Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host?
Abstract
1. Introduction
2. Basic Knowledge on Malaria-Related Immunosuppression
3. Host Regulatory Cells in Malaria Infections
3.1. CD8+ Regulatory T Cells
3.2. CD4+ Regulatory T Cells
3.3. Regulatory B Cells
3.3.1. BAFF System Molecules
3.3.2. Atypical Memory B Cells
3.4. Follicular Regulatory T Cells and Follicular Helper T Cells
3.5. Dendritic Cells
3.6. Myeloid Regulatory Cells and Myeloid-Derived Suppressor Cells
4. Concluding Remarks
- Only during or shortly after infection with Plasmodium spp.;
- Genuinely caused by Pf and not by a concomitant infection, malnutrition, or as a response to fever;
- Prolonged effect, about 2–4 weeks (up to 8 in severe malaria) following acute infection before returning to normal;
- The immunosuppression develops in the host during the growth of the parasite or is established through continuous and repetitive infections;
- The contributions of the different stages of the parasite’s lifecycle are distinct;
- During malaria, there are plasmatic components with suppressive properties (parasite cellular components);
- Malaria affects cellular as well as humoral responses in the host, suppressing T cell proliferation and antibody production.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241565721. [Google Scholar]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Beeson, J.G.; Kurtovic, L.; Dobaño, C.; Opi, D.H.; Chan, J.-A.; Feng, G.; Good, M.F.; Reiling, L.; Boyle, M.J. Challenges and strategies for developing efficacious and long-lasting malaria vaccines. Sci. Transl. Med. 2019, 11, eaau1458. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.L.; Vekemans, J.; Richie, T.L.; Duffy, P.E. The march toward malaria vaccines. Vaccine 2015, 33 (Suppl. 4), D13–D23. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- Mordmüller, B.; Surat, G.; Lagler, H.; Chakravarty, S.; Ishizuka, A.S.; Lalremruata, A.; Gmeiner, M.; Campo, J.J.; Esen, M.; Ruben, A.J.; et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017, 542, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Itsara, L.S.; Zhou, Y.; Do, J.; Grieser, A.M.; Vaughan, A.M.; Ghosh, A.K. The Development of Whole Sporozoite Vaccines for Plasmodium falciparum Malaria. Front. Immunol. 2018, 9, 2748. [Google Scholar] [CrossRef]
- Zheng, J.; Pan, H.; Gu, Y.; Zuo, X.; Ran, N.; Yuan, Y.; Zhang, C.; Wang, F. Prospects for Malaria Vaccines: Pre-Erythrocytic Stages, Blood Stages, and Transmission-Blocking Stages. BioMed Res. Int. 2019, 2019, 9751471. [Google Scholar] [CrossRef]
- van den Berg, M.; Ogutu, B.; Sewankambo, N.K.; Biller-Andorno, N.; Tanner, M. RTS, S malaria vaccine pilot studies: Addressing the human realities in large-scale clinical trials. Trials 2019, 20, 316. [Google Scholar] [CrossRef]
- Kumar, R.; Ng, S.; Engwerda, C. The Role of IL-10 in Malaria: A Double Edged Sword. Front. Immunol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Lyke, K.E.; Dabo, A.; Arama, C.; Diarra, I.; Plowe, C.V.; Doumbo, O.K.; Sztein, M.B. Long-term Maintenance of CD4 T Cell Memory Responses to Malaria Antigens in Malian Children Coinfected with Schistosoma haematobium. Front. Immunol. 2017, 8, 1995. [Google Scholar] [CrossRef]
- Nacher, M. Malaria vaccine trials in a wormy world. Trends Parasitol. 2001, 17, 563–565. [Google Scholar] [CrossRef]
- Jongo, S.A.; Shekalaghe, S.A.; Church, L.W.P.; Ruben, A.J.; Schindler, T.; Zenklusen, I.; Rutishauser, T.; Rothen, J.; Tumbo, A.; Mkindi, C.; et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am. J. Trop. Med. Hyg. 2018, 99, 338–349. [Google Scholar] [CrossRef]
- Olotu, A.; Urbano, V.; Hamad, A.; Eka, M.; Chemba, M.; Nyakarungu, E.; Raso, J.; Eburi, E.; Mandumbi, D.O.; Hergott, D.; et al. Advancing Global Health through Development and Clinical Trials Partnerships: A Randomized, Placebo-Controlled, Double-Blind Assessment of Safety, Tolerability, and Immunogenicity of PfSPZ Vaccine for Malaria in Healthy Equatoguinean Men. Am. J. Trop. Med. Hyg. 2018, 98, 308–318. [Google Scholar] [CrossRef]
- Langhorne, J.; Ndungu, F.M.; Sponaas, A.-M.; Marsh, K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008, 9, 725–732. [Google Scholar] [CrossRef]
- Pistone, T.; Diallo, A.; Mechain, M.; Receveur, M.-C.; Malvy, D. Epidemiology of imported malaria give support to the hypothesis of ‘long-term’ semi-immunity to malaria in sub-Saharan African migrants living in France. Travel Med. Infect. Dis. 2014, 12, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Portugal, S.; Tran, T.M.; Ongoiba, A.; Bathily, A.; Li, S.; Doumbo, S.; Skinner, J.; Doumtabe, D.; Kone, Y.; Sangala, J.; et al. Treatment of Chronic Asymptomatic Plasmodium falciparum Infection Does Not Increase the Risk of Clinical Malaria Upon Reinfection. Clin. Infect. Dis. 2017, 64, 645–653. [Google Scholar] [CrossRef]
- Overstreet, M.G.; Cockburn, I.A.; Chen, Y.-C.; Zavala, F. Protective CD8 T cells against Plasmodium liver stages: Immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 2008, 225, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Gatton, M.L.; Cheng, Q. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am. J. Trop. Med. Hyg. 2002, 66, 467–473. [Google Scholar] [CrossRef]
- Shanks, G.D. Tolerance May Be More Appropriate Than Immunity When Describing Chronic Malaria Infections. Am. J. Trop. Med. Hyg. 2019, 100, 497–500. [Google Scholar] [CrossRef]
- Gupta, S.; Snow, R.W.; Donnelly, C.A.; Marsh, K.; Newbold, C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 1999, 5, 340–343. [Google Scholar] [CrossRef]
- Ademolue, T.W.; Awandare, G.A. Evaluating antidisease immunity to malaria and implications for vaccine design. Immunology 2018, 153, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Lell, B.; Mordmüller, B.; Dejon Agobe, J.-C.; Honkpehedji, J.; Zinsou, J.; Mengue, J.B.; Loembe, M.M.; Adegnika, A.A.; Held, J.; Lalremruata, A.; et al. Impact of Sickle Cell Trait and Naturally Acquired Immunity on Uncomplicated Malaria after Controlled Human Malaria Infection in Adults in Gabon. Am. J. Trop. Med. Hyg. 2018, 98, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Dejon-Agobe, J.C.; Ateba-Ngoa, U.; Lalremruata, A.; Homoet, A.; Engelhorn, J.; Nouatin, O.P.; Edoa, J.R.; Fernandes, J.F.; Esen, M.; Mouwenda, Y.D.; et al. Controlled Human Malaria Infection of Healthy Adults with Lifelong Malaria Exposure to Assess Safety, Immunogenicity, and Efficacy of the Asexual Blood Stage Malaria Vaccine Candidate GMZ2. Clin. Infect. Dis. 2019, 69, 1377–1384. [Google Scholar] [CrossRef]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease tolerance as a defense strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Galatas, B.; Bassat, Q.; Mayor, A. Malaria Parasites in the Asymptomatic: Looking for the Hay in the Haystack. Trends Parasitol. 2016, 32, 296–308. [Google Scholar] [CrossRef]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef]
