Curry Leaf Triggers Cell Death of P. gingivalis with Membrane Blebbing
Abstract
:1. Introduction
2. Results
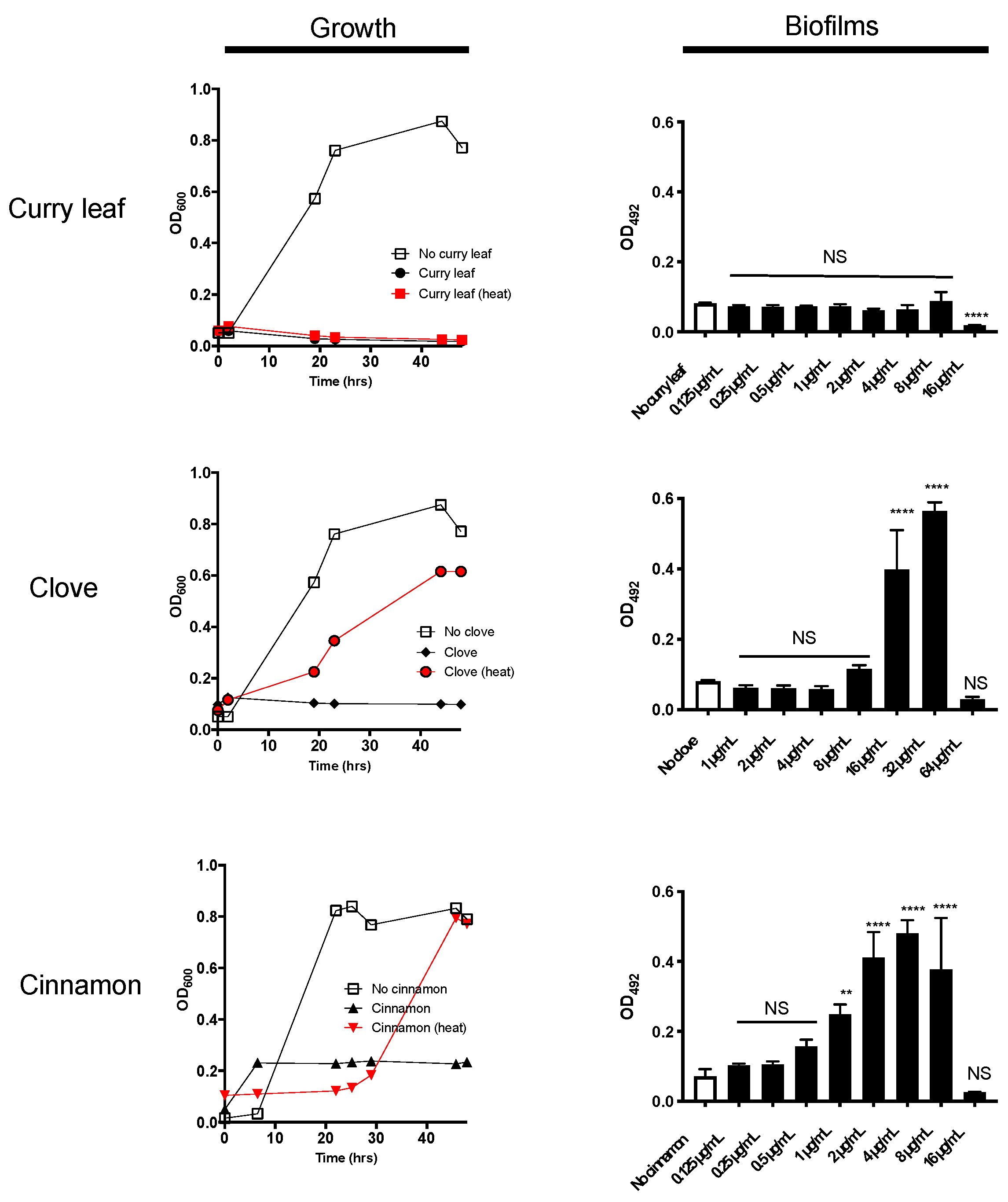
2.1. Curry Leaf Extract (CLE) Shows Anti-Bacterial Activity Together with Thermo-Stability
2.2. P. gingivalis Is Highly Susceptible to Treatment with CLE
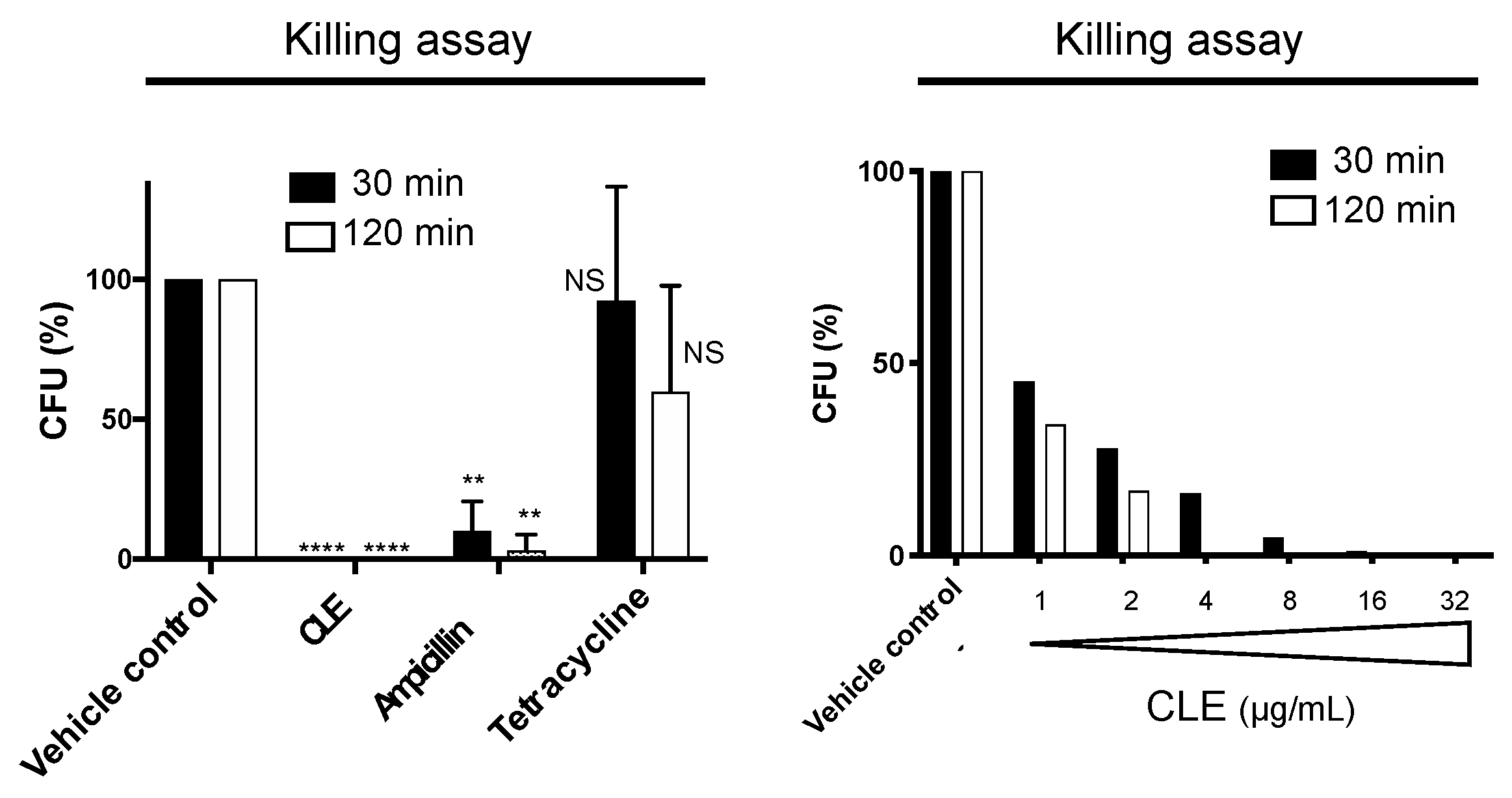
2.3. CLE Showed Strong and Rapid Bactericidal Activity
