Monitoring Risk: Tick and Borrelia burgdorferi Public Participatory Surveillance in the Canadian Maritimes, 2012–2020
Abstract
:1. Introduction
2. Results
2.1. Tick Submissions
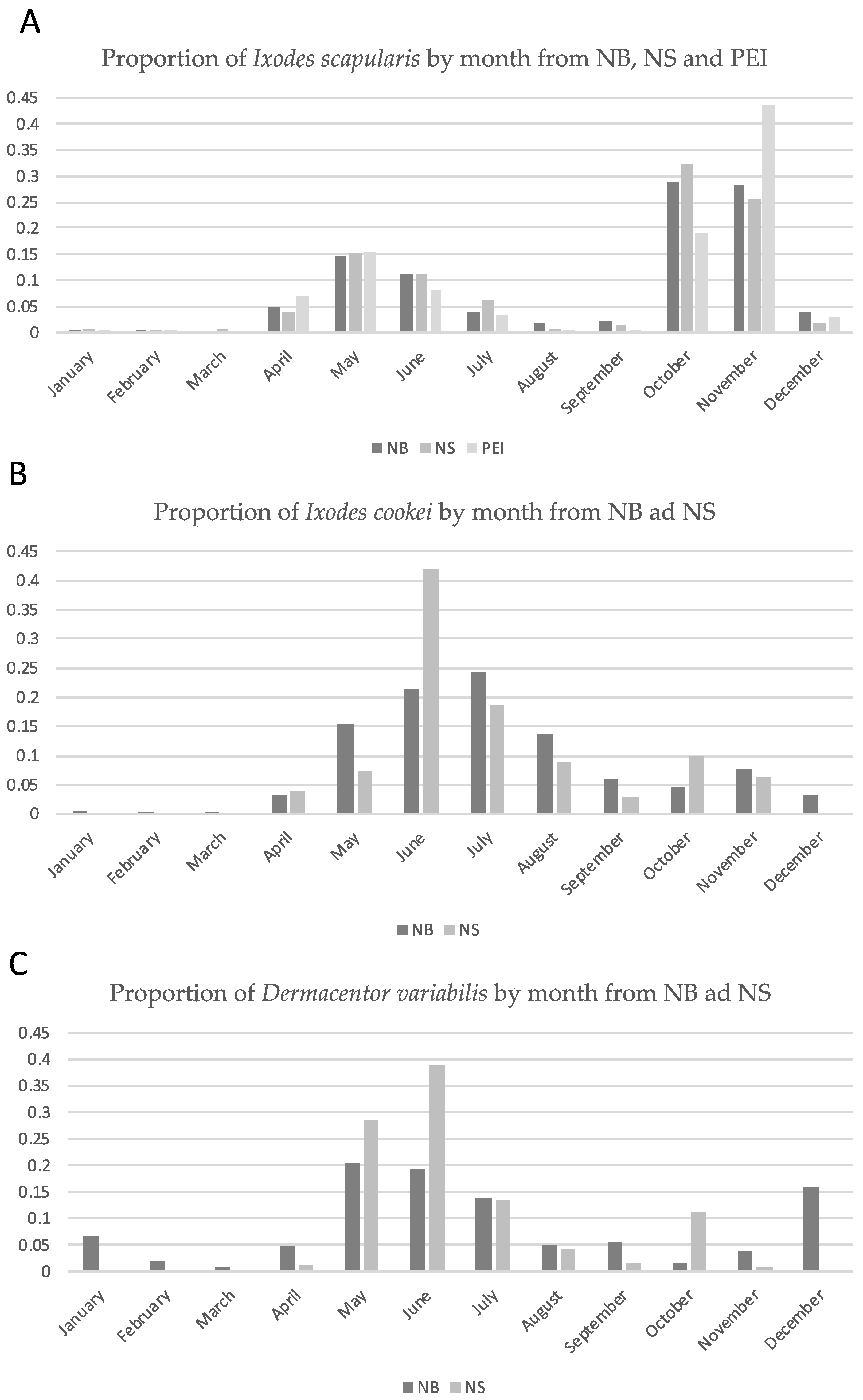
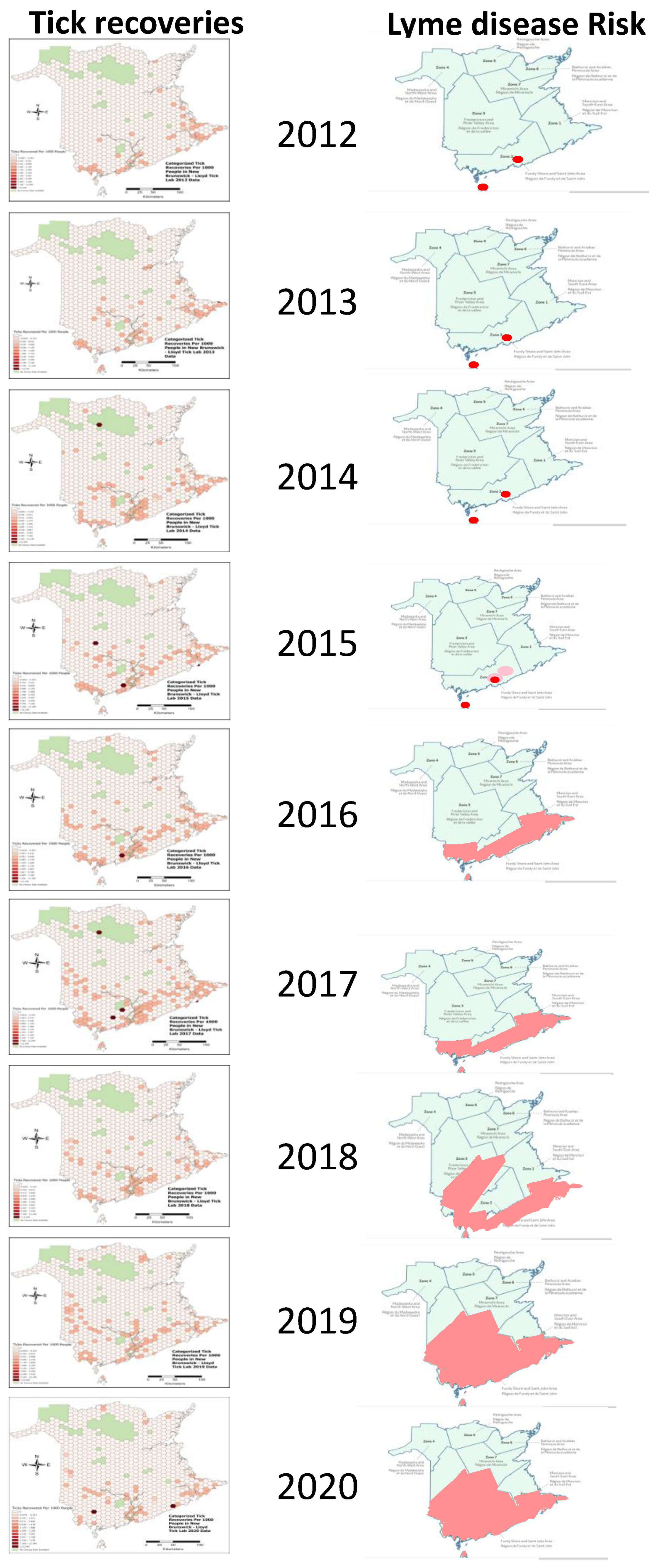
2.2. Geographic Distribution of I. scapularis Ticks and Borrelia burgdorferi
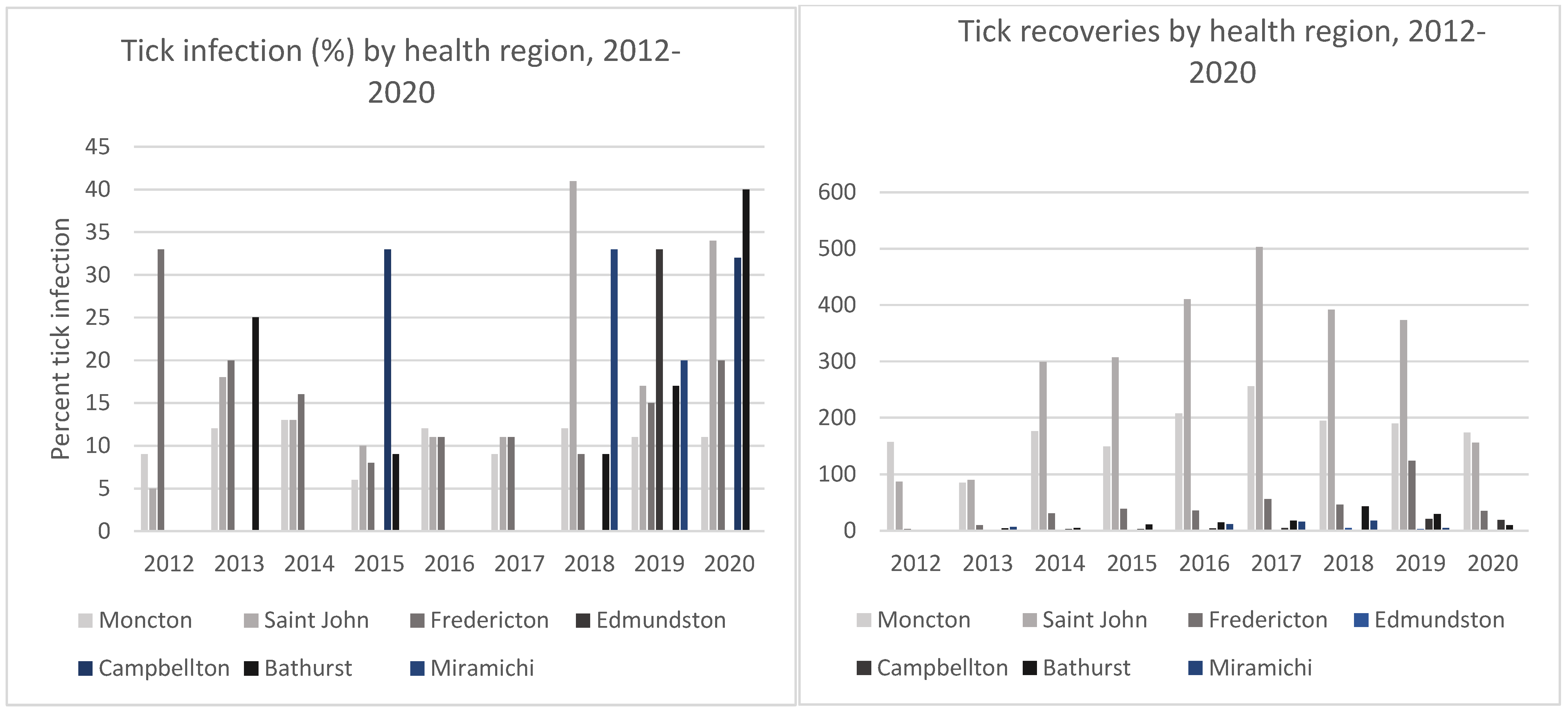
2.3. Borrelia burgdorferi Infection Prevalence
3. Discussion
3.1. Borrelia burgdorferi and Other Borrelia Species
3.2. Tick Surveillance
3.3. Lyme Disease Risk Predictions
| Reference | Province(s) Studied | Tick Collection Method(s) | Number of I. scapularis Collected | Borrelia Infection Rate (If Any) |
|---|---|---|---|---|
| Banerjee et al., 2000 [43] | ON | Passive surveillance (dogs) (1997–1999) | 139 | 2% (n = 121) by culture confirmed by monoclonal antibodies and PCR 6% (n = 121) by PCR |
| Morshed et al., 2003 [90] | ON | Flagging Trapping small mammals (1999–2000) | 263 199 | 14% of tick pools 1 (n = 86 pools) by PCR 40% of white-footed mice (n = 15) by PCR |
| Scott et al., 2004 [91] | ON | Flagging Trapping small mammals (2001–2002) | 254 59 | 45% of cultured pools 2 (n = 53) by PCR 25% of white-footed mice (n = 4) by culturing confirmed with PCR |
| Ogden et al., 2006a [53] | SK, MB, ON, QC, NB, NS, PEI, and NL | Passive surveillance (1996–2003) | 1816 | 10% (n = 349) in MB 11% (n = 45) in ON 13% (n = 984) in QC 16% (n = 151) in NB 15% (n = 86) in NS 11% (n = 180) in PE I19% (n = 21) in NL 13% (n = 1816) in Canada, all by PCR |
| Scott et al., 2007 [92] | ON | Flagging (2005–2006) | 46 | 67% of pools 3 (n = 15 tick pools) by culture confirmed with PCR |
| Ogden et al., 2008 [24] | ON, QC, and NS | Birds capturing (2005–2006) | 263 | 15% (n = 205 nymphs) and 0% (n = 53 larvae) in ON No tick was recovered from QC 25% (n = 4 nymphs) in NS, all by PCR |
| Scott and Durden, 2009 [93] | ON | Bird capturing | 7 | 43% (n = 7) by culture and confirmed with PCR |
| Ogden et al., 2010 [71] | QC | Flagging and rodent capture (2007–2008) Passive surveillance (1996–2004) | 2259 for active surveillance For passive surveillance, numbers are not given but submission per year is shown in their Figure 1 | 1% (n = 1169) by serological analysis of rodents 1.8–3.3% (n = 675) by PCR of seropositive rodents and ticks; 11 ticks from 1 rodent were pooled |
| Bouchard et al., 2011 [94] | QC | Rodent trapping (2007–2008) | 855 | 5% (n = 848) of ticks by PCR 1% of rodents were seropositive (n = 887) by immunofluorescence, ELISA, and Western blot |
| Krakowetz et al., 2011 [95] | MB, ON, and NS | Drag sampling | 153 | No infection rates provided |
| Dibernardo et al., 2014 [44] | AB, MB, ON, QC, NB, NS, PEI, and NL | Passive surveillance (2012) | 4938 | 14% (n = 87) in AB 9% (n = 170) in MB 16% (n = 2591) in ON 14% (n = 1479) in QC 7% (n = 366) in NB 12% (n = 34) in NS 10% (n = 178) in PEI 27% (n = 33) in NL All by PCR |
| Nelder et al., 2014 [45] | ON | Passive surveillance (2008–2012) | 7842 | 15% of pools 4 (n = 6046 tick pools) by PCR |
| Ogden et al., 2014 [96] | AB, SK, MB, QC, ON, NB, NS, PEI, and NL | Drag sampling (2008–2013) and trapping of small mammals Rodent capturing (2007 and May-Oct.2008) Passive surveillance (2004–2012) | Not provided in the report but a map of sites where at least one I. scapularis was found is given Rodent results are presented in the article by Bouchard et al., 2011 221 | No infection rates provided in this report but ticks were tested at the National Microbiology Laboratory |
