; Mashkour, N.; Ariel, E. Molecular evidence for horizontal transmission of chelonid alphaherpesvirus 5 at green turtle (Chelonia mydas) foraging grounds in Queensland, Australia. PLoS ONE 2020, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Work, T.; Dagenais, J.; Balazs, G.H.; Schumacher, J.; Lewis, T.D.; Leong, J.-A.C.; Casey, R.N.; Casey, J.W. In vitro biology of fibropapilloma-associated turtle herpesvirus and host cells in Hawaiian green turtles (Chelonia mydas). J. Gen. Virol. 2009, 90, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Dagenais, J.; Weatherby, T.M.; Balazs, G.H.; Ackermann, M. In Vitro Replication of Chelonid Herpesvirus 5 in organotypic skin cultures from hawaiian green turtles (Chelonia mydas). J. Virol. 2017, 91, e00404-17. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.; Vaclavik, T.; Manceur, A.M.; Šprtová, L.; Von Wehrden, H.; Welk, E.; Cord, A. glUV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Häder, D.-P.; Williamson, C.E.; Wängberg, S.-Å.; Rautio, M.; Rose, K.C.; Gao, K.; Helbling, E.W.; Sinha, R.P.; Worrest, R. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 108–126. [Google Scholar] [CrossRef]
- Duffy, D.J.; Schnitzler, C.; Karpinski, L.; Thomas, R.; Whilde, J.; Eastman, C.; Yang, C.; Krstic, A.; Rollinson, D.; Zirkelbach, B.; et al. Sea turtle fibropapilloma tumors share genomic drivers and therapeutic vulnerabilities with human cancers. Commun. Biol. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Seminoff, J.; Resendiz, A.; Nichols, W. Home range of green turtles Chelonia mydas at a coastal foraging area in the Gulf of California, Mexico. Mar. Ecol. Prog. Ser. 2002, 242, 253–265. [Google Scholar] [CrossRef]
- Tucker, A.D.; Lutz, P.L.; Musick, J.A. The Biology of Sea Turtles. Copeia 1998, 1998, 803. [Google Scholar] [CrossRef]
- Tedetti, M.; Sempéré, R. Penetration of ultraviolet radiation in the marine environment: A review. Photochem. Photobiol. 2006, 82, 389. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Grall, J.; Chauvaud, L. Marine eutrophication and benthos: The need for new approaches and concepts. Glob. Chang. Biol. 2002, 8, 813–830. [Google Scholar] [CrossRef]
- Arthur, K.; Limpus, C.; Balazs, G.; Capper, A.; Udy, J.; Shaw, G.; Keuper-Bennett, U.; Bennett, P. The exposure of green turtles (Chelonia mydas) to tumour promoting compounds produced by the cyanobacterium Lyngbya majuscula and their potential role in the aetiology of fibropapillomatosis. Harmful Algae 2008, 7, 114–125. [Google Scholar] [CrossRef]
- Arthur, K.; Shaw, G.; Limpus, C.; Udy, J. A review of the potential role of tumour-promoting compounds produced by Lyngbya majusculain in marine turtle fibropapillomatosis. Afr. J. Mar. Sci. 2006, 28, 441–446. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Balazs, G.H.; Steidinger, K.A.; Baden, D.G.; Work, T.M.; Russell, D.J. The Potential role of natural tumor promoters in marine turtle fibropapillomatosis. J. Aquat. Anim. Health 1999, 11, 199–210. [Google Scholar] [CrossRef]
- Perrault, J.R.; Stacy, N.; Lehner, A.F.; Mott, C.R.; Hirsch, S.; Gorham, J.C.; Buchweitz, J.P.; Bresette, M.J.; Walsh, C.J. Potential effects of brevetoxins and toxic elements on various health variables in Kemp’s ridley (Lepidochelys kempii) and green (Chelonia mydas) sea turtles after a red tide bloom event. Sci. Total. Environ. 2017, 605, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Delisle, L.; Petton, B.; Burguin, J.F.; Morga, B.; Corporeau, C.; Pernet, F. Temperature modulate disease susceptibility of the Pacific oyster Crassostrea gigas and virulence of the Ostreid herpesvirus type Fish. Shellfish Immunol. 2018, 80, 71–79. [Google Scholar] [CrossRef]