- Starkl Renar, K.; Iskra, J.; Križaj, I. Understanding malarial toxins. Toxicon 2016, 119, 319–329. [Google Scholar] [CrossRef]
- Boutlis, C.S.; Yeo, T.W.; Anstey, N.M. Malaria tolerance--for whom the cell tolls? Trends Parasitol. 2006, 22, 371–377. [Google Scholar] [CrossRef]
- Rubenstein, M.; Mulholland, J.H.; Jeffery, G.M.; Wolff, S.M. Malaria induced endotoxin tolerance. Proc. Soc. Exp. Biol. Med. 1965, 118, 283–287. [Google Scholar] [CrossRef]
- Sinton, J.A. Immunity or Tolerance in Malarial Infections. Proc. R. Soc. Med. 1938, 31, 1298–1302. [Google Scholar] [CrossRef]
- Giglioli, G. Paratyphoid C an Endemic Disease of British Guiana: A Clinical and Pathological Outline. B. Paratyphosum C as a Pyogenic Organism. Proc. R. Soc. Med. 1929, 23, 165–177. [Google Scholar] [CrossRef]
- Greenwood, B.M. Autoimmune disease and parasitic infections in Nigerians. Lancet 1968, 292, 380–382. [Google Scholar] [CrossRef]
- Floyd, S.; Pönnighaus, J.M.; Bliss, L.; Nkhosa, P.; Sichali, L.; Msiska, G.; Fine, P.E.M. Kinetics of delayed-type hypersensitivity to tuberculin induced by bacille Calmette-Guérin vaccination in northern Malawi. J. Infect. Dis. 2002, 186, 807–814. [Google Scholar] [CrossRef]
- Ayieko, C.; Ogola, B.S.; Ochola, L.; Ngwena, G.A.M.; Ayodo, G.; Hodges, J.S.; Noland, G.S.; John, C.C. Interferon-γ responses to Plasmodium falciparum vaccine candidate antigens decrease in the absence of malaria transmission. PeerJ 2017, 5, e2855. [Google Scholar] [CrossRef] [PubMed]
- Maclennan, C.A. Out of Africa: Links between invasive nontyphoidal Salmonella disease, typhoid fever, and malaria. Clin. Infect. Dis. 2014, 58, 648–650. [Google Scholar] [CrossRef]
- Bediako, Y.; Ngoi, J.M.; Nyangweso, G.; Wambua, J.; Opiyo, M.; Nduati, E.W.; Bejon, P.; Marsh, K.; Ndungu, F.M. The effect of declining exposure on T cell-mediated immunity to Plasmodium falciparum—An epidemiological “natural experiment”. BMC Med. 2016, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, A.J.; Riley, E.M. Suppression of vaccine responses by malaria: Insignificant or overlooked? Expert Rev. Vaccines 2010, 9, 409–429. [Google Scholar] [CrossRef] [PubMed]
- McGregor, I.A.; Barr, M. Antibody response to tetanus toxoid inoculation in malarious and non-malarious Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1962, 56, 364–367. [Google Scholar] [CrossRef]
- van Ginderachter, J.A.; Beschin, A.; de Baetselier, P.; Raes, G. Myeloid-derived suppressor cells in parasitic infections. Eur. J. Immunol. 2010, 40, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Williamson, W.A.; Greenwood, B.M. Impairment of the immune response to vaccination after acute malaria. Lancet 1978, 311, 1328–1329. [Google Scholar] [CrossRef]
- Riley, E.M.; Jobe, O.; Blackman, M.; Whittle, H.C.; Greenwood, B.M. Plasmodium falciparum schizont sonic extracts suppress lymphoproliferative responses to mitogens and antigens in malaria-immune adults. Infect. Immun. 1989, 57, 3181–3188. [Google Scholar] [CrossRef]
- Bejon, P.; Mwacharo, J.; Kai, O.; Todryk, S.; Keating, S.; Lowe, B.; Lang, T.; Mwangi, T.W.; Gilbert, S.C.; Peshu, N.; et al. The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J. Immunol. 2007, 179, 4193–4201. [Google Scholar] [CrossRef]
- Illingworth, J.; Butler, N.S.; Roetynck, S.; Mwacharo, J.; Pierce, S.K.; Bejon, P.; Crompton, P.D.; Marsh, K.; Ndungu, F.M. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 2013, 190, 1038–1047. [Google Scholar] [CrossRef]
- Berkley, J.A.; Bejon, P.; Mwangi, T.; Gwer, S.; Maitland, K.; Williams, T.N.; Mohammed, S.; Osier, F.; Kinyanjui, S.; Fegan, G.; et al. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin. Infect. Dis. 2009, 49, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef]
- Greenwood, B.M.; Palit, A.; Bradley-Moore, A.; Bryceson, A. Immunosuppression in children with malaria. Lancet 1972, 299, 169–172. [Google Scholar] [CrossRef]
- Nyirenda, T.S.; Nyirenda, J.T.; Tembo, D.L.; Storm, J.; Dube, Q.; Msefula, C.L.; Jambo, K.C.; Mwandumba, H.C.; Heyderman, R.S.; Gordon, M.A.; et al. Loss of Humoral and Cellular Immunity to Invasive Nontyphoidal Salmonella during Current or Convalescent Plasmodium falciparum Infection in Malawian Children. Clin. Vaccine Immunol. 2017, 24, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Usen, S.; Milligan, P.; Ethevenaux, C.; Greenwood, B.; Mulholland, K. Effect of fever on the serum antibody response of Gambian children to Haemophilus influenzae type b conjugate vaccine. Pediatr. Infect. Dis. J. 2000, 19, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Clarke, S.E.; Gosling, R.; Hamainza, B.; Killeen, G.; Magill, A.; O’Meara, W.; Price, R.N.; Riley, E.M. “Asymptomatic” Malaria: A Chronic and Debilitating Infection That Should Be Treated. PLoS Med. 2016, 13, e1001942. [Google Scholar] [CrossRef]
- Belkaid, Y.; Rouse, B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005, 6, 353–360. [Google Scholar] [CrossRef]
- Belkaid, Y. Regulatory T cells and infection: A dangerous necessity. Nat. Rev. Immunol. 2007, 7, 875–888. [Google Scholar] [CrossRef]
- Ocaña-Morgner, C.; Mota, M.M.; Rodriguez, A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 2003, 197, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Playfair, J.H.; Torrigiani, G. Immunosuppression in murine malaria. I. General characteristics. Clin. Exp. Immunol. 1971, 8, 467–478. [Google Scholar] [PubMed]
- Ho, M.; Webster, H.K.; Looareesuwan, S.; Supanaranond, W.; Phillips, R.E.; Chanthavanich, P.; Warrell, D.A. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J. Infect. Dis. 1986, 153, 763–771. [Google Scholar] [CrossRef]
- Walsh, D.S.; Looareesuwan, S.; Vaniganonta, S.; Viravan, C.; Webster, H. Cutaneous delayed-type hypersensitivity responsiveness in patients during and after Plasmodium falciparum and Plasmodium vivax infections. Clin. Immunol. Immunopathol. 1995, 77, 89–94. [Google Scholar] [CrossRef]