| Simon et al., 2014 [97] | QC | Drag sampling Trapping small mammals | 1417 (total for both collection methods) | 14% (n = 311) of ticks from dragging, by PCR 1% of ticks from mammals, by PCR |
| Werden et al., 2014 [98] | ON | Drag sampling (2009–2010) Trapping small mammals (2009–2010) | 1354 (total for both collection methods) | Infection rate ranged from 12 to 30% (n = 1354) between sites by PCR |
| Gabriele-Rivet et al., 2015 [74] | NB | Drag sampling (2014) | 5 | 25% (n = 4) by PCR |
| Scott et al., 2016 [99] | ON | Flagging | 29 | 41% (n = 29) by PCR |
4. Conclusions
5. Materials and Methods
5.1. Tick Collection, Identification, and Photography
5.2. DNA Extraction
5.3. Nested Polymerase Chain Reaction
5.4. Geomapping Tick Recoveries
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-borne zoonoses in the United States: Persistent and emerging threats to human health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmelař, J.; Kotál, J.; Karim, S.; Kopacek, P.; Francischetti, I.M.B.; Pedra, J.H.F.; Kotsyfakis, M. Silomes and Mialomes: A system-biology view of tick tissues and tick-host interactions. Trends Parasitol. 2016, 32, 242–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, J.R.; McSwain, J.L.; Bowman, A.S.; Essenberg, R.C. Tick salivary gland physiology. Annu. Rev. Etomol. 1995, 40, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Sperling, J.L.H.; Sperling, F.A.H. Lyme borreliosis in Canada: Biological diversity and diagnostic complexity from an entomological perspective. Can. Entomol. 2009, 141, 521–549. [Google Scholar] [CrossRef]
- Sperling, J.L.H.; Middelveen, M.J.; Klein, D.; Sperling, F.A.H. Evolving perspectives on Lyme borreliosis in Canada. Open Neurol. J. 2012, 6, 94–103. [Google Scholar] [CrossRef]
- McCausland, F.R.; Niedermaier, S.; Bijol, V.; Rennke, H.G.; Choi, M.E.; Forman, J.P. Lyme disease-associated glomerulonephritis. Nephrol. Dial. Transplant. 2011, 26, 3054–3056. [Google Scholar] [CrossRef] [Green Version]
- Littman, M.P. Lyme nephritis. J. Vet. Emerg. Crit. Care 2013, 23, 163–173. [Google Scholar] [CrossRef]
- Bransfield, R.C. Suicide and Lyme and associated diseases. Neuropsychiatr. Dis. Treat. 2017, 13, 1575–1587. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.; Baranchuk, A. Systematic Approach to the Diagnosis and Treatment of Lyme Carditis and High-Degree Atrioventricular Block. Healthcare 2018, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, E.; Galloway, T.D.; Artsob, H.; Lindsay, L.R.; Drebot, M.; Wood, H.; Robbins, R.G. A Handbook to the Ticks of Canada (Ixodida: Ixodidae, Argasidae); Biological Survey of Canada: Sackville, NB, Canada, 2016. [Google Scholar]
- Piesman, J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J. Infect. Dis. 1993, 167, 1082–1085. [Google Scholar] [CrossRef]
- Hall, J.E.; Amrine, J.W., Jr.; Gais, R.D.; Kolanko, V.P.; Hagenbuch, B.E.; Gerencser, V.F.; Clark, S.M. Parasitization of humans in West Virginia by Ixodes cookei (Acari: Ixodidae), a potential vector of Lyme borreliosis. J. Med. Entomol. 1991, 28, 186–189. [Google Scholar] [CrossRef]
- Barker, I.K.; Lindsay, L.R.; Campbell, G.D.; Surgeoner, G.A.; McEwen, S.A. The groundhog tick Ixodes cookei (Acari: Ixodidae): A poor potential vector of Lyme borreliosis. J. Wildl. Dis. 1993, 29, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Powassan Virus, 2015a. Available online: http://www.cdc.gov/powassan/symptoms.html (accessed on 8 September 2016).