- Haines, H.; Kleese, W.C. Effect of water temperature on a herpesvirus infection of sea turtles. Infect. Immun. 1977, 15, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Asashima, M.; Oinuma, T.; Matsuyama, H.; Nagano, M. Effects of temperature on papilloma growth in the Newt, Cynops pyrrhogaster. Cancer Res. 1985, 45, 1198–1205. [Google Scholar] [PubMed]
- Bowser, P.R.; Martineau, D.; Wooster, G.A. Effects of water temperature on experimental transmission of dermal sarcoma in fingerling walleyes. J. Aquat. Anim. Health 1990, 2, 157–161. [Google Scholar] [CrossRef]
- Huang, B.; Liu, C.; Banzon, V.; Freeman, E.; Graham, G.; Hankins, B.; Smith, T.; Zhang, H.-M. Improvements of the daily optimum interpolation sea surface temperature (DOISST) version 2. J. Clim. 2021, 34, 1–47. [Google Scholar] [CrossRef]
- Greenblatt, R.J.; Work, T.; Balazs, G.H.; Sutton, C.A.; Casey, R.N.; Casey, J.W. The Ozobranchus leech is a candidate mechanical vector for the fibropapilloma-associated turtle herpesvirus found latently infecting skin tumors on Hawaiian green turtles (Chelonia mydas). Virology 2004, 321, 101–110. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Q.; Zamzow, J.P.; Wang, Y.; Losey, G.S.; Balazs, G.H.; Nerurkar, V.R.; Yanagihara, R. Detection of Green Turtle Herpesviral Sequence in Saddleback Wrasse Thalassoma duperrey: A Possible Mode of Transmission of Green Turtle Fibropapilloma. J. Aquat. Anim. Health 2000, 12, 58–63. [Google Scholar] [CrossRef]
- Rittenburg, L.T.; Kelley, J.R.; Mansfield, K.L.; Savage, E.A. Marine leech parasitism of sea turtles varies across host species, seasons, and the tumor disease fibropapillomatosis. Dis. Aquat. Org. 2021, 143, 1–12. [Google Scholar] [CrossRef]
- Farrell, J.A.; Yetsko, K.; Whitmore, L.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Linser, P.; Creer, S.; Burkhalter, B.; et al. Environmental DNA monitoring of oncogenic viral shedding and genomic profiling of sea turtle fibropapillomatosis reveals unusual viral dynamics. Commun. Biol. 2021, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chu, H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology 2020, 31, 713–717. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Statistics for biology and health. In Mixed Effects Models and Extensions in Ecology with R.; Springer: Berlin, Germany, 2009. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Szumilas, M. Explaining odds ratios. J. Can. Acad. Child. Adolesc. Psychiatry 2010, 19, 227–229. [Google Scholar]
- Tredennick, A.T.; Hooker, G.; Ellner, S.P.; Adler, P.B. A practical guide to selecting models for exploration, inference, and prediction in ecology. Ecology 2021, 102, e03336. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Hargrove, S.K.; Balazs, G.H. Land use, macroalgae, and a tumor-forming disease in marine turtles. PLoS ONE 2010, 5, e12900. [Google Scholar] [CrossRef]
- Lüdecke, D. Ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Halsey, L.G.; Curran-Everett, D.; Vowler, S.L.; Drummond, G.B. The fickle p-value generates irreproducible results. Nat. Methods 2015, 12, 179–185. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2020. [Google Scholar]
- Mollie, E.B.; Kristensen, K.; Koen, J.; Magnusson, A.; Casper, W.B.; Nielsen, A.; Hans, J.S.; Mächler, M.; Benjamin, M.B. Glmmtmb balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; University of Regensburg: Regensburg, Germany, 2020. [Google Scholar]
- Esteban, N.; Mortimer, J.A.; Stokes, H.J.; Laloë, J.-O.; Unsworth, R.K.F.; Hays, G.C. A global review of green turtle diet: Sea surface temperature as a potential driver of omnivory levels. Mar. Biol. 2020, 167, 1–17. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Dortch, Q.; Gobler, C.; Heil, C.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Hecky, R.E.; Kilham, P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 1988, 33, 796–822. [Google Scholar] [CrossRef]