- Riley, E.M.; Andersson, G.; Otoo, L.N.; Jepsen, S.; Greenwood, B.M. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin. Exp. Immunol. 1988, 73, 17–22. [Google Scholar]
- Urban, B.C.; Cordery, D.; Shafi, M.J.; Bull, P.C.; Newbold, C.I.; Williams, T.N.; Marsh, K. The frequency of BDCA3-positive dendritic cells is increased in the peripheral circulation of Kenyan children with severe malaria. Infect. Immun. 2006, 74, 6700–6706. [Google Scholar] [CrossRef]
- Müller, I.; Genton, B.; Rare, L.; Kiniboro, B.; Kastens, W.; Zimmerman, P.; Kazura, J.; Alpers, M.; Smith, T.A. Three different Plasmodium species show similar patterns of clinical tolerance of malaria infection. Malar. J. 2009, 8, 158. [Google Scholar] [CrossRef]
- Wamae, K.; Wambua, J.; Nyangweso, G.; Mwambingu, G.; Osier, F.; Ndung’u, F.; Bejon, P.; Ochola-Oyier, L.I. Transmission and Age Impact the Risk of Developing Febrile Malaria in Children with Asymptomatic Plasmodium falciparum Parasitemia. J. Infect. Dis. 2019, 219, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Bediako, Y.; Adams, R.; Reid, A.J.; Valletta, J.J.; Ndungu, F.M.; Sodenkamp, J.; Mwacharo, J.; Ngoi, J.M.; Kimani, D.; Kai, O.; et al. Repeated clinical malaria episodes are associated with modification of the immune system in children. BMC Med. 2019, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mazliah, D.; Ndungu, F.M.; Aye, R.; Langhorne, J. B-cell memory in malaria: Myths and realities. Immunol. Rev. 2020, 293, 57–69. [Google Scholar] [CrossRef] [PubMed]
- van Braeckel-Budimir, N.; Kurup, S.P.; Harty, J.T. Regulatory issues in immunity to liver and blood-stage malaria. Curr. Opin. Immunol. 2016, 42, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Salwati, E.; Minigo, G.; Woodberry, T.; Piera, K.A.; de Silva, H.D.; Kenangalem, E.; Tjitra, E.; Coppel, R.L.; Price, R.N.; Anstey, N.M.; et al. Differential cellular recognition of antigens during acute Plasmodium falciparum and Plasmodium vivax malaria. J. Infect. Dis. 2011, 203, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- White, C.E.; Villarino, N.F.; Sloan, S.S.; Ganusov, V.V.; Schmidt, N.W. Plasmodium suppresses expansion of T cell responses to heterologous infections. J. Immunol. 2015, 194, 697–708. [Google Scholar] [CrossRef]
- Hviid, L.; Theander, T.G.; Abu-Zeid, Y.A.; Abdulhadi, N.H.; Jakobsen, P.H.; Saeed, B.O.; Jepsen, S.; Bayoumi, R.A.; Jensen, J.B. Loss of cellular immune reactivity during acute Plasmodium falciparum malaria. FEMS Microbiol. Immunol. 1991, 3, 219–227. [Google Scholar] [CrossRef]
- Nwuba, R.I.; Sodeinde, O.; Anumudu, C.I.; Omosun, Y.O.; Odaibo, A.B.; Holder, A.A.; Nwagwu, M. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect. Immun. 2002, 70, 5328–5331. [Google Scholar] [CrossRef]
- McNally, A.; Hill, G.R.; Sparwasser, T.; Thomas, R.; Steptoe, R.J. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc. Natl. Acad. Sci. USA 2011, 108, 7529–7534. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Araujo Furlan, C.L.; Tosello Boari, J.; Rodriguez, C.; Canale, F.P.; Fiocca Vernengo, F.; Boccardo, S.; Beccaria, C.G.; Adoue, V.; Joffre, O.; Gruppi, A.; et al. Limited Foxp3+ Regulatory T Cells Response During Acute Trypanosoma cruzi Infection Is Required to Allow the Emergence of Robust Parasite-Specific CD8+ T Cell Immunity. Front. Immunol. 2018, 9, 2555. [Google Scholar] [CrossRef]
- Adalid-Peralta, L.; Fragoso, G.; Fleury, A.; Sciutto, E. Mechanisms underlying the induction of regulatory T cells and its relevance in the adaptive immune response in parasitic infections. Int. J. Biol. Sci. 2011, 7, 1412–1426. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Ng, S.S.; Bunn, P.T. The Regulation of CD4(+) T Cell Responses during Protozoan Infections. Front. Immunol. 2014, 5, 498. [Google Scholar] [CrossRef]
- Finney, O.C.; Riley, E.M.; Walther, M. Regulatory T cells in malaria—Friend or foe? Trends Immunol. 2010, 31, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, P.; Eccles-James, I.; Bowen, K.; Nankya, F.; Auma, A.; Wamala, S.; Ebusu, C.; Muhindo, M.K.; Arinaitwe, E.; Briggs, J.; et al. IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog. 2014, 10, e1003864. [Google Scholar] [CrossRef]
- Scholzen, A.; Mittag, D.; Rogerson, S.J.; Cooke, B.M.; Plebanski, M. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. 2009, 5, e1000543. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, A.; Cooke, B.M.; Plebanski, M. Plasmodium falciparum induces Foxp3hi CD4 T cells independent of surface PfEMP1 expression via small soluble parasite components. Front. Microbiol. 2014, 5, 200. [Google Scholar] [CrossRef]
- Clemente, A.; Caporale, R.; Sannella, A.R.; Majori, G.; Severini, C.; Fadigati, G.; Cirelli, D.; Bonini, P.; Garaci, E.; Cozzolino, F.; et al. Plasmodium falciparum soluble extracts potentiate the suppressive function of polyclonal T regulatory cells through activation of TGFβ-mediated signals. Cell. Microbiol. 2011, 13, 1328–1338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omer, F.M.; de Souza, J.B.; Corran, P.H.; Sultan, A.A.; Riley, E.M. Activation of transforming growth factor beta by malaria parasite-derived metalloproteinases and a thrombospondin-like molecule. J. Exp. Med. 2003, 198, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, B.R.; Bangirana, P.; Opoka, R.O.; Park, G.S.; John, C.C. Thrombocytopenia May Mediate Disease Severity in Plasmodium falciparum Malaria Through Reduced Transforming Growth Factor Beta-1 Regulation of Proinflammatory and Anti-inflammatory Cytokines. Pediatr. Infect. Dis. J. 2015, 34, 783–788. [Google Scholar] [CrossRef]
- Riley, E.M.; Wahl, S.; Perkins, D.J.; Schofield, L. Regulating immunity to malaria. Parasite Immunol. 2006, 28, 35–49. [Google Scholar] [CrossRef]
- Walther, M.; Tongren, J.E.; Andrews, L.; Korbel, D.; King, E.; Fletcher, H.; Andersen, R.F.; Bejon, P.; Thompson, F.; Dunachie, S.J.; et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 2005, 23, 287–296. [Google Scholar] [CrossRef]
- Scholzen, A.; Sauerwein, R.W. Immune activation and induction of memory: Lessons learned from controlled human malaria infection with Plasmodium falciparum. Parasitology 2016, 143, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Drewry, L.L.; Harty, J.T. Balancing in a black box: Potential immunomodulatory roles for TGF-β signaling during blood-stage malaria. Virulence 2020, 11, 159–169. [Google Scholar] [CrossRef]