- Centers for Disease Control and Prevention. Rocky Mountain Spotted Fever, 2015b. Available online: https://www.cdc.gov/rmsf/ (accessed on 8 September 2016).
- Centers for Disease Control and Prevention. Tularemia, 2015c. Available online: https://www.cdc.gov/tularemia/signssymptoms/index.html (accessed on 8 September 2016).
- De la Fuente, J.; Blouin, E.F.; Kocan, K.M. Infection exclusion of the rickettsial pathogen anaplasma marginale in the tick vector Dermacentor variabilis. Clin. Diagn. Lab. Immunol. 2003, 10, 182–184. [Google Scholar] [CrossRef] [Green Version]
- Fritzen, C.M.; Huang, J.; Westby, K.; Freye, J.D.; Dunlap, B.; Yabsley, M.J.; Schardein, M.; Dunn, J.R.; Jones, T.F.; Moncayo, A.C. Infection prevalences of common tick-borne pathogens in adult lone star ticks (Amblyomma americanum) and American dog ticks (Dermacentor variabilis) in Kentucky. Am. J. Trop. Med. Hyg. 2011, 85, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Dodds, D.G.; Martell, A.M.; Yescott, R.E. Ecology of the American dog tick, Dermacentor variabilis (Say), in Nova Scotia. Can. J. Zool. 1969, 47, 171–181. [Google Scholar] [CrossRef]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Geddes, G.; McNabb, A.; Mak, S.; Durden, L.A. Distribution and characterization of Borrelia burgdorferi isolates from Ixodes scapularis and presence in mammalian hosts in Ontario, Canada. J. Med. Entomol. 2006, 43, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.S.; Buckley, P.A.; Balmforth, M.G.; Zhioua, E.; Mitra, S.; Buckley, F.G. Reservoir competence of native North American birds for the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 2005, 42, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Baker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and rage expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, N.H.; Barker, I.K.; Francis, C.A.; Heagy, A.; Lindsay, L.R.; Hobson, K.A. How far north are migrant birds transporting the tick Ixodes scapularis in Canada? Insights from stable hydrogen isotope analyses of feathers. Ticks Tick-Borne Dis. 2015, 6, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Durden, L.A. New record of the Lyme disease bacterium in ticks collected from songbirds in Central and Eastern Canada. Int. J. Acarol. 2015, 41, 241–249. [Google Scholar] [CrossRef]
- Scott, J.D. Studies abound on how far north Ixodes scapularis ticks are transported by birds. Tick Tick-Borne Dis. 2016, 7, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Neelakanta, G.; Sultana, H.; Fish, D.; Anderson, J.F.; Fikrig, E. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express and antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Invest. 2010, 120, 3179–3190. [Google Scholar] [CrossRef] [Green Version]
- Talbot, B.; Slatculescu, A.; Thickstun, C.R.; Koffi, J.K.; Leighton, P.A.; McKay, R.; Kulkarni, M.A. Landscape determinants of density of blacklegged ticks, vectors of Lyme disease, at the northern edge of their distribution in Canada. Sci. Rep. 2019, 9, 16652. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 28, 452–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddell, L.A.; Greig, J.; Lindsay, L.R.; Hinckley, A.F.; Ogden, N.H. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS ONE 2018, 13, e0207067. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Surveillance of Lyme Disease. Available online: http://healthycanadians.gc.ca/diseases-conditions-maladies-affections/disease-maladie/lyme/surveillance-eng.php (accessed on 1 December 2020).
- Leeflang, M.M.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Van Bortel, W.; Brandenburg, A.H.; Van Burgel, N.D.; Van Dam, A.P.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 140. [Google Scholar] [CrossRef] [Green Version]
- Koffi, J.K.; Leighton, P.A.; Pelcat, Y.; Trudel, L.; Lindsay, L.R.; Milord, F.; Ogden, N.H. Passive surveillance for I. scapularis ticks: Enhanced analysis for early detection of emerging Lyme disease risk. J. Med. Entomol. 2012, 49, 400–409. [Google Scholar] [CrossRef]
- Gherman, C.M.; Mihalca, A.D.; Dumitrache, M.O.; Gyoörke, A.; Oroian, I.; Sandor, M.; Cozma, V. CO2 flagging—An improved method for the collection of questing ticks. Parasites Vectors 2012, 5, 125. [Google Scholar] [CrossRef] [Green Version]
- Lieske, D.J.; Lloyd, V.K. Combining public participatory surveillance and occupancy modelling to predict the distributional response of Ixodes scapularis to climate change. Ticks Tick-Borne Dis. 2018, 9, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Boudreau, C.R.; Patterson, J.W.; Bradet-Legris, J.; Lloyd, V.K. Citizen science and community engagement in tick surveillance-A Canadian case study. Healthcare 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, N.H.; Lindsay, L.R.; Morshed, M.; Sockett, P.N.; Artsob, H.A. The emergence of Lyme disease in Canada. Can. Med. Assoc. J. 2009, 180, 1221–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nova Scotia. Communicable Disease and Prevention Control; Nova Scotia: Halifax, NS, Canada, 2013. [Google Scholar]
- New Brunswick Disease Watch Bulletin. 2015. Available online: http://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/Publications/NBDiseaseWatchBulletin_vol21.pdf (accessed on 3 December 2020).
- New Brunswick Office of the Chief Medical Officer of Health (Public Health). Lyme Disease—Brief Reference for New Brunswick Clinicians. Available online: https://www2.gnb.ca/content/gnb/en/departments/ocmoh/cdc/content/vectorborne_andzoonotic/Tick-Borne_Diseases/brief.html (accessed on 3 December 2020).
- Cawthorn, R.J.; Horney, B.S.; Maloney, R. Lyme disease vector, Ixodes dammini (the northern deer tick), identified in Prince Edward Island. Can. Vet. J. 1990, 31, 220. [Google Scholar]
- Banerjee, S.N.; Banerjee, M.; Fernando, K.; Scott, J.D.; Mann, R.; Morshed, M.G. Presence of spirochete causing Lyme disease, Borrelia burgdorferi, in the blacklegged tick, Ixodes scapularis, in Southern Ontario. Can. Med. Assoc. J. 2000, 162, 1567–1569. [Google Scholar]
- Dibernardo, A.; Cote, T.; Ogden, N.H.; Lindsay, L.R. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasites Vectors 2014, 7, 183. [Google Scholar] [CrossRef] [Green Version]
- Nelder, M.P.; Russell, C.; Lindsay, L.R.; Dhar, B.; Patel, S.N.; Johnson, S.; Moore, S.; Kristjanson, E.; Li, Y.; Ralevski, F. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS ONE 2014, 9, e105358. [Google Scholar] [CrossRef]
- Ogden, N.H.; Bigras-Poulin, M.; O’Callaghan, C.J.; Barker, I.K.; Lindsay, L.R.; Maarouf, A.; Smoyer-Tomic, K.E.; Waltner-Toews, D.; Charron, D. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 2006, 35, 375–389. [Google Scholar] [CrossRef]
- Foley-Eby, A.H.; Savidge, C.; Lloyd, V.K. Ixodes scapularis ticks and Borrelia burgdorferi on Prince Edward Island: Passive tick surveillance and canine seroprevalence. Can. Vet. J. 2020, 61, 1107–1110. [Google Scholar]
- Global News, 2017: New Blacklegged Tick Risk Areas Identified in New Brunswick. Available online: https://globalnews.ca/news/3762878/new-tick-risk-areas-new-brunswick/ (accessed on 9 December 2020).