- O’Neil, J.; Davis, T.; Burford, M.; Gobler, C. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Egge, J.; Aksnes, D. Silicate as regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Anderson, C.R.; Berdalet, E.; Kudela, R.M.; Cusack, C.K.; Silke, J.; O’Rourke, E.; Dugan, D.; McCammon, M.; Newton, J.A.; Moore, S.K.; et al. Scaling up from regional case studies to a global harmful algal bloom observing system. Front. Mar. Sci. 2019, 6, 250. [Google Scholar] [CrossRef]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef]
- Walsh, C.J.; Leggett, S.R.; Carter, B.J.; Colle, C. Effects of brevetoxin exposure on the immune system of loggerhead sea turtles. Aquat. Toxicol. 2010, 97, 293–303. [Google Scholar] [CrossRef]
- Capper, A.; Flewelling, L.J.; Arthur, K. Dietary exposure to harmful algal bloom (HAB) toxins in the endangered manatee (Trichechus manatus latirostris) and green sea turtle (Chelonia mydas) in Florida, USA. Harmful Algae 2013, 28, 1–9. [Google Scholar] [CrossRef]
- Nechifor, M.; Neagu, T.-M.; Manda, G. Reactive oxygen species, cancer and anti-cancer therapies. Curr. Chem. Biol. 2012, 3, 22–46. [Google Scholar] [CrossRef]
- Perrault, J.R.; Perkins, C.R.; Ajemian, M.J.; Bresette, M.J.; Mott, C.R.; Page-Karjian, A. Harmful algal and cyanobacterial toxins in foraging green turtles (Chelonia mydas) in Florida’s Big Bend. Toxicon X 2020, 5, 100020. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M.; Dagenais, J.; Balazs, G.H.; Schettle, N.; Ackermann, M. Dynamics of Virus shedding and in situ confirmation of chelonid herpesvirus 5 in Hawaiian green turtles with fibropapillomatosis. Vet. Pathol. 2015, 52, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.S.; Brown, D.R.; Gaskin, J.M.; Jacobson, E.R.; Ehrhart, L.M.; Blahak, S.; Herbst, L.H.; Klein, P.A. Persistent infectivity of a disease-associated herpesvirus in green turtles after exposure to seawater. J. Wildl. Dis. 2000, 36, 792–797. [Google Scholar] [CrossRef]
- Ene, A.; Su, M.; Lemaire, S.; Rose, C.; Schaff, S.; Moretti, R.; Lenz, J.; Herbst, L.H. Distribution of chelonid fibro papillomatosis-associated herpesvirus variants in Florida: Molecular genetic evidence for infection of turtles following recruitment to neritic developmental habitats. J. Wildl. Dis. 2005, 41, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Schofield, G.; Papafitsoros, K.; Haughey, R.; Katselidis, K. Aerial and underwater surveys reveal temporal variation in cleaning-station use by sea turtles at a temperate breeding area. Mar. Ecol. Prog. Ser. 2017, 575, 153–164. [Google Scholar] [CrossRef]
- Losey, G.S.; Balazs, G.H.; Privitera, L.A. Cleaning symbiosis between the wrasse, Thalassoma duperry, and the green turtle, Chelonia mydas. Copeia 1994, 1994, 684–690. [Google Scholar] [CrossRef]
- Dujon, A.M.; Ierodiaconou, D.; Geeson, J.J.; Arnould, J.P.Y.; Allan, B.M.; Katselidis, K.A.; Schofield, G. Machine learning to detect marine animals in UAV imagery: Effect of morphology, spacing, behaviour and habitat. Remote. Sens. Ecol. Conserv. 2021, 7, 341–354. [Google Scholar] [CrossRef]
- Work, T.M.; Dagenais, J.; Willimann, A.; Balazs, G.; Mansfield, K.; Ackermann, M. Differences in antibody responses against Chelonid Alpha herpesvirus 5 (ChHV5) suggest differences in virus biology in ChHV5-seropositive green turtles from Hawaii and ChHV5-seropositive green turtles from Florida. J. Virol. 2019, 94, 1–15. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Perrault, J.; Zirkelbach, B.; Pescatore, J.; Riley, R.; Stadler, M.; Zachariah, T.T.; Marks, W.; Norton, T.M. Tumor re-growth, case outcome, and tumor scoring systems in rehabilitated green turtles with fibropapillomatosis. Dis. Aquat. Org. 2019, 137, 101–108. [Google Scholar] [CrossRef]