- Edwards, C.L.; Ng, S.S.; Corvino, D.; Montes de Oca, M.; de Labastida Rivera, F.; Nones, K.; Lakis, V.; Waddell, N.; Amante, F.H.; McCarthy, J.S.; et al. Early Changes in CD4+ T-Cell Activation During Blood-Stage Plasmodium falciparum Infection. J. Infect. Dis. 2018, 218, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.G.; Santarlasci, V.; Cosmi, L.; Clemente, A.; Maggi, L.; Mangano, V.D.; Verra, F.; Bancone, G.; Nebie, I.; Sirima, B.S.; et al. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 2008, 105, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Torres, K.J.; Villasis, E.; Bendezú, J.; Chauca, J.; Vinetz, J.M.; Gamboa, D. Relationship of regulatory T cells to Plasmodium falciparum malaria symptomatology in a hypoendemic region. Malar. J. 2014, 13, 108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walther, M.; Jeffries, D.; Finney, O.C.; Njie, M.; Ebonyi, A.; Deininger, S.; Lawrence, E.; Ngwa-Amambua, A.; Jayasooriya, S.; Cheeseman, I.H.; et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009, 5, e1000364. [Google Scholar] [CrossRef]
- Minigo, G.; Woodberry, T.; Piera, K.A.; Salwati, E.; Tjitra, E.; Kenangalem, E.; Price, R.N.; Engwerda, C.R.; Anstey, N.M.; Plebanski, M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009, 5, e1000402. [Google Scholar] [CrossRef]
- Cohen, J.L.; Wood, K.J. TNFR2: The new Treg switch? Oncoimmunology 2017, 7, e1373236. [Google Scholar] [CrossRef]
- Boyle, M.J.; Jagannathan, P.; Farrington, L.A.; Eccles-James, I.; Wamala, S.; McIntyre, T.I.; Vance, H.M.; Bowen, K.; Nankya, F.; Auma, A.; et al. Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria. PLoS Pathog. 2015, 11, e1005041. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Jagannathan, P.; Bowen, K.; McIntyre, T.I.; Vance, H.M.; Farrington, L.A.; Greenhouse, B.; Nankya, F.; Rek, J.; Katureebe, A.; et al. Effector Phenotype of Plasmodium falciparum-Specific CD4+ T Cells Is Influenced by Both Age and Transmission Intensity in Naturally Exposed Populations. J. Infect. Dis. 2015, 212, 416–425. [Google Scholar] [CrossRef]
- Boyle, M.J.; Jagannathan, P.; Bowen, K.; McIntyre, T.I.; Vance, H.M.; Farrington, L.A.; Schwartz, A.; Nankya, F.; Naluwu, K.; Wamala, S.; et al. The Development of Plasmodium falciparum-Specific IL10 CD4 T Cells and Protection from Malaria in Children in an Area of High Malaria Transmission. Front. Immunol. 2017, 8, 1329. [Google Scholar] [CrossRef] [PubMed]
- Portugal, S.; Moebius, J.; Skinner, J.; Doumbo, S.; Doumtabe, D.; Kone, Y.; Dia, S.; Kanakabandi, K.; Sturdevant, D.E.; Virtaneva, K.; et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog. 2014, 10, e1004079. [Google Scholar] [CrossRef] [PubMed]
- Bousema, T.; Okell, L.; Felger, I.; Drakeley, C. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nat. Rev. Microbiol. 2014, 12, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, P.; Bowen, K.; Nankya, F.; McIntyre, T.I.; Auma, A.; Wamala, S.; Sikyomu, E.; Naluwu, K.; Nalubega, M.; Boyle, M.J.; et al. Effective Antimalarial Chemoprevention in Childhood Enhances the Quality of CD4+ T Cells and Limits Their Production of Immunoregulatory Interleukin 10. J. Infect. Dis. 2016, 214, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, R.; Karunarathne, D.S.; Horne-Debets, J.M.; Wykes, M. The Contribution of Co-signaling Pathways to Anti-malarial T Cell Immunity. Front. Immunol. 2018, 9, 2926. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef]
- de Jong, S.E.; Asscher, V.E.R.; Wammes, L.J.; Wiria, A.E.; Hamid, F.; Sartono, E.; Supali, T.; Smits, H.H.; Luty, A.J.F.; Yazdanbakhsh, M. Longitudinal study of changes in γδ T cells and CD4+ T cells upon asymptomatic malaria infection in Indonesian children. Sci. Rep. 2017, 7, 8844. [Google Scholar] [CrossRef]
- Mackroth, M.S.; Abel, A.; Steeg, C.; Zur Schulze Wiesch, J.; Jacobs, T. Acute Malaria Induces PD1+CTLA4+ Effector T Cells with Cell-Extrinsic Suppressor Function. PLoS Pathog. 2016, 12, e1005909. [Google Scholar] [CrossRef]
- Finney, O.C.; Lawrence, E.; Gray, A.P.; Njie, M.; Riley, E.M.; Walther, M. Freeze-thaw lysates of Plasmodium falciparum-infected red blood cells induce differentiation of functionally competent regulatory T cells from memory T cells. Eur. J. Immunol. 2012, 42, 1767–1777. [Google Scholar] [CrossRef]
- Ding, T.; Yan, F.; Cao, S.; Ren, X. Regulatory B cell: New member of immunosuppressive cell club. Hum. Immunol. 2015, 76, 615–621. [Google Scholar] [CrossRef]
- Zhang, X. Regulatory functions of innate-like B cells. Cell. Mol. Immunol. 2013, 10, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.-M.; Jamin, C.; Amrouche, K.; Le Goff, B.; Maugars, Y.; Youinou, P. Regulatory B cells play a key role in immune system balance. Jt. Bone Spine 2013, 80, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, J.; Zhang, Y.; Zhang, Y.; Cao, H.; Cao, Y.; Qi, Z. Potential Role for Regulatory B Cells as a Major Source of Interleukin-10 in Spleen from Plasmodium chabaudi-Infected Mice. Infect. Immun. 2018, 86, e00016-18. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.; Antinarelli, L.M.R.; Abramo, C.; Macedo, G.C.; Coimbra, E.S.; Scopel, K.K.G. What do we know about the role of regulatory B cells (Breg) during the course of infection of two major parasitic diseases, malaria and leishmaniasis? Pathog. Glob. Health 2017, 111, 107–115. [Google Scholar] [CrossRef]
- Bao, L.Q.; Huy, N.T.; Kikuchi, M.; Yanagi, T.; Senba, M.; Shuaibu, M.N.; Honma, K.; Yui, K.; Hirayama, K. CD19(+) B cells confer protection against experimental cerebral malaria in semi-immune rodent model. PLoS ONE 2013, 8, e64836. [Google Scholar] [CrossRef]
- Silveira, E.L.V.; Dominguez, M.R.; Soares, I.S. To B or Not to B: Understanding B Cell Responses in the Development of Malaria Infection. Front. Immunol. 2018, 9, 2961. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Wang, S.; Ko, K.-H.; Xu, H.; Zheng, B.-J.; Cao, X.; Lu, L. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J. Immunol. 2010, 184, 3321–3325. [Google Scholar] [CrossRef]
- Borhis, G.; Richard, Y. Subversion of the B-cell compartment during parasitic, bacterial, and viral infections. BMC Immunol. 2015, 16, 15. [Google Scholar] [CrossRef]
- Sakai, J.; Akkoyunlu, M. The Role of BAFF System Molecules in Host Response to Pathogens. Clin. Microbiol. Rev. 2017, 30, 991–1014. [Google Scholar] [CrossRef]
- Dechkhajorn, W.; Benjathummarak, S.; Glaharn, S.; Chaisri, U.; Viriyavejakul, P.; Maneerat, Y. The activation of BAFF/APRIL system in spleen and lymph nodes of Plasmodium falciparum infected patients. Sci. Rep. 2020, 10, 3865. [Google Scholar] [CrossRef] [PubMed]