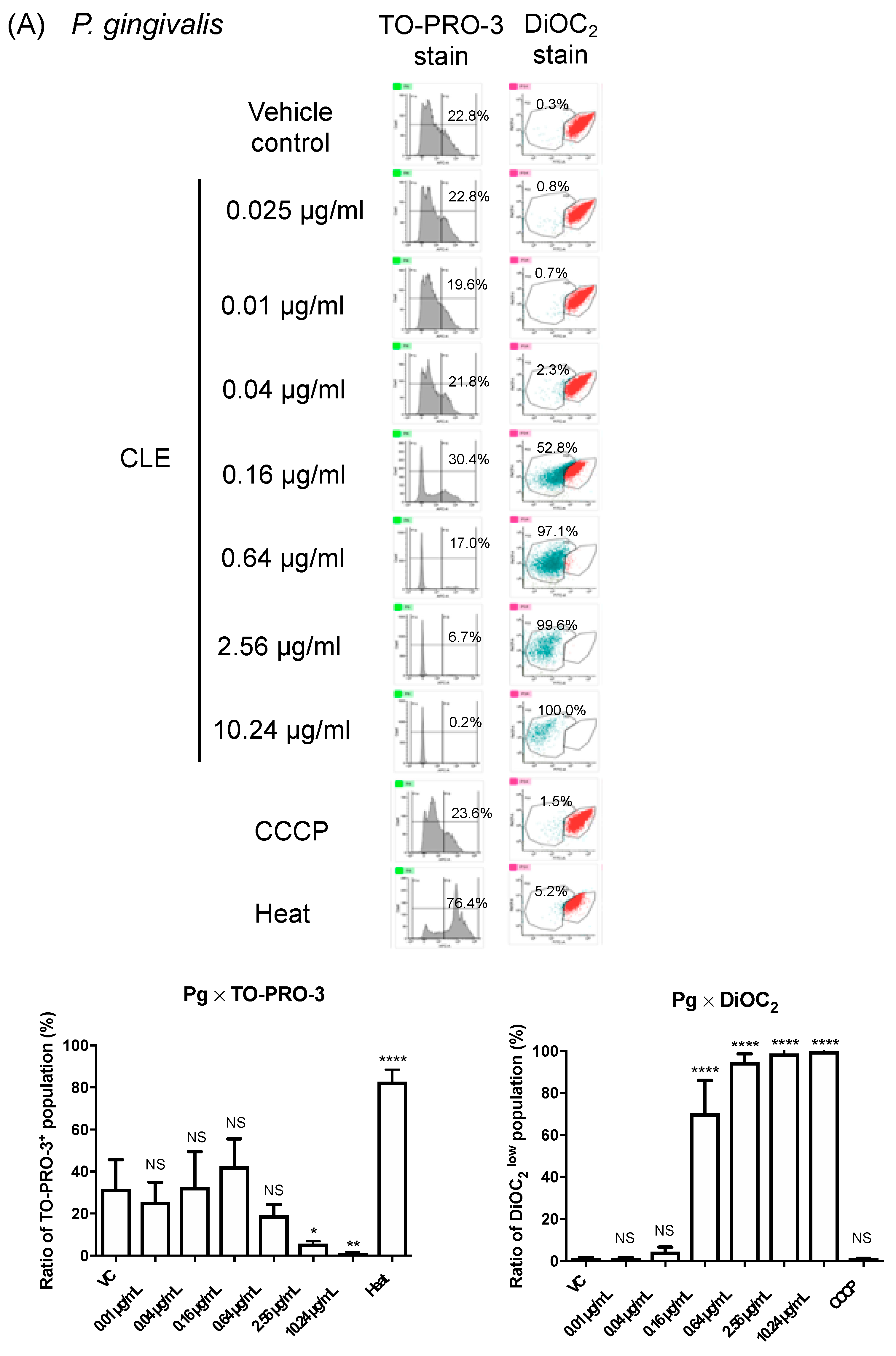
2.4. CLE Attacks Bacterial Outer Membrane
3. Discussion
4. Materials and Methods
4.1. Herbs and Preparation of Ethanol-Extracted Herb
4.2. Bacterial Strains and Growth Conditions
4.3. Growth Assay and Biofilm Formation Assay
4.4. Killing Assay to Assess Bactericidal Activity
4.5. Scanning Electron Microscopy (SEM)
4.6. Spatiotemporal Analysis Using High-Speed Atomic Force Microscopy (HS-AFM)
4.7. Cytotoxicity toward Oral Epithelial Cells
4.8. Bacterial Membrane Potential Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G.N.; Jenkinson, H.F. Oral Microbiology and Immunology, 2nd ed.; ASM Press: Washington, DC, USA, 2013. [Google Scholar]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontology 2000 2021, 86, 210–230. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 6 October 2021).