- Adrion, E.R.; Aucott, J.; Lemke, K.W.; Weiner, J.P. Health care costs, utilization and patterns of care following Lyme disease. PLoS ONE 2015, 10, e0116767. [Google Scholar] [CrossRef]
- Van den Wijngaard, C.C.; Hofhuis, A.; Wong, A.; Harms, M.G.; de Wit, G.A.; Lugner, A.K.; Suijkerbuijk, A.W.M.; Mangen, M.-J.J.; van Pelt, W. The cost of Lyme borreliosis. Eur. J. Public Health 2017, 27, 538–547. [Google Scholar] [CrossRef]
- Davidsson, M. The Financial Implications of a Well-Hidden and Ignored Chronic Lyme Disease Pandemic. Healthcare 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mac, S.; da Silva, S.R.; Sander, B. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: A scoping review. PLoS ONE 2019, 14, e0210280. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Trudel, L.; Artsob, H.; Barker, I.K.; Beauchamp, G.; Charron, D.F.; Drebot, M.A.; Galloway, T.D.; O’Handley, R.; Thompson, R.A.; et al. Ixodes scapularis ticks collected by passive surveillance in Canada: Analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 2006, 43, 600–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoles, G.A.; Papero, M.; Beati, L.; Fish, D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001, 1, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick-Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Han, S.; Lubelczyk, C.; Hickling, G.J.; Belperron, A.A.; Bockenstedt, L.K.; Tsao, J.I. Vertical transmission rates of Borrelia miyamotoi in Ixodes scapularis collected from white-tailed deer . Ticks Tick Borne Dis. 2019, 10, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Johnson, R.C.; Magnarelli, L.A.; Hyde, F.W. Identification of endemic foci of Lyme disease: Isolation of Borrelia burgdorferi from feral rodents and ticks (Dermacentor variabilis). J. Clin. Microbiol. 1985, 22, 36–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, L.R.; Barker, I.K.; Surgeoner, G.A.; McEwen, S.A.; Elliott, L.A.; Kolar, J. Apparent incompetence of Dermacentor variabilis (Acari: Ixodidae) and fleas (Insecta: Siphonaptera) as vectors of Borrelia burgdorferi in an Ixodes scapularis endemic area of Ontario, Canada. J. Med. Entomol. 1991, 28, 750–753. [Google Scholar] [CrossRef]
- Scott, J.; Clark, K.L.; Anderson, J.F.; Foley, J.E.; Young, M.R.; Durden, L.A. Lyme Disease Bacterium, Borrelia burgdorferi sensu lato, Detected in Multiple Tick Species at Kenora, Ontario, Canada. J. Bacteriol. Parasitol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Patterson, J.W.; Duncan, A.M.; McIntyre, K.C.; Lloyd, V.K. Evidence for genetic hybridization between Ixodes scapularis and Ixodes cookei. Can. J. Zool. 2017, 95, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Piesman, J.; Sinsky, R.J. Ability of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum (Acari: Ixodidae) to acquire, maintain, and transmit Lyme disease spirochete (Borrelia burgdorferi). J. Med. Entomol. 1988, 25, 336–338. [Google Scholar] [CrossRef]
- Sanders, F.H., Jr.; Oliver, J.H., Jr. Evaluation of Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis (Acari: Ixodidae) from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 1995, 32, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Kocan, A.A.; Mukolwe, S.W.; Murphy, G.L.; Barker, R.W.; Kocan, K.M. Isolation of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) from Ixodes scapularis and Dermacentoralbipictus ticks (Acari: Ixodidae) in Oklahoma. J. Med. Entomol. 1992, 29, 630–633. [Google Scholar] [CrossRef]
- Johns, R.; Sonenshine, D.E.; Hynes, W.L. Identification of a defensin from the hemolymph of the American dog tick, Dermacentor variabilis. Insect Biochem. Mol. Biol. 2001, 31, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Johns, R.; Ohnishi, J.; Broadwater, A.; Sonenshine, D.E.; De Silva, A.M.; Hynes, W.L. Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: Immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae). J. Med. Entomol. 2001, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Crowder, C.D.; Matthews, H.E.; Schutzer, S.; Rounds, M.A.; Luft, B.J.; Nolte, O.; Campbell, S.R.; Phillipson, C.A.; Li, F.; Sampath, R.; et al. Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS ONE 2010, 5, e10650. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.P.; Muzaffar, S.B.; Lavers, J.; Lacombe, E.H.; Cahill, B.K.; Lubelczyk, C.B.; Kinsler, A.; Mathers, A.J.; Rand, P.W. Borrelia garinii in seabird ticks (Ixodes uriae), Atlantic coast, North America. Emerg. Infect. Dis. 2006, 12, 1909–1911. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.; Lloyd, V.K. Identification of Borrelia bissettii in Ixodes scapularis ticks from New Brunswick, Canada. Can. J. Microbiol. 2019, 65, 155–161. [Google Scholar] [CrossRef]
- Pachner, A.R.; Dail, D.; Bai, Y.; Sondey, M.; Pak, L.; Narayan, K.; Cadavid, D. Genotype determines phenotype in experimental Lyme borreliosis. Ann. Neurol. 2004, 56, 361–370. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex—Clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ. Health Perspect. 2010, 118, 909–914. [Google Scholar] [CrossRef] [Green Version]
- Quigley, R. Little local worry about tick populations. J. Pioneer 2011. Available online: https://www.journalpioneer.com/news/local/little-local-worry-about-tick-populations-52682/ (accessed on 11 December 2020).
- Lloyd, V.K.; Hawkins, R.G. Under-Detection of Lyme Disease in Canada. Healthcare 2018, 6, 125. [Google Scholar] [CrossRef] [Green Version]
- Gabriele-Rivet, V.; Arsenault, J.; Badcock, J.; Cheng, A.; Edsall, J.; Goltz, J.; Kennedy, J.; Lindsay, L.R.; Pelcat, Y.; Ogden, N.H. Different ecological niches for ticks of public health significance in Canada. PLoS ONE 2015, 10, e0131282. [Google Scholar] [CrossRef]
- Bjurman, N.K.; Bradet, G.; Lloyd, V.K. Assessing the risk of Borrelia infection in New Brunswick: Using dogs as a sentinel species. Can. Vet. J. 2016, 57, 1–4. [Google Scholar]
- McGowan, C.V. The Seroprevalence of Borrelia burgdorferi in New Brunswick and Nova Scotia Cows (Bos taurus) and Transmission of Borrelia to Unpasteurized Milk. B.Sc. Honours Thesis, Mount Allison University, Sackville, NB, Canada, 2019. [Google Scholar]
- Bush, E. Borrelia Infection in Maritime Horses, 2018. B.Sc. Honours Thesis, Mount Allison University, Sackville, NB, Canada, 2018. [Google Scholar]
- Lindenmayer, J.M.; Marshall, D.; Onderdonk, A.B. Dogs as sentinels for Lyme disease in Massachusetts. Am. J. Public Health 1991, 81, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Soucy, J.R.; Slatculescu, A.M.; Nyiraneza, C.; Ogden, N.H.; Leighton, P.A.; Kerr, J.T.; Kulkarni, M.A. High-Resolution Ecological Niche Modeling of Ixodes scapularis Ticks Based on Passive Surveillance Data at the Northern Frontier of Lyme Disease Emergence in North America. Vector-Borne Zoonotic Dis. 2018, 18, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slatculescu, A.M.; Clow, K.M.; McKay, R.; Talbot, B.; Logan, J.J.; Thickstun, C.R.; Jardine, C.M.; Ogden, N.H.; Knudby, A.J.; Kulkarni, M.A. Species distribution models for the eastern blacklegged tick, Ixodes scapularis, and the Lyme disease pathogen, Borrelia burgdorferi, in Ontario, Canada. PLoS ONE 2020, 15, e0238126. [Google Scholar] [CrossRef] [PubMed]
- Kotchi, S.O.; Bouchard, C.; Brazeau, S.; Ogden, N.H. Earth Observation-Informed Risk Maps of the Lyme Disease Vector Ixodes scapularis in Central and Eastern Canada. Remote Sens. 2021, 13, 524. [Google Scholar] [CrossRef]
- Ogden, N.H.; Maarouf, A.; Barker, I.K.; Bigras-Poulin, M.; Lindsay, L.R.; Morshed, M.G.; O’Callaghan, C.J.; Ramay, F.; Waltner-Toew, D.; Charron, D.F. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol. 2006, 36, 63–70. [Google Scholar] [CrossRef]
- Ogden, N.H.; Radojević, M.; Wu, X.; Duvvuri, V.R.; Leighton, P.A.; Wu, J. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ. Health Perspect. 2014, 122, 631–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disease Watch 26: 06/17. 2017. Available online: https://www2.gnb.ca/content/gnb/en/departments/ocmoh/publications.html (accessed on 21 September 2021).