- Muehlenbachs, A.; Fried, M.; Lachowitzer, J.; Mutabingwa, T.K.; Duffy, P.E. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J. Immunol. 2007, 179, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.T.; Embury, P.; Anderson, T.; Mungai, P.; Malhotra, I.; King, C.; Kazura, J.; Dent, A. HIV, Cytomegalovirus, and Malaria Infections during Pregnancy Lead to Inflammation and Shifts in Memory B Cell Subsets in Kenyan Neonates. J. Immunol. 2019, 202, 1465–1478. [Google Scholar] [CrossRef]
- Craxton, A.; Magaletti, D.; Ryan, E.J.; Clark, E.A. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 2003, 101, 4464–4471. [Google Scholar] [CrossRef] [PubMed]
- Nduati, E.; Gwela, A.; Karanja, H.; Mugyenyi, C.; Langhorne, J.; Marsh, K.; Urban, B.C. The plasma concentration of the B cell activating factor is increased in children with acute malaria. J. Infect. Dis. 2011, 204, 962–970. [Google Scholar] [CrossRef]
- Scholzen, A.; Sauerwein, R.W. How malaria modulates memory: Activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol. 2013, 29, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, A.; Teirlinck, A.C.; Bijker, E.M.; Roestenberg, M.; Hermsen, C.C.; Hoffman, S.L.; Sauerwein, R.W. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J. Immunol. 2014, 192, 3719–3729. [Google Scholar] [CrossRef]
- Liu, X.Q.; Stacey, K.J.; Horne-Debets, J.M.; Cridland, J.A.; Fischer, K.; Narum, D.; Mackay, F.; Pierce, S.K.; Wykes, M.N. Malaria infection alters the expression of B-cell activating factor resulting in diminished memory antibody responses and survival. Eur. J. Immunol. 2012, 42, 3291–3301. [Google Scholar] [CrossRef]
- Rönnberg, C.; Lugaajju, A.; Nyman, A.; Hammar, U.; Bottai, M.; Lautenbach, M.J.; Sundling, C.; Kironde, F.; Persson, K.E.M. A longitudinal study of plasma BAFF levels in mothers and their infants in Uganda, and correlations with subsets of B cells. PLoS ONE 2021, 16, e0245431. [Google Scholar] [CrossRef]
- Pinna, R.A.; Dos Santos, A.C.; Perce-da-Silva, D.S.; da Silva, L.A.; da Silva, R.N.R.; Alves, M.R.; Santos, F.; de Oliveira Ferreira, J.; Lima-Junior, J.C.; Villa-Verde, D.M.; et al. Correlation of APRIL with production of inflammatory cytokines during acute malaria in the Brazilian Amazon. Immun. Inflamm. Dis. 2018, 6, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Hansen, D.S. Development of B Cell Memory in Malaria. Front. Immunol. 2019, 10, 559. [Google Scholar] [CrossRef]
- Portugal, S.; Obeng-Adjei, N.; Moir, S.; Crompton, P.D.; Pierce, S.K. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell. Immunol. 2017, 321, 18–25. [Google Scholar] [CrossRef]
- Yap, X.Z.; Hustin, L.S.P.; Sauerwein, R.W. TH1-Polarized TFH Cells Delay Naturally-Acquired Immunity to Malaria. Front. Immunol. 2019, 10, 1096. [Google Scholar] [CrossRef]
- Reddy, S.B.; Nagy, N.; Rönnberg, C.; Chiodi, F.; Lugaajju, A.; Heuts, F.; Szekely, L.; Wahlgren, M.; Persson, K.E.M. Publisher Correction to: Direct contact between Plasmodium falciparum and human B-cells in a novel co-culture increases parasite growth and affects B-cell growth. Malar. J. 2021, 20, 323. [Google Scholar] [CrossRef]
- Karnell, J.L.; Kumar, V.; Wang, J.; Wang, S.; Voynova, E.; Ettinger, R. Role of CD11c+ T-bet+ B cells in human health and disease. Cell. Immunol. 2017, 321, 40–45. [Google Scholar] [CrossRef]
- Wipasa, J.; Suphavilai, C.; Okell, L.C.; Cook, J.; Corran, P.H.; Thaikla, K.; Liewsaree, W.; Riley, E.M.; Hafalla, J.C.R. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010, 6, e1000770. [Google Scholar] [CrossRef]
- Pérez-Mazliah, D.; Gardner, P.J.; Schweighoffer, E.; McLaughlin, S.; Hosking, C.; Tumwine, I.; Davis, R.S.; Potocnik, A.J.; Tybulewicz, V.L.; Langhorne, J. Plasmodium-specific atypical memory B cells are short-lived activated B cells. Elife 2018, 7, e39800. [Google Scholar] [CrossRef] [PubMed]
- Muellenbeck, M.F.; Ueberheide, B.; Amulic, B.; Epp, A.; Fenyo, D.; Busse, C.E.; Esen, M.; Theisen, M.; Mordmüller, B.; Wardemann, H. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J. Exp. Med. 2013, 210, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zander, R.A.; Obeng-Adjei, N.; Guthmiller, J.J.; Kulu, D.I.; Li, J.; Ongoiba, A.; Traore, B.; Crompton, P.D.; Butler, N.S. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe 2015, 17, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Adjei, N.; Portugal, S.; Holla, P.; Li, S.; Sohn, H.; Ambegaonkar, A.; Skinner, J.; Bowyer, G.; Doumbo, O.K.; Traore, B.; et al. Malaria-induced interferon-γ drives the expansion of Tbethi atypical memory B cells. PLoS Pathog. 2017, 13, e1006576. [Google Scholar] [CrossRef]
- Arroyo, E.N.; Pepper, M. B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection. J. Exp. Med. 2020, 217, e20190849. [Google Scholar] [CrossRef]
- Powell, M.D.; Read, K.A.; Sreekumar, B.K.; Jones, D.M.; Oestreich, K.J. IL-12 signaling drives the differentiation and function of a TH1-derived TFH1-like cell population. Sci. Rep. 2019, 9, 13991. [Google Scholar] [CrossRef] [PubMed]
- Lönnberg, T.; Svensson, V.; James, K.R.; Fernandez-Ruiz, D.; Sebina, I.; Montandon, R.; Soon, M.S.F.; Fogg, L.G.; Nair, A.S.; Liligeto, U.; et al. Single-cell RNA-seq and computational analysis using temporal mixture modelling resolves Th1/Tfh fate bifurcation in malaria. Sci. Immunol. 2017, 2, eaal2192. [Google Scholar] [CrossRef]
- Ly, A.; Liao, Y.; Pietrzak, H.; Ioannidis, L.J.; Sidwell, T.; Gloury, R.; Doerflinger, M.; Triglia, T.; Qin, R.Z.; Groom, J.R.; et al. Transcription Factor T-bet in B Cells Modulates Germinal Center Polarization and Antibody Affinity Maturation in Response to Malaria. Cell Rep. 2019, 29, 2257–2269. [Google Scholar] [CrossRef]
- Chan, J.-A.; Loughland, J.R.; de Labastida Rivera, F.; SheelaNair, A.; Andrew, D.W.; Dooley, N.L.; Wines, B.D.; Amante, F.H.; Webb, L.; Hogarth, P.M.; et al. Th2-like T Follicular Helper Cells Promote Functional Antibody Production during Plasmodium falciparum Infection. Cell Rep. Med. 2020, 1, 100157. [Google Scholar] [CrossRef]
- Obeng-Adjei, N.; Portugal, S.; Tran, T.M.; Yazew, T.B.; Skinner, J.; Li, S.; Jain, A.; Felgner, P.L.; Doumbo, O.K.; Kayentao, K.; et al. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell Rep. 2015, 13, 425–439. [Google Scholar] [CrossRef]