- Yetsko, K.; Farrell, J.A.; Blackburn, N.B.; Whitmore, L.; Stammnitz, M.R.; Whilde, J.; Eastman, C.B.; Ramia, D.R.; Thomas, R.; Krstic, A.; et al. Molecular characterization of a marine turtle tumor epizootic, profiling external, internal and postsurgical regrowth tumors. Commun. Biol. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Hazen, E.L.; Scales, K.L.; Maxwell, S.M.; Briscoe, D.K.; Welch, H.; Bograd, S.J.; Bailey, H.; Benson, S.R.; Eguchi, T.; Dewar, H.; et al. A dynamic ocean management tool to reduce bycatch and support sustainable fisheries. Sci. Adv. 2018, 4, eaar3001. [Google Scholar] [CrossRef]
- Howell, E.A.; Hoover, A.; Benson, S.R.; Bailey, H.; Polovina, J.J.; Seminoff, J.A.; Dutton, P.H. Enhancing the TurtleWatch product for leatherback sea turtles, a dynamic habitat model for ecosystem-based management. Fish. Oceanogr. 2015, 24, 57–68. [Google Scholar] [CrossRef]
- Hamede, R.; Owen, R.; Siddle, H.; Peck, S.; Jones, M.; Dujon, A.; Giraudeau, M.; Roche, B.; Ujvari, B.; Thomas, F. The ecology and evolution of wildlife cancers: Applications for management and conservation. Evol. Appl. 2020, 13, 1719–1732. [Google Scholar] [CrossRef]
- Dheilly, N.M.; Ewald, P.W.; Brindley, P.J.; Fichorova, R.N.; Thomas, F. Parasite-microbe-host interactions and cancer risk. PLoS Pathog. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mazaris, A.D.; Gkazinou, C.; Almpanidou, V.; Balazs, G. The sociology of sea turtle research: Evidence on a global expansion of co-authorship networks. Biodivers. Conserv. 2018, 27, 1503–1516. [Google Scholar] [CrossRef]
- Vilca, F.Z.; Rossi, S.; Olinda, R.; Sánchez-Sarmiento, A.M.; Prioste, F.E.S.; Matushima, E.R.; Tornisielo, V.L. Concentrations of polycyclic aromatic hydrocarbons in liver samples of juvenile green sea turtles from Brazil: Can these compounds play a role in the development of fibropapillomatosis? Mar. Pollut. Bull. 2018, 130, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.B.; Ismail, N.; Sassoubre, L.M.; Andruszkiewicz, E.A. Oceans in peril: Grand Challenges in applied water quality research for the 21st century. Environ. Eng. Sci. 2017, 34, 3–15. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Hurley, B.J.; Finkbeiner, E.M.; Bolten, A.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Amorocho, D.; Bjorndal, K.; et al. Regional management units for marine turtles: A Novel framework for prioritizing conservation and research across multiple scales. PLoS ONE 2010, 5, 1–11. [Google Scholar] [CrossRef]
- The International Cancer Genome Consortium. International network of cancer genome projects. Nature 2010, 464, 993–998. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujon, A.M.; Schofield, G.; Venegas, R.M.; Thomas, F.; Ujvari, B. Sea Turtles in the Cancer Risk Landscape: A Global Meta-Analysis of Fibropapillomatosis Prevalence and Associated Risk Factors. Pathogens 2021, 10, 1295. https://doi.org/10.3390/pathogens10101295
Dujon AM, Schofield G, Venegas RM, Thomas F, Ujvari B. Sea Turtles in the Cancer Risk Landscape: A Global Meta-Analysis of Fibropapillomatosis Prevalence and Associated Risk Factors. Pathogens. 2021; 10(10):1295. https://doi.org/10.3390/pathogens10101295
Chicago/Turabian StyleDujon, Antoine M., Gail Schofield, Roberto M. Venegas, Frédéric Thomas, and Beata Ujvari. 2021. "Sea Turtles in the Cancer Risk Landscape: A Global Meta-Analysis of Fibropapillomatosis Prevalence and Associated Risk Factors" Pathogens 10, no. 10: 1295. https://doi.org/10.3390/pathogens10101295
APA StyleDujon, A. M., Schofield, G., Venegas, R. M., Thomas, F., & Ujvari, B. (2021). Sea Turtles in the Cancer Risk Landscape: A Global Meta-Analysis of Fibropapillomatosis Prevalence and Associated Risk Factors. Pathogens, 10(10), 1295. https://doi.org/10.3390/pathogens10101295