- Maritime Tick Information Portal. Available online: http://www.maritimetickmaps.ca/ (accessed on 21 September 2021).
- Open Data New Brunswick. Tick Data—2012 to 2018/Données Relatives Aux Tiques—2012 à 2018—Tick Timeline. 2019. Available online: https://gnb.socrata.com/Health-and-Wellness/Tick-Data-2012-to-2018-Donn-es-relatives-aux-tique/3mpw-72pb.Tick Timeline_Chronologie de tiques 2012-2018.mp4 (accessed on 8 December 2020).
- Kopsco, H.L.; Duhaime, R.J.; Mather, T.N.; Diuk-Wasser, M. Crowdsourced Tick Image-Informed Updates to U.S. County Records of Three Medically Important Tick Species. J. Med. Entomol. 2021, tjab082. [Google Scholar] [CrossRef] [PubMed]
- Cull, B. Potential for online crowdsourced biological recording data to complement surveillance for arthropod vectors. PLoS ONE 2021, 16, e0250382. [Google Scholar] [CrossRef] [PubMed]
- Koffi, J.; Savage, J.; Thivierge, K.; Lindsay, L.R.; Bouchard, C.; Pelcat, Y.N.; Ogden, N. Evaluating the submission of digital images as a method of surveillance for Ixodes scapularis ticks. Parasitology 2017, 144, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morshed, M.G.; Scott, J.D.; Fernando, K.; Mann, R.B.; Durden, L.A. Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park, Ontario. J. Med. Entomol. 2003, 40, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Fernando, K.; Durden, L.A.; Morshed, M.G. Lyme disease spirochete, Borrelia burgdorferi, endemic in epicenter at Turkey Point, Ontario. J. Med. Entomol. 2004, 41, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Lee, M.-K.; Fernando, K.; Jorgensen, D.R.; Durden, L.A.; Morshed, M.G. Rapid introduction of Lyme disease spirochete, Borrelia burgdorferi sensu stricto, in Ixodes scapularis (Acari: Ixodidae) established at Turkey Point Provincial Park, Ontario, Canada. J. Vector Ecol. 2007, 33, 64–69. [Google Scholar] [CrossRef]
- Scott, J.D.; Durden, L.A. First isolation of Lyme disease spirochete, Borrelia burgdorferi, from ticks collected from songbirds in Ontario, Canada. N. Am. Bird Bander 2009, 34, 97–101. [Google Scholar]
- Bouchard, C.; Beauchamp, G.; Nguon, S.; Trudel, L.; Milord, F.; Lindsay, L.R.; Bélanger, D.; Ogden, N.H. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in Southeastern Canada: Implications for Borrelia burgdorferi transmission. Ticks Tick-Borne Dis. 2011, 2, 183–190. [Google Scholar] [CrossRef]
- Krakowetz, C.N.; Lindsay, L.R.; Chilton, N.B. Genetic diversity in Ixodes scapularis (Acari: Ixodidae) from six established populations in Canada. Tick Tick-Borne Dis. 2011, 2, 143–150. [Google Scholar] [CrossRef]
- Ogden, N.H.; Koffi, J.K.; Pelcat, Y.; Lindsay, L.R. Environmental risk from Lyme disease in central and Eastern Canada: A summary of recent surveillance information. Can. Comm. Dis. Rep. 2014, 40, 74. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Marrotte, R.R.; Desrosiers, N.; Fiset, J.; Gaitan, J.; Gonzalez, A.; Koffi, J.K.; Lapointe, F.-J.; Leighton, P.A.; Lindsay, L.R.; et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol. Appl. 2014, 7, 750–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werden, L.; Barker, I.K.; Bowman, J.; Gonzales, E.K.; Leighton, P.A.; Lindsay, L.R.; Jardine, C.M. Geography, deer, and host biodiversity shape the pattern of Lyme disease emergence in the thousand islands archipelago of Ontario, Canada. PLoS ONE 2014, 9, e85640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.D.; Anderson, J.F.; Durden, L.A.; Smith, M.L.; Manord, J.M.; Clark, K.L. Prevalence of the Lyme disease spirochete, Borrelia burgdorferi, in blacklegged ticks, Ixodes scapularis at Hamilton-Wentworth, Ontario. Int. J. Med. Sci. 2016, 13, 316–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keirans, J.E.; Litwak, T.R. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi river. J. Med. Entomol. 1989, 26, 435–448. [Google Scholar] [CrossRef]
- Wills, M.K.; Kirby, A.M.; Lloyd, V.K. Detecting the Lyme Disease Spirochete, Borrelia Burgdorferi, in Ticks Using Nested PCR. J. Vis. Exp. 2018, 132, e56471. [Google Scholar] [CrossRef] [Green Version]
- Zinck, C.B.; Priest, J.M.; Shutler, D.; Boudreau, M.; Lloyd, V.K. Detection of Borrelia spp., Ehrlichia canis, Anaplasma phagocytophylum, and Dirofilaria immitis in Eastern Coyotes (Canis latrans) in Nova Scotia, Canada. J. Wildl. Dis. 2021, 57, 678–682. [Google Scholar] [CrossRef]
- Nolte, O. Nucleic acid amplification based diagnostic of Lyme (neuro-) borreliosis—Lost in the jungle of methods, targets, and assays? Open Neurol. J. 2012, 6, 129–139. [Google Scholar] [CrossRef] [PubMed]
| I. scapularis | I. cookei | D. variabilis | Unknown 1 | Other Tick Species 2 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult Female | Adult Male | Nymph | Larvae | Total I. scapularis | Adult Female | Adult Male | Nymph | Larvae | Total I. cookei | Adult Female | Adult Male | Nymph | Larvae | Total D. variabilis | Adult Female | Adult Male | Nymph | Larvae | Total Unknown | Adult Female | Adult Male | Nymph | Larvae | Total Other Species | Total All Species | ||
| 2012 | Dog | 167 | 8 | 2 | 0 | 177 | 14 | 0 | 0 | 0 | 14 | 3 | 4 | 0 | 0 | 7 | 8 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 207 |
| Cat | 63 | 4 | 3 | 0 | 70 | 16 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 11 | 2 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 99 | |
| Human | 13 | 1 | 0 | 20 | 34 | 3 | 0 | 0 | 0 | 3 | 3 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 43 | |
| Other host 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 | |
| Undetermined 4 | 8 | 2 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | |
| Total 2012 | 251 | 15 | 5 | 20 | 291 | 36 | 0 | 2 | 0 | 38 | 6 | 5 | 0 | 0 | 11 | 21 | 3 | 1 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 365 | |
| 2013 | Dog | 311 | 15 | 4 | 0 | 330 | 23 | 0 | 4 | 6 | 33 | 15 | 4 | 0 | 0 | 19 | 3 | 1 | 0 | 4 | 8 | 2 | 1 | 0 | 0 | 1 | 391 |
| Cat | 43 | 3 | 2 | 0 | 48 | 48 | 0 | 1 | 0 | 49 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 98 | |
| Human | 26 | 1 | 3 | 6 | 36 | 6 | 6 | 0 | 0 | 12 | 17 | 2 | 2 | 5 | 26 | 1 | 0 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 77 | |
| Other host 3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Undetermined 4 | 4 | 0 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | |
| Total 2013 | 385 | 19 | 10 | 6 | 420 | 77 | 6 | 5 | 6 | 94 | 46 | 6 | 2 | 5 | 59 | 4 | 1 | 0 | 6 | 11 | 3 | 1 | 0 | 0 | 1 | 585 | |
| 2014 | Dog | 426 | 18 | 5 | 0 | 449 | 59 | 2 | 4 | 14 | 79 | 12 | 2 | 0 | 0 | 14 | 22 | 0 | 0 | 6 | 28 | 0 | 0 | 0 | 0 | 0 | 570 |
| Cat | 71 | 3 | 2 | 0 | 76 | 23 | 0 | 11 | 5 | 39 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 121 | |
| Human | 33 | 4 | 5 | 0 | 42 | 1 | 0 | 4 | 0 | 5 | 10 | 6 | 0 | 0 | 16 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 64 | |
| Other host 3 | 5 | 1 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | |
| Undetermined 4 | 8 | 0 | 1 | 0 | 9 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 12 | |
| Total 2014 | 543 | 26 | 13 | 0 | 582 | 83 | 2 | 20 | 19 | 124 | 23 | 9 | 0 | 0 | 32 | 29 | 0 | 1 | 6 | 36 | 0 | 0 | 0 | 0 | 0 | 774 | |
| 2015 | Dog | 400 | 9 | 9 | 4 | 422 | 33 | 0 | 16 | 1 | 50 | 38 | 18 | 0 | 0 | 56 | 7 | 0 | 4 | 0 | 11 | 1 | 0 | 0 | 0 | 539 | |
| Cat | 76 | 2 | 12 | 0 | 90 | 19 | 0 | 19 | 0 | 38 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 0 | 3 | 133 | |
| Human | 29 | 3 | 14 | 0 | 46 | 2 | 0 | 4 | 0 | 6 | 16 | 18 | 2 | 0 | 36 | 1 | 1 | 3 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 93 | |
| Other host 3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 5 | 1 | 0 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 10 | |
| Undetermined 4 | 11 | 0 | 2 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | |
| Total 2015 | 517 | 14 | 37 | 4 | 572 | 54 | 0 | 39 | 1 | 94 | 58 | 39 | 2 | 0 | 99 | 11 | 1 | 11 | 0 | 23 | 1 | 3 | 0 | 0 | 3 | 791 | |
| 2016 | Dog | 520 | 24 | 6 | 0 | 550 | 14 | 0 | 8 | 12 | 34 | 12 | 3 | 1 | 0 | 16 | 14 | 5 | 2 | 0 | 21 | 9 | 1 | 0 | 0 | 1 | 622 |
| Cat | 132 | 6 | 6 | 1 | 145 | 20 | 0 | 22 | 11 | 53 | 4 | 0 | 0 | 0 | 4 | 4 | 0 | 1 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 210 | |
| Human | 77 | 6 | 8 | 1 | 92 | 2 | 0 | 2 | 0 | 4 | 24 | 13 | 0 | 0 | 37 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 135 | |
| Other host 3 | 7 | 1 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 11 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 20 | |
| Undetermined 4 | 18 | 9 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 30 | |
| Total 2016 | 754 | 46 | 20 | 2 | 822 | 36 | 0 | 32 | 23 | 91 | 53 | 16 | 1 | 0 | 70 | 21 | 6 | 3 | 3 | 33 | 9 | 1 | 0 | 0 | 1 | 1017 | |
| 2017 | Dog | 607 | 44 | 5 | 1 | 657 | 7 | 0 | 26 | 7 | 40 | 29 | 13 | 0 | 0 | 42 | 18 | 7 | 8 | 0 | 33 | 4 | 3 | 1 | 0 | 4 | 776 |
| Cat | 140 | 9 | 10 | 0 | 159 | 10 | 0 | 16 | 0 | 26 | 1 | 0 | 0 | 0 | 1 | 10 | 1 | 2 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 199 | |
| Human | 160 | 7 | 38 | 0 | 205 | 3 | 0 | 6 | 0 | 9 | 27 | 32 | 0 | 1 | 60 | 4 | 2 | 6 | 0 | 12 | 1 | 0 | 1 | 0 | 1 | 287 | |
| Other host 3 | 8 | 0 | 2 | 0 | 10 | 0 | 0 | 8 | 1 | 9 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 21 | |
| Undetermined 4 | 37 | 4 | 1 | 0 | 42 | 0 | 0 | 2 | 0 | 2 | 8 | 5 | 0 | 1 | 14 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 60 | |
| Total 2017 | 952 | 64 | 56 | 1 | 1073 | 20 | 0 | 58 | 8 | 86 | 65 | 50 | 1 | 2 | 118 | 35 | 10 | 16 | 0 | 61 | 7 | 3 | 2 | 0 | 5 | 1343 | |
| 2018 | Dog | 309 | 27 | 2 | 0 | 338 | 19 | 2 | 16 | 5 | 42 | 20 | 13 | 0 | 0 | 33 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 3 | 417 |
| Cat | 98 | 2 | 17 | 0 | 117 | 20 | 0 | 26 | 1 | 47 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 167 | |
| Human | 71 | 12 | 18 | 0 | 101 | 5 | 0 | 11 | 0 | 16 | 41 | 22 | 0 | 1 | 64 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 2 | 0 | 2 | 183 | |
| Other host 3 | 5 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 7 | |
| Undetermined 4 | 11 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 8 | 0 | 11 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 23 | |
| Total 2018 | 494 | 41 | 37 | 0 | 572 | 45 | 2 | 53 | 6 | 106 | 67 | 35 | 8 | 1 | 111 | 1 | 1 | 0 | 0 | 2 | 17 | 4 | 2 | 0 | 6 | 797 | |
| 2019 | Dog | 471 | 29 | 2 | 0 | 502 | 24 | 0 | 9 | 2 | 35 | 28 | 15 | 1 | 0 | 44 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 2 | 584 |
| Cat | 115 | 2 | 2 | 0 | 119 | 14 | 0 | 21 | 4 | 39 | 0 | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 161 | |
| Human | 217 | 7 | 31 | 0 | 255 | 4 | 0 | 7 | 0 | 11 | 39 | 32 | 2 | 0 | 73 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 3 | 0 | 3 | 344 | |
| Other host 3 | 4 | 0 | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 9 | |
| Undetermined 4 | 50 | 7 | 2 | 0 | 59 | 0 | 0 | 2 | 0 | 2 | 8 | 5 | 0 | 1 | 14 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 77 | |
| Total 2019 | 857 | 45 | 37 | 0 | 939 | 45 | 0 | 39 | 6 | 90 | 76 | 55 | 3 | 1 | 135 | 2 | 2 | 1 | 1 | 6 | 6 | 1 | 4 | 0 | 5 | 1175 | |
| 2020 | Dog | 243 | 23 | 0 | 0 | 266 | 15 | 0 | 6 | 0 | 21 | 8 | 4 | 0 | 0 | 12 | 2 | 0 | 5 | 0 | 7 | 0 | 1 | 0 | 0 | 1 | 307 |
| Cat | 23 | 1 | 2 | 0 | 26 | 1 | 0 | 8 | 1 | 10 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 43 | |