- Hansen, D.S.; Obeng-Adjei, N.; Ly, A.; Ioannidis, L.J.; Crompton, P.D. Emerging concepts in T follicular helper cell responses to malaria. Int. J. Parasitol. 2017, 47, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ryg-Cornejo, V.; Ioannidis, L.J.; Ly, A.; Chiu, C.Y.; Tellier, J.; Hill, D.L.; Preston, S.P.; Pellegrini, M.; Yu, D.; Nutt, S.L.; et al. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep. 2016, 14, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, G.P.; van Bruggen, R.; Grobusch, M.P.; Dobaño, C. Plasmodium falciparum malaria and invasive bacterial co-infection in young African children: The dysfunctional spleen hypothesis. Malar. J. 2014, 13, 335. [Google Scholar] [CrossRef]
- Baeza Garcia, A.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.J.; Ulmer, J.B.; et al. Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat. Commun. 2018, 9, 2714. [Google Scholar] [CrossRef] [PubMed]
- Zander, R.A.; Vijay, R.; Pack, A.D.; Guthmiller, J.J.; Graham, A.C.; Lindner, S.E.; Vaughan, A.M.; Kappe, S.H.I.; Butler, N.S. Th1-like Plasmodium-Specific Memory CD4+ T Cells Support Humoral Immunity. Cell Rep. 2017, 21, 1839–1852. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Wang, H.; Ba, X.; Shen, P.; Lin, W.; Wang, Y.; Qin, K.; Huang, Y.; Tu, S. Follicular regulatory T cells: A novel target for immunotherapy? Clin. Transl. Immunol. 2020, 9, e1106. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.P.; Obeng-Adjei, N.; Anthony, S.M.; Traore, B.; Doumbo, O.K.; Butler, N.S.; Crompton, P.D.; Harty, J.T. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat. Med. 2017, 23, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Berretta, F.; Piccirillo, C.A.; Stevenson, M.M. Plasmodium chabaudi AS Infection Induces CD4+ Th1 Cells and Foxp3+T-bet+ Regulatory T Cells That Express CXCR3 and Migrate to CXCR3 Ligands. Front. Immunol. 2019, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.M.; Dent, A.L. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front. Immunol. 2018, 9, 1536. [Google Scholar] [CrossRef]
- Ding, T.; Su, R.; Wu, R.; Xue, H.; Wang, Y.; Su, R.; Gao, C.; Li, X.; Wang, C. Frontiers of Autoantibodies in Autoimmune Disorders: Crosstalk Between Tfh/Tfr and Regulatory B Cells. Front. Immunol. 2021, 12, 641013. [Google Scholar] [CrossRef]
- Gensous, N.; Charrier, M.; Duluc, D.; Contin-Bordes, C.; Truchetet, M.-E.; Lazaro, E.; Duffau, P.; Blanco, P.; Richez, C. T Follicular Helper Cells in Autoimmune Disorders. Front. Immunol. 2018, 9, 1637. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Plebanski, M.; Akinwunmi, P.; Lee, E.A.; Reece, W.H.; Robson, K.J.; Hill, A.V.; Pinder, M. Broadly distributed T cell reactivity, with no immunodominant loci, to the pre-erythrocytic antigen thrombospondin-related adhesive protein of Plasmodium falciparum in West Africans. Eur. J. Immunol. 1999, 29, 1943–1954. [Google Scholar] [CrossRef]
- Ludwig, R.J.; Vanhoorelbeke, K.; Leypoldt, F.; Kaya, Z.; Bieber, K.; McLachlan, S.M.; Komorowski, L.; Luo, J.; Cabral-Marques, O.; Hammers, C.M.; et al. Mechanisms of Autoantibody-Induced Pathology. Front. Immunol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Babatunde, K.A.; Yesodha Subramanian, B.; Ahouidi, A.D.; Martinez Murillo, P.; Walch, M.; Mantel, P.-Y. Role of Extracellular Vesicles in Cellular Cross Talk in Malaria. Front. Immunol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Mantel, P.-Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef]
- Couper, K.N.; Barnes, T.; Hafalla, J.C.R.; Combes, V.; Ryffel, B.; Secher, T.; Grau, G.E.; Riley, E.M.; de Souza, J.B. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010, 6, e1000744. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.M.; McConkey, C.A.; Shin, S. Outrunning the Red Queen: Bystander activation as a means of outpacing innate immune subversion by intracellular pathogens. Cell. Mol. Immunol. 2017, 14, 14–21. [Google Scholar] [CrossRef]
- Mourão, L.C.; Cardoso-Oliveira, G.P.; Braga, É.M. Autoantibodies and Malaria: Where We Stand? Insights into Pathogenesis and Protection. Front. Cell. Infect. Microbiol. 2020, 10, 262. [Google Scholar] [CrossRef]
- Gong, F.; Zheng, T.; Zhou, P. T Follicular Helper Cell Subsets and the Associated Cytokine IL-21 in the Pathogenesis and Therapy of Asthma. Front. Immunol. 2019, 10, 2918. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Leonard, W.J. IL-21 and T follicular helper cells. Int. Immunol. 2010, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Ma, C.S. Regulation of the germinal center and humoral immunity by interleukin-21. J. Exp. Med. 2020, 217, e20191638. [Google Scholar] [CrossRef] [PubMed]
- Solaymani-Mohammadi, S.; Eckmann, L.; Singer, S.M. Interleukin (IL)-21 in Inflammation and Immunity during Parasitic Diseases. Front. Cell. Infect. Microbiol. 2019, 9, 401. [Google Scholar] [CrossRef]
- Pérez-Mazliah, D.; Ng, D.H.L.; Freitas do Rosário, A.P.; McLaughlin, S.; Mastelic-Gavillet, B.; Sodenkamp, J.; Kushinga, G.; Langhorne, J. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog. 2015, 11, e1004715. [Google Scholar] [CrossRef]
- Mooney, J.P.; Wassmer, S.C.; Hafalla, J.C. Type I Interferon in Malaria: A Balancing Act. Trends Parasitol. 2017, 33, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-C.; Zhong, J.; Xu, J.-F. Regulatory B cells in infectious disease (Review). Mol. Med. Rep. 2017, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yap, X.Z.; Lundie, R.J.; Beeson, J.G.; O’Keeffe, M. Dendritic Cell Responses and Function in Malaria. Front. Immunol. 2019, 10, 357. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Urban, B.C.; Ferguson, D.J.; Pain, A.; Willcox, N.; Plebanski, M.; Austyn, J.M.; Roberts, D.J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 1999, 400, 73–77. [Google Scholar] [CrossRef]
- Elliott, S.R.; Spurck, T.P.; Dodin, J.M.; Maier, A.G.; Voss, T.S.; Yosaatmadja, F.; Payne, P.D.; McFadden, G.I.; Cowman, A.F.; Rogerson, S.J.; et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 2007, 75, 3621–3632. [Google Scholar] [CrossRef]
- Wykes, M.N.; Good, M.F. What really happens to dendritic cells during malaria? Nat. Rev. Microbiol. 2008, 6, 864–870. [Google Scholar] [CrossRef]
- Azeem, W.; Bakke, R.M.; Appel, S.; Øyan, A.M.; Kalland, K.-H. Dual Pro- and Anti-Inflammatory Features of Monocyte-Derived Dendritic Cells. Front. Immunol. 2020, 11, 438. [Google Scholar] [CrossRef]