- Kerdar, T.; Rabienejad, N.; Alikhani, Y.; Moradkhani, S.; Dastan, D. Clinical, in vitro and phytochemical, studies of Scrophularia striata mouthwash on chronic periodontitis disease. J. Ethnopharmacol. 2019, 239, 111872. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.; Varghese, J.; Shetty, S.; Bhat, V.; Durgekar, T.; Lobo, R.; Nayak, U.Y.; Vishwanath, U. Evaluation of a mouthrinse containing guava leaf extract as part of comprehensive oral care regimen—A randomized placebo-controlled clinical trial. BMC Complement. Altern. Med. 2019, 19, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, S.K.; Mohanty, S.; Padhi, A.; Pati, R.; Sonawane, A. Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement. Altern. Med. 2014, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Vats, M.; Singh, H.; Sardana, S. Phytochemical screening and antimicrobial activity of roots of Murraya koenigii (Linn.) Spreng. (Rutaceae). Braz. J. Microbiol. 2011, 42, 1569–1573. [Google Scholar] [CrossRef] [Green Version]
- Keluskar, P.; Ingle, S. Ethnopharmacology guided screening of traditional Indian herbs for selective inhibition of Plasmodium specific lactate dehydrogenase. J. Ethnopharmacol. 2012, 144, 201–207. [Google Scholar] [CrossRef]
- Singh, A.P.; Wilson, T.; Luthria, D.; Freeman, M.R.; Scott, R.M.; Bilenker, D.; Shah, S.; Somasundaram, S.; Vorsa, N. LC-MS–MS characterisation of curry leaf flavonols and antioxidant activity. Food Chem. 2011, 127, 80–85. [Google Scholar] [CrossRef]
- Novo, D.J.; Perlmutter, N.G.; Hunt, R.H.; Shapiro, H.M. Multiparameter Flow Cytometric Analysis of Antibiotic Effects on Membrane Potential, Membrane Permeability, and Bacterial Counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob. Agents Chemother. 2000, 44, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Olsen, I.; Lambris, J.; Hajishengallis, G. Porphyromonas gingivalis disturbs host–commensal homeostasis by changing complement function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of beta-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio 2012, 3, e00198-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashiro, Y.; Inagaki, A.; Ono, K.; Inaba, T.; Yawata, Y.; Uchiyama, H.; Nomura, N. Low concentrations of ethanol stimulate biofilm and pellicle formation in Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 2014, 78, 178–181. [Google Scholar] [CrossRef]
- Weiser, J.; Henke, H.A.; Hector, N.; Both, A.; Christner, M.; Büttner, H.; Kaplan, J.B.; Rohde, H. Sub-inhibitory tigecycline concentrations induce extracellular matrix binding protein Embp dependent Staphylococcus epidermidis biofilm formation and immune evasion. Int. J. Med Microbiol. 2016, 306, 471–478. [Google Scholar] [CrossRef]
- Goneau, L.W.; Hannan, T.J.; MacPhee, R.A.; Schwartz, D.; Macklaim, J.M.; Gloor, G.B.; Razvi, H.; Reid, G.; Hultgren, S.J.; Burton, J.P. Subinhibitory Antibiotic Therapy Alters Recurrent Urinary Tract Infection Pathogenesis through Modulation of Bacterial Virulence and Host Immunity. mBio 2015, 6, e00356-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hards, K.; McMillan, D.; Schurig-Briccio, L.A.; Gennis, R.B.; Lill, H.; Bald, D.; Cook, G.M. Ionophoric effects of the antitubercular drug bedaquiline. Proc. Natl. Acad. Sci. USA 2018, 115, 7326–7331. [Google Scholar] [CrossRef] [Green Version]
- Tempelaars, M.H.; Rodrigues, S.; Abee, T. Comparative Analysis of Antimicrobial Activities of Valinomycin and Cereulide, the Bacillus cereus Emetic Toxin. Appl. Environ. Microbiol. 2011, 77, 2755–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, K.S.; Jagadeesh, S.; Baishya, G.; Rao, P.G.; Barua, N.C.; Bhattacharya, S.; Banerjee, P.P. A Naturally Derived Small Molecule Disrupts Ligand-Dependent and Ligand-Independent Androgen Receptor Signaling in Human Prostate Cancer Cells. Mol. Cancer Ther. 2014, 13, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Dutta, D.; Samanta, S.K.; Bhattacharya, K.; Pal, B.C.; Li, J.; Datta, K.; Mandal, C.; Mandal, C. Oxidative inhibition of Hsp90 disrupts the super-chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int. J. Cancer 2013, 132, 695–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, A.; Sikha, M.S.; Ramesh, K.; Venkatesh, P.; Godugu, C. Modulation of cerulein-induced pancreatic inflammation by hydroalcoholic extract of curry leaf (Murraya koenigii). Phytother. Res. 2019, 33, 1510–1525. [Google Scholar] [CrossRef]