| Human | 46 | 6 | 2 | 0 | 54 | 1 | 0 | 3 | 0 | 4 | 6 | 1 | 0 | 1 | 8 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 66 | |
| Other host 3 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | |
| Undetermined 4 | 9 | 0 | 0 | 0 | 9 | 1 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | |
| Total 2020 | 325 | 30 | 4 | 0 | 359 | 18 | 0 | 18 | 2 | 38 | 14 | 5 | 0 | 1 | 20 | 4 | 0 | 10 | 0 | 14 | 1 | 1 | 0 | 0 | 1 | 432 | |
| Year of Collection | Collection Location | Percentage of Tick Infection (%) and Samples Size (n) by Tick Species: | ||||
|---|---|---|---|---|---|---|
| I. scapularis | I. cookei | D. variabilis | Unknown Species 1 | Other Species 2 | ||
| 2012 | NB | 8 (n = 20/249) | 7 (n = 1/14) | 11 (n = 1/9) | 15 (n = 4/26) | 0 (n = 0/0) |
| NS | 23 (n = 9/39) | 25 (n = 1/4) | 100 (n = 1/1) | 0 (n = 0/1) | 0 (n = 0/0) | |
| PEI | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | |
| Other 3 | 33 (n = 1/3) | 0 (n = 0/0) | 50 (n = 1/2) | 0 (n = 0/0) | 0 (n = 0/0) | |
| 2013 | NB | 22 (n = 71/323) | 4 (n = 3/84) | 0 (n = 0/35) | 0 (n = 0/9) | 0 (n = 0/1) |
| NS | 14 (n = 8/58) | 0 (n = 0/2) | 0 (n = 0/15) | 0 (n = 0/2) | 0 (n = 0/0) | |
| PEI | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | |
| Other 3 | 33 (n = 1/3) | 0 (n = 0/0) | 33 (n = 1/3) | 0 (n = 0/0) | 0 (n = 0/0) | |
| 2014 | NB | 13 (n = 67/516) | 5 (n = 5/109) | 7 (n = 1/14) | 9 (n = 3/34) | 0 (n = 0/0) |
| NS | 17 (n = 9/53) | 13 (n = 1/8) | 0 (n = 0/9) | 0 (n = 0/2) | 0 (n = 0/0) | |
| PEI | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/8) | 0 (n = 0/0) | |
| Other 3 | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/1) | 0 (n = 0/0) | |
| 2015 | NB | 10 (n = 47/465) | 2 (n = 2/90) | 0 (n = 0/28) | 0 (n = 0/21) | 0 (n = 0/3) |
| NS | 16 (n = 13/82) | 0 (n = 0/7) | 0 (n = 0/60) | 0 (n = 0/0) | 0 (n = 0/1) | |
| PEI | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | |
| Other 3 | 13 (n = 1/8) | 0 (n = 0/2) | 0 (n = 0/10) | 0 (n = 0/3) | 0 (n = 0/0) | |
| 2016 | NB | 11 (n = 75/682) | 9 (n = 7/76) | 4 (n = 1/24) | 5 (n = 1/22) | 0 (n = 0/10) |
| NS | 26 (n = 29/113) | 0 (n = 0/4) | 0 (n = 0/31) | 0 (n = 0/3) | 0 (n = 0/0) | |
| PEI | 33 (n = 1/3) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/1) | 0 (n = 0/0) | |
| Other 3 | 25 (n = 2/8) | 0 (n = 0/0) | 0 (n = 0/16) | 0 (n = 0/14) | 0 (n = 0/1) | |
| 2017 | NB | 10 (n = 84/841) | 2 (n = 1/66) | 0 (n = 0/36) | 0 (n = 0/52) | 0 (n = 0/12) |
| NS | 21 (n = 38/179) | 0 (n = 0/8) | 0 (n = 0/59) | 0 (n = 0/9) | 0 (n = 0/0) | |
| PEI | 0 (n = 0/1) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/0) | |
| Other 3 | 15 (n = 6/41) | 0 (n = 0/1) | 0 (n = 0/13) | 0 (n = 0/7) | 0 (n = 0/0) | |
| 2018 | NB | 22 (n = 144/519) | 5 (n = 4/88) | 5 (n = 2/40) | 22 (n = 2/9) | 0 (n = 0/24) |
| NS | 25 (n = 28/112) | 0 (n = 0/12) | 0 (n = 0/55) | 0 (n = 0/0) | 0 (n = 0/0) | |
| PEI | 25 (n = 4/16) | 0 (n = 0/0) | 0 (n = 0/2) | 0 (n = 0/0) | 100 (n1/1) | |
| Other 3 | 24 (n = 8/33) | 33 (n = 1/3) | 0 (n = 0/7) | 0 (n = 0/0) | 0 (n = 0/0) | |
| 2019 | NB | 17 (n = 103/604) | 2 (n = 2/81) | 0 (n = 0/39) | 7 (n = 1/14) | 0 (n = 0/8) |
| NS | 21 (n = 31/147) | 0 (n = 0/8) | 2 (n = 2/89) | 0 (n = 0/7) | 0 (n = 0/0) | |
| PEI | 8 (n = 1/13) | 0 (n = 0/0) | 0 (n = 0/1) | 0 (n = 0/1) | 0 (n = 0/0) | |
| Other 3 | 30 (n = 6/20) | 0 (n = 0/1) | 0 (n = 0/1) | 0 (n = 0/0) | 0 (n = 0/0) | |
| 2020 | NB | 18 (n = 55/307) | 5 (n = 2/37) | 15 (n = 2/13) | 19 (n = 3/16) | 0 (n = 0/1) |
| NS | 18 (n = 7/40) | 0 (n = 0/0) | 0 (n = 0/5) | 0 (n = 0/0) | 0 (n = 0/0) | |
| PEI | 8 (n = 2/25) | 0 (n = 0/0) | 0 (n = 0/1) | 0 (n = 0/0) | 0 (n = 0/0) | |
| Other 3 | 0 (n = 0/4) | 0 (n = 0/0) | 0 (n = 0/1) | 0 (n = 0/0) | 0 (n = 0/1) | |
| Year of Collection | Percentage of Tick, All Species, Infection (%) and Sample Size (n) by Health Regions: | ||||||
|---|---|---|---|---|---|---|---|
| Moncton | Saint John | Fredericton | Edmundston | Campbellton | Bathurst | Miramichi | |
| 2012 | 9 (n = 14/157) | 5 (n = 4/87) | 33 (n = 1/3) | 0 (n = 0/0) | 0 (n = 0/0) | 0 (n = 0/2) | 0 (n = 0/0) |
| 2013 | 12 (n = 10/85) | 18 (n = 16/90) | 20 (n = 2/10) | 0 (n = 0/0) | 0 (n = 0/1) | 25 (n = 1/4) | 0 (n = 0/7) |
| 2014 | 13 (n = 23/176) | 13 (n = 39/299) | 16 (n = 5/31) | 0 (n = 0/2) | 0 (n = 0/3) | 0 (n = 0/5) | 0 (n = 0/0) |
| 2015 | 6 (n = 9/149) | 10 (n = 31/307) | 8 (n = 3/39) | 0 (n = 0/1) | 33 (n = 1/3) | 9 (n = 1/11) | 0 (n = 0/2) |
| 2016 | 12 (n = 25/208) | 11 (n = 45/410) | 11 (n = 4/36) | 0 (n = 0/1) | 0 (n = 0/4) | 0 (n = 0/15) | 0 (n = 0/12) |
| 2017 | 9 (n = 23/256) | 11 (n = 55/503) | 11 (n = 6/56) | 0 (n = 0/2) | 0 (n = 0/5) | 0 (n = 0/18) | 0 (n = 0/16) |
| 2018 | 12 (n= 23/195) | 41 (n = 161/392) | 9 (n = 4/46) | 0 (n = 0/5) | 0 (n = 0/2) | 9 (n = 4/43) | 33 (n = 6/18) |
| 2019 | 11 (n = 21/190) | 17 (n = 63/373) | 15 (n = 19/124) | 33 (n = 1/3) | 0 (n = 0/21) | 17 (n = 5/30) | 20 (n = 1/5) |
| 2020 | 11 (n = 19/174) | 34 (n = 53/156) | 20 (n = 7/35) | 0 (n = 0/1) | 32 (n = 6/19) | 40 (n = 4/10) | 0 (n = 0/1) |
| Year of Collection | Percentage of Tick Infection (%) and Samples Size (n) by Host: | ||||
|---|---|---|---|---|---|
| Dog | Cat | Human | Other Hosts 1 | Unknown Hosts 2 | |
| 2012 | 9 (n = 16/177) | 14 (n = 10/70) | 3 (n = 1/34) | 50 (n = 1/2) | 25 (n = 2/8) |
| 2013 | 15 (n = 26/172) | 10 (n = 3/29) | 27 (n = 7/26) | 0 (n = 0/1) | 33 (n = 1/3) |
| 2014 | 12 (n = 50/415) | 16 (n = 10/64) | 16 (n = 4/25) | 0 (n = 0/6) | 33 (n = 2/6) |
| 2015 | 10 (n = 42/423) | 12 (n = 11/91) | 9 (n = 4/46) | 0 (n = 0/1) | 39 (n = 5/13) |
| 2016 | 11 (n = 61/550) | 14 (n = 20/145) | 27 (n = 25/92) | 0 (n = 0/9) | 6 (n = 1/18) |