- Robbins, S.H.; Walzer, T.; Dembélé, D.; Thibault, C.; Defays, A.; Bessou, G.; Xu, H.; Vivier, E.; Sellars, M.; Pierre, P.; et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008, 9, R17. [Google Scholar] [CrossRef]
- Hirako, I.C.; Assis, P.A.; Galvão-Filho, B.; Luster, A.D.; Antonelli, L.R.; Gazzinelli, R.T. Monocyte-derived dendritic cells in malaria. Curr. Opin. Microbiol. 2019, 52, 139–150. [Google Scholar] [CrossRef]
- Woodberry, T.; Minigo, G.; Piera, K.A.; Amante, F.H.; Pinzon-Charry, A.; Good, M.F.; Lopez, J.A.; Engwerda, C.R.; McCarthy, J.S.; Anstey, N.M. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J. Infect. Dis. 2012, 206, 333–340. [Google Scholar] [CrossRef]
- Pinzon-Charry, A.; Woodberry, T.; Kienzle, V.; McPhun, V.; Minigo, G.; Lampah, D.A.; Kenangalem, E.; Engwerda, C.; López, J.A.; Anstey, N.M.; et al. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J. Exp. Med. 2013, 210, 1635–1646. [Google Scholar] [CrossRef]
- Loughland, J.R.; Minigo, G.; Sarovich, D.S.; Field, M.; Tipping, P.E.; Montes de Oca, M.; Piera, K.A.; Amante, F.H.; Barber, B.E.; Grigg, M.J.; et al. Plasmacytoid dendritic cells appear inactive during sub-microscopic Plasmodium falciparum blood-stage infection, yet retain their ability to respond to TLR stimulation. Sci. Rep. 2017, 7, 2596. [Google Scholar] [CrossRef] [PubMed]
- Loughland, J.R.; Minigo, G.; Burel, J.; Tipping, P.E.; Piera, K.A.; Amante, F.H.; Engwerda, C.R.; Good, M.F.; Doolan, D.L.; Anstey, N.M.; et al. Profoundly Reduced CD1c+ Myeloid Dendritic Cell HLA-DR and CD86 Expression and Increased Tumor Necrosis Factor Production in Experimental Human Blood-Stage Malaria Infection. Infect. Immun. 2016, 84, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Kho, S.; Marfurt, J.; Handayuni, I.; Pava, Z.; Noviyanti, R.; Kusuma, A.; Piera, K.A.; Burdam, F.H.; Kenangalem, E.; Lampah, D.A.; et al. Characterization of blood dendritic and regulatory T cells in asymptomatic adults with sub-microscopic Plasmodium falciparum or Plasmodium vivax infection. Malar. J. 2016, 15, 328. [Google Scholar] [CrossRef]
- Breitling, L.P.; Fendel, R.; Mordmueller, B.; Adegnika, A.A.; Kremsner, P.G.; Luty, A.J.F. Cord blood dendritic cell subsets in African newborns exposed to Plasmodium falciparum in utero. Infect. Immun. 2006, 74, 5725–5729. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.M.; Salmazi, K.C.; Santos, B.A.N.; Bastos, M.S.; Rocha, S.C.; Boscardin, S.B.; Silber, A.M.; Kallás, E.G.; Ferreira, M.U.; Scopel, K.K.G. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: Do different parasite species elicit similar host responses? Infect. Immun. 2010, 78, 4763–4772. [Google Scholar] [CrossRef]
- Götz, A.; Tang, M.S.; Ty, M.C.; Arama, C.; Ongoiba, A.; Doumtabe, D.; Traore, B.; Crompton, P.D.; Loke, P.; Rodriguez, A. Atypical activation of dendritic cells by Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2017, 114, E10568–E10577. [Google Scholar] [CrossRef]
- Loharungsikul, S.; Troye-Blomberg, M.; Amoudruz, P.; Pichyangkul, S.; Yongvanitchit, K.; Looareesuwan, S.; Mahakunkijcharoen, Y.; Sarntivijai, S.; Khusmith, S. Expression of toll-like receptors on antigen-presenting cells in patients with falciparum malaria. Acta Trop. 2008, 105, 10–15. [Google Scholar] [CrossRef]
- Yap, X.Z.; Lundie, R.J.; Feng, G.; Pooley, J.; Beeson, J.G.; O’Keeffe, M. Different Life Cycle Stages of Plasmodium falciparum Induce Contrasting Responses in Dendritic Cells. Front. Immunol. 2019, 10, 32. [Google Scholar] [CrossRef]
- Montes de Oca, M.; Kumar, R.; Rivera, F.d.L.; Amante, F.H.; Sheel, M.; Faleiro, R.J.; Bunn, P.T.; Best, S.E.; Beattie, L.; Ng, S.S.; et al. Type I Interferons Regulate Immune Responses in Humans with Blood-Stage Plasmodium falciparum Infection. Cell Rep. 2016, 17, 399–412. [Google Scholar] [CrossRef]
- Zander, R.A.; Guthmiller, J.J.; Graham, A.C.; Pope, R.L.; Burke, B.E.; Carr, D.J.J.; Butler, N.S. Type I Interferons Induce T Regulatory 1 Responses and Restrict Humoral Immunity during Experimental Malaria. PLoS Pathog. 2016, 12, e1005945. [Google Scholar] [CrossRef]
- Sebina, I.; Haque, A. Effects of type I interferons in malaria. Immunology 2018, 155, 176–185. [Google Scholar] [CrossRef]
- Haque, A.; Best, S.E.; Ammerdorffer, A.; Desbarrieres, L.; de Oca, M.M.; Amante, F.H.; de Labastida Rivera, F.; Hertzog, P.; Boyle, G.M.; Hill, G.R.; et al. Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur. J. Immunol. 2011, 41, 2688–2698. [Google Scholar] [CrossRef]
- Kempaiah, P.; Anyona, S.B.; Raballah, E.; Davenport, G.C.; Were, T.; Hittner, J.B.; Ong’echa, J.M.; Perkins, D.J. Reduced interferon (IFN)-α conditioned by IFNA2 (-173) and IFNA8 (-884) haplotypes is associated with enhanced susceptibility to severe malarial anemia and longitudinal all-cause mortality. Hum. Genet. 2012, 131, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, E.; Fooksman, D.; Moore, J.M.; Saidi, A.; Feintuch, C.M.; Reizis, B.; Chorro, L.; Daily, J.; Lauvau, G. STING-Licensed Macrophages Prime Type I IFN Production by Plasmacytoid Dendritic Cells in the Bone Marrow during Severe Plasmodium yoelii Malaria. PLoS Pathog. 2016, 12, e1005975. [Google Scholar] [CrossRef] [PubMed]
- Amodio, G.; Cichy, J.; Conde, P.; Matteoli, G.; Moreau, A.; Ochando, J.; Oral, B.H.; Pekarova, M.; Ryan, E.J.; Roth, J.; et al. Role of myeloid regulatory cells (MRCs) in maintaining tissue homeostasis and promoting tolerance in autoimmunity, inflammatory disease and transplantation. Cancer Immunol. Immunother. 2019, 68, 661–672. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Pawelec, G.; Verschoor, C.P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Not Only in Tumor Immunity. Front. Immunol. 2019, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Qi, G.; Tu, G.; Yang, C.; Xu, M. Myeloid-derived suppressor cells in transplantation: The dawn of cell therapy. J. Transl. Med. 2018, 16, 19. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Hartl, D. Myeloid-Derived Suppressor Cells in Infection: A General Overview. J. Innate Immun. 2018, 10, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Glaría, E.; Garcia-Tellez, T.; Nieuwenhuizen, N.E.; Zelinskyy, G.; Favier, B.; Singh, A.; Ehrchen, J.; Gujer, C.; Münz, C.; et al. MDSCs in infectious diseases: Regulation, roles, and readjustment. Cancer Immunol. Immunother. 2019, 68, 673–685. [Google Scholar] [CrossRef]
- Ouaissi, A. Regulatory cells and immunosuppressive cytokines: Parasite-derived factors induce immune polarization. J. Biomed. Biotechnol. 2007, 2007, 94971. [Google Scholar] [CrossRef] [PubMed]