- Babu, H.M.; Varghese, A.; Kukkera, P.N. Comparative evaluation of efficacy of Murraya koenigii and chlorhexidine gluconate in the treatment of gingivitis: A randomized controlled clinical trial. J. Indian Soc. Periodontol. 2018, 22, 427–432. [Google Scholar] [CrossRef]
- Adebajo, A.; Iwalewa, E.; Obuotor, E.; Ibikunle, G.; Omisore, N.; Adewunmi, C.; Obaparusi, O.; Klaes, M.; Adetogun, G.; Schmidt, T.; et al. Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: Anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 2009, 122, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- CLSI, Clinical and Laboratory Standards Institute. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 8th ed.; M11-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- CLSI, Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Information. M100-S23; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Nakao, R.; Senpuku, H.; Watanabe, H. Porphyromonas gingivalis galE Is Involved in Lipopolysaccharide O-Antigen Synthesis and Biofilm Formation. Infect. Immun. 2006, 74, 6145–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef]
- Nakao, R.; Hasegawa, H.; Ochiai, K.; Takashiba, S.; Ainai, A.; Ohnishi, M.; Watanabe, H.; Senpuku, H. Outer Membrane Vesicles of Porphyromonas gingivalis Elicit a Mucosal Immune Response. PLoS ONE 2011, 6, e26163. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Sakai, N.; Yoshida, A.; Uekusa, Y.; Yagi, A.; Imaoka, Y.; Ito, S.; Karaki, K.; Takeyasu, K. High-speed atomic force microscopy combined with inverted optical microscopy for studying cellular events. Sci. Rep. 2013, 3, srep02131. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Sakai, N.; Uekusa, Y.; Deguchi, K.; Gilmore, J.L.; Kumeta, M.; Ito, S.; Takeyasu, K. Probing in vivo dynamics of mitochondria and cortical actin networks using high-speed atomic force/fluorescence microscopy. Genes Cells 2015, 20, 85–94. [Google Scholar] [CrossRef]
- Yoshida, A.; Sakai, N.; Uekusa, Y.; Imaoka, Y.; Itagaki, Y.; Suzuki, Y.; Yoshimura, S.H. Morphological changes of plasma membrane and protein assembly during clathrin-mediated endocytosis. PLoS Biol. 2018, 16, e2004786. [Google Scholar] [CrossRef] [Green Version]


| Strains | MICs |
|---|---|
| Porphyromonas gingivalis ATCC 33277 | 16 |
| Porphyromonas gingivalis W83 | 16 |
| Porphyromonas gingivalis W50 | 32 |
| Fusobacterium nucleatum #20 | >64 |
| Fusobacterium nucleatum ATCC 23726 | >64 |
| Prevotella loescheii ATCC 15930 | >64 |
| Prevotella nigrescens ATCC 33563 | >64 |
| Aggregatibacter actinomycetemcomitans Y4 | >64 |
| Aggregatibacter actinomycetemcomitans ATCC 29522 | 64 |
| Streptococcus anginosus ATCC 33397 | >64 |
| Streptococcus cristatus ATCC 51100 | >64 |
| Streptococcus gordonii ATCC 10558 | 64 |
| Streptococcus mitis ATCC 6245 | >64 |
| Streptococcus mutans UA159 | >64 |
| Streptococcus oralis No. 10 | 64 |
| Streptococcus salivalius ATCC 9759 | >64 |
| Streptococcus sanguinis ATCC 10556 | >64 |
| Streptococcus sobrinus ATCC 6715 | 64 |
| Escherichia coli BW25113 | >64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakao, R.; Ikeda, T.; Furukawa, S.; Morinaga, Y. Curry Leaf Triggers Cell Death of P. gingivalis with Membrane Blebbing. Pathogens 2021, 10, 1286. https://doi.org/10.3390/pathogens10101286
Nakao R, Ikeda T, Furukawa S, Morinaga Y. Curry Leaf Triggers Cell Death of P. gingivalis with Membrane Blebbing. Pathogens. 2021; 10(10):1286. https://doi.org/10.3390/pathogens10101286
Chicago/Turabian StyleNakao, Ryoma, Tsuyoshi Ikeda, Soichi Furukawa, and Yasushi Morinaga. 2021. "Curry Leaf Triggers Cell Death of P. gingivalis with Membrane Blebbing" Pathogens 10, no. 10: 1286. https://doi.org/10.3390/pathogens10101286
APA StyleNakao, R., Ikeda, T., Furukawa, S., & Morinaga, Y. (2021). Curry Leaf Triggers Cell Death of P. gingivalis with Membrane Blebbing. Pathogens, 10(10), 1286. https://doi.org/10.3390/pathogens10101286