| 2017 | 10 (n = 67/665) | 12 (n = 19/161) | 17 (n = 36/209) | 18 (n = 2/11) | 21 (n = 9/42) |
| 2018 | 20 (n = 85/424) | 20 (n = 23/117) | 25 (n = 32/129) | 0 (n = 0/3) | 21 (n = 3/14) |
| 2019 | 16 (n = 81/509) | 23 (n = 26/114) | 21 (n = 24/114) | 40 (n = 2/5) | 16 (n = 5/32) |
| 2020 | 17 (n = 49/287) | 19 (n = 5/26) | 16 (n = 9/55) | 33 (n = 1/3) | 10 (n = 1/10) |
| Year of Collection | Percentage of Tick Infection (%) and Samples Size (n) by Developmental Stage: | |||
|---|---|---|---|---|
| Adult | Nymph | Larvae | Unknown | |
| 2012 | 11 (n = 29/266) | 40 (n = 2/5) | 0 (n = 0/20) | 0 (n = 0/0) |
| 2013 | 17 (n = 37/216) | 13 (n = 1/8) | 0 (n = 0/6) | 0 (n = 0/0) |
| 2014 | 13 (n = 74/569) | 8 (n = 1/12) | 0 (n = 0/0) | 0 (n = 0/0) |
| 2015 | 11 (n = 58/531) | 8 (n = 3/37) | 0 (n = 0/4) | 0 (n = 0/3) |
| 2016 | 14 (n = 112/801) | 5 (n = 1/20) | 0 (n = 0/2) | 0 (n = 0/0) |
| 2017 | 13 (n = 123/945) | 11 (n = 6/56) | 0 (n = 0/1) | 0 (n = 0/0) |
| 2018 | 20 (n = 135/675) | 20 (n = 6/30) | 0 (n = 0/0) | 0 (n = 0/0) |
| 2019 | 17 (n = 129/760) | 28 (n = 12/43) | 0 (n = 0/0) | 0 (n = 0/0) |
| 2020 | 17 (n = 64/377) | 0 (n = 0/4) | 0 (n = 0/0) | 0 (n = 0/0) |
| Year of Collection | Percentage of Tick Infection (%) and Sample Size (n) by Engorgement Status:. | ||
|---|---|---|---|
| Non-Engorged | Engorged | Highly Engorged | |
| 2012 | 8 (n = 3/36) | 11 (n = 21/193) | 8 (n = 5/63) |
| 2013 | 40 (n = 8/20) | 11 (n = 14/130) | 19 (n = 15/80) |
| 2014 | 4 (n = 1/26) | 14 (n = 53/380) | 13 (n = 23/176) |
| 2015 | 10 (n = 2/21) | 13 (n = 46/354) | 7 (n = 14/200) |
| 2016 | 13 (n = 6/47) | 17 (n = 65/385) | 10 (n = 39/389) |
| 2017 | 20 (n = 13/65) | 12 (n = 73/606) | 11 (n = 46/417) |
| 2018 | 33 (n = 1854) | 22 (n = 81/368) | 17 (n = 44/260) |
| 2019 | 7 (n = 5/68) | 18 (n = 86/477) | 26 (n = 60/230) |
| 2020 | 0 (n = 0/30) | 18 (n = 37/208) | 19 (n = 27/143) |
| Year | Primer Name | Target Gene | Sequence (5′-3′) | Annealing Temperature (℃) | Amplicon Size (bp) | Source |
|---|---|---|---|---|---|---|
| 2012 | OspA out 1 R1 | OspA | GTTAGCAGCCTTGACGAGA | 60 | 272 | Ogden et al. 2006 [53] (OspA1b) |
| OspA out F1 | GATACTAGTGTTTTGCCATC | Ogden et al. 2006 [53] (OspA4b) | ||||
| OspA in 1 R1 | OspA | GCGTTTCAGTAGATTTGCCTG | 60 | 214 | Ogden et al. 2006 [53] (OspA2b) | |
| OspA in F1 | TCAAGTGTGGTTTGACCTAG | Ogden et al. 2006 [53] (OspA3b) | ||||
| FlagB out R1 | FlagB | AATTGCATACTCAGTACTATTCTTTATAGAT | 60 | 601 | Ogden et al. 2006 [53] (fla outer 2) | |
| FlagB out F1 | AAGTAGAAAAAGTCTTAGTAAGAATGAAGGA | Ogden et al. 2006 [53] (fla outer 1) | ||||
| FlagB in R1 | FlagB | GAAGGTGCTGTAGCAGGTGCTGGCTGT | 60 | 390 | Ogden et al. 2006 [53] (fla inner 2) | |
| FlagB in F1 | CACATATTCAGATGCAGACAGAGGTTCTA | Ogden et al. 2006 [53] (fla inner 1) | ||||
| 2013 | OspA out R4 | OspA | ACAAGAGCAGACGGAACCAG | 60 | 358 | This work |
| OspA out F4 | CCCCTCTAATTTGGTGCCAT | |||||
| OspA in R4 | OspA | CACAGGAATTAAAAGCGATGG | 60 | 220 | This work | |
| OspA in F4 | AGTGCCTGAATTCCAAGCTG | |||||
| OspA out R3 | OspA | GTAATTTCAACTGCTGACCCC | 60 | 561 | This work | |
| OspA out F3 | TGAAGGCGTAAAAGCTGAC | |||||
| OspA in R3 | OspA | TTGGTGCCATTTGAGTCGTA | 60 | 330 | This work | |
| OspA in F3 | ACTTGAATACACAGGAATTA | |||||
| FlagB out R2 | FlagB | TGGGGAACTTGATTAGCCTG | 60 | 493 | This work | |
| FlagB out F2 | TCATTGCCATTGCAGATTGT | |||||
| FlagB in R2 | FlagB | TCATTGCCATTGCAGATTGT | 60 | 437 | This work | |
| FlagB in F2 | CTTTAAGAGTTCATGTTGGAG | |||||
| 2014 to 2017 | OspA out R2 | OspA | CAACTGCTGACCCCTCTAAT | 55 | 487 | This work |
| OspA out F2 | CTTGAAGTTTTCAAAGAAGAT | |||||
| OspA in R2 | OspA | TTGGTGCCATTTGAGTCGTA | 58 | 350 | This work | |
| OspA in F2 | ACAAGAGCAGACGGAACCAG | |||||
| FlagB out R3 | FlagB | GCATCACTTTCAGGGTCTCA | 55 | 503 | This work | |
| FlagB out F3 | TGGGGAACTTGATTAGCCTG | |||||
| FlagB in R3 | FlagB | CTTTAAGAGTTCATGTTGGAG | 58 | 447 | This work | |
| FlagB in F3 | TCATTGCCATTGCAGATTGT | |||||
| 2017 | OspA out R2 | OspA | CAACTGCTGACCCCTCTAAT | 55 | 487 | This work |
| OspA out F2 | CTTGAAGTTTTCAAAGAAGAT | |||||
| 2018 to 2020 | 23S out F | Borrelia spp. 23S rRNA | GTATGTTTAGTGAGGGGGGTG | 50 | 587 | Dibernardo et al. 2014 [44] |
| 23S out R | GGATCATAGCTAGGTGGTTAG | |||||
| 23S in F | Borrelia spp. 23S rRNA | ATGTATTCCATTGTTTTAATTACG | 51 | 340 | Zinck et al. 2021 [102] | |
| 23S in R | GACAAGTATTGTAGCGAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, J.; Kirby, A.M.; Harris, K.D.; Filiaggi, C.L.; Foley-Eby, A.; Mann, M.; Lieske, D.; Lloyd, V.K. Monitoring Risk: Tick and Borrelia burgdorferi Public Participatory Surveillance in the Canadian Maritimes, 2012–2020. Pathogens 2021, 10, 1284. https://doi.org/10.3390/pathogens10101284
Lewis J, Kirby AM, Harris KD, Filiaggi CL, Foley-Eby A, Mann M, Lieske D, Lloyd VK. Monitoring Risk: Tick and Borrelia burgdorferi Public Participatory Surveillance in the Canadian Maritimes, 2012–2020. Pathogens. 2021; 10(10):1284. https://doi.org/10.3390/pathogens10101284
Chicago/Turabian StyleLewis, Julie, Andrea M. Kirby, Kami Dawn Harris, Cory L. Filiaggi, Alexandra Foley-Eby, Malcolm Mann, David Lieske, and Vett K. Lloyd. 2021. "Monitoring Risk: Tick and Borrelia burgdorferi Public Participatory Surveillance in the Canadian Maritimes, 2012–2020" Pathogens 10, no. 10: 1284. https://doi.org/10.3390/pathogens10101284
APA StyleLewis, J., Kirby, A. M., Harris, K. D., Filiaggi, C. L., Foley-Eby, A., Mann, M., Lieske, D., & Lloyd, V. K. (2021). Monitoring Risk: Tick and Borrelia burgdorferi Public Participatory Surveillance in the Canadian Maritimes, 2012–2020. Pathogens, 10(10), 1284. https://doi.org/10.3390/pathogens10101284