- Lamsfus Calle, C.; Fendel, R.; Singh, A.; Richie, T.L.; Hoffman, S.L.; Kremsner, P.G.; Mordmüller, B. Expansion of Functional Myeloid-Derived Suppressor Cells in Controlled Human Malaria Infection. Front. Immunol. 2021, 12, 625712. [Google Scholar] [CrossRef]
- Belyaev, N.N.; Biró, J.; Langhorne, J.; Potocnik, A.J. Extramedullary myelopoiesis in malaria depends on mobilization of myeloid-restricted progenitors by IFN-γ induced chemokines. PLoS Pathog. 2013, 9, e1003406. [Google Scholar] [CrossRef]
- Liu, J.; Shao, D.; Lin, Y.; Luo, M.; Wang, Z.; Yao, M.; Hao, X.; Wei, C.; Gao, Y.; Deng, W.; et al. PyMIF enhances the inflammatory response in a rodent model by stimulating CD11b(+) Ly6C(+) cells accumulation in spleen. Parasite Immunol. 2016, 38, 377–383. [Google Scholar] [CrossRef]
- Kilunga Kubata, B.; Eguchi, N.; Urade, Y.; Yamashita, K.; Mitamura, T.; Tai, K.; Hayaishi, O.; Horii, T. Plasmodium falciparum produces prostaglandins that are pyrogenic, somnogenic, and immunosuppressive substances in humans. J. Exp. Med. 1998, 188, 1197–1202. [Google Scholar] [CrossRef]
- Rodríguez-Ubreva, J.; Català-Moll, F.; Obermajer, N.; Álvarez-Errico, D.; Ramirez, R.N.; Company, C.; Vento-Tormo, R.; Moreno-Bueno, G.; Edwards, R.P.; Mortazavi, A.; et al. Prostaglandin E2 Leads to the Acquisition of DNMT3A-Dependent Tolerogenic Functions in Human Myeloid-Derived Suppressor Cells. Cell Rep. 2017, 21, 154–167. [Google Scholar] [CrossRef]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef]
- Clark, I.A.; Rockett, K.A. Nitric Oxide and Parasitic Disease. In Advances in Parasitology; Elsevier: Burlington, MA, USA, 1996; Volume 37, pp. 1–56. [Google Scholar] [CrossRef]
- Choi, B.-S.; Martinez-Falero, I.C.; Corset, C.; Munder, M.; Modolell, M.; Müller, I.; Kropf, P. Differential impact of L-arginine deprivation on the activation and effector functions of T cells and macrophages. J. Leukoc. Biol. 2009, 85, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.P.; Ilies, M.; Olszewski, K.L.; Portugal, S.; Mota, M.M.; Llinás, M.; Christianson, D.W. Crystal structure of arginase from Plasmodium falciparum and implications for L-arginine depletion in malarial infection. Biochemistry 2010, 49, 5600–5608. [Google Scholar] [CrossRef] [PubMed]
- Omodeo-Salè, F.; Cortelezzi, L.; Vommaro, Z.; Scaccabarozzi, D.; Dondorp, A.M. Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme. Am. J. Physiol. Cell Physiol. 2010, 299, C148–C154. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, K.L.; Morrisey, J.M.; Wilinski, D.; Burns, J.M.; Vaidya, A.B.; Rabinowitz, J.D.; Llinás, M. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe 2009, 5, 191–199. [Google Scholar] [CrossRef]
- Markowitz, J.; Wang, J.; Vangundy, Z.; You, J.; Yildiz, V.; Yu, L.; Foote, I.P.; Branson, O.E.; Stiff, A.R.; Brooks, T.R.; et al. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration. Sci. Rep. 2017, 7, 15424. [Google Scholar] [CrossRef]
- Ballbach, M.; Hall, T.; Brand, A.; Neri, D.; Singh, A.; Schaefer, I.; Herrmann, E.; Hansmann, S.; Handgretinger, R.; Kuemmerle-Deschner, J.; et al. Induction of Myeloid-Derived Suppressor Cells in Cryopyrin-Associated Periodic Syndromes. J. Innate Immun. 2016, 8, 493–506. [Google Scholar] [CrossRef]
- Kalantari, P.; DeOliveira, R.B.; Chan, J.; Corbett, Y.; Rathinam, V.; Stutz, A.; Latz, E.; Gazzinelli, R.T.; Golenbock, D.T.; Fitzgerald, K.A. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 2014, 6, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, P. The Emerging Role of Pattern Recognition Receptors in the Pathogenesis of Malaria. Vaccines 2018, 6, 13. [Google Scholar] [CrossRef]
- Lang, T.; Lee, J.P.W.; Elgass, K.; Pinar, A.A.; Tate, M.D.; Aitken, E.H.; Fan, H.; Creed, S.J.; Deen, N.S.; Traore, D.A.K.; et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat. Commun. 2018, 9, 2223. [Google Scholar] [CrossRef]
- Shao, D.; Han, Z.; Lin, Y.; Zhang, L.; Zhong, X.; Feng, M.; Guo, Y.; Wang, H. Detection of Plasmodium falciparum derived macrophage migration inhibitory factor homologue in the sera of malaria patients. Acta Trop. 2008, 106, 9–15. [Google Scholar] [CrossRef]
- Gottschlich, A.; Endres, S.; Kobold, S. Therapeutic Strategies for Targeting IL-1 in Cancer. Cancers 2021, 13, 477. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Simpson, K.D.; Templeton, D.J.; Cross, J.V. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J. Immunol. 2012, 189, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Otvos, B.; Silver, D.J.; Mulkearns-Hubert, E.E.; Alvarado, A.G.; Turaga, S.M.; Sorensen, M.D.; Rayman, P.; Flavahan, W.A.; Hale, J.S.; Stoltz, K.; et al. Cancer Stem Cell-Secreted Macrophage Migration Inhibitory Factor Stimulates Myeloid Derived Suppressor Cell Function and Facilitates Glioblastoma Immune Evasion. Stem Cells 2016, 34, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Li, S.; Doumbo, S.; Doumtabe, D.; Huang, C.-Y.; Dia, S.; Bathily, A.; Sangala, J.; Kone, Y.; Traore, A.; et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin. Infect. Dis. 2013, 57, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, M.T.; Maire, N.; Felger, I.; Owusu-Agyei, S.; Smith, T. Asymptomatic Plasmodium falciparum infections may not be shortened by acquired immunity. Malar. J. 2015, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Quin, J.E.; Bujila, I.; Chérif, M.; Sanou, G.S.; Qu, Y.; Vafa Homann, M.; Rolicka, A.; Sirima, S.B.; O’Connell, M.A.; Lennartsson, A.; et al. Major transcriptional changes observed in the Fulani, an ethnic group less susceptible to malaria. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Obiero, J.M.; Shekalaghe, S.; Hermsen, C.C.; Mpina, M.; Bijker, E.M.; Roestenberg, M.; Teelen, K.; Billingsley, P.F.; Sim, B.K.L.; James, E.R.; et al. Impact of malaria preexposure on antiparasite cellular and humoral immune responses after controlled human malaria infection. Infect. Immun. 2015, 83, 2185–2196. [Google Scholar] [CrossRef]
- Gowda, D.C.; Wu, X. Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria. Front. Immunol. 2018, 9, 3006. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. A vaccine against malaria: Five minutes with Richard Bucala. BMJ 2021, 372, n651. [Google Scholar] [CrossRef]
- Naran, K.; Nundalall, T.; Chetty, S.; Barth, S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018, 9, 3158. [Google Scholar] [CrossRef] [PubMed]
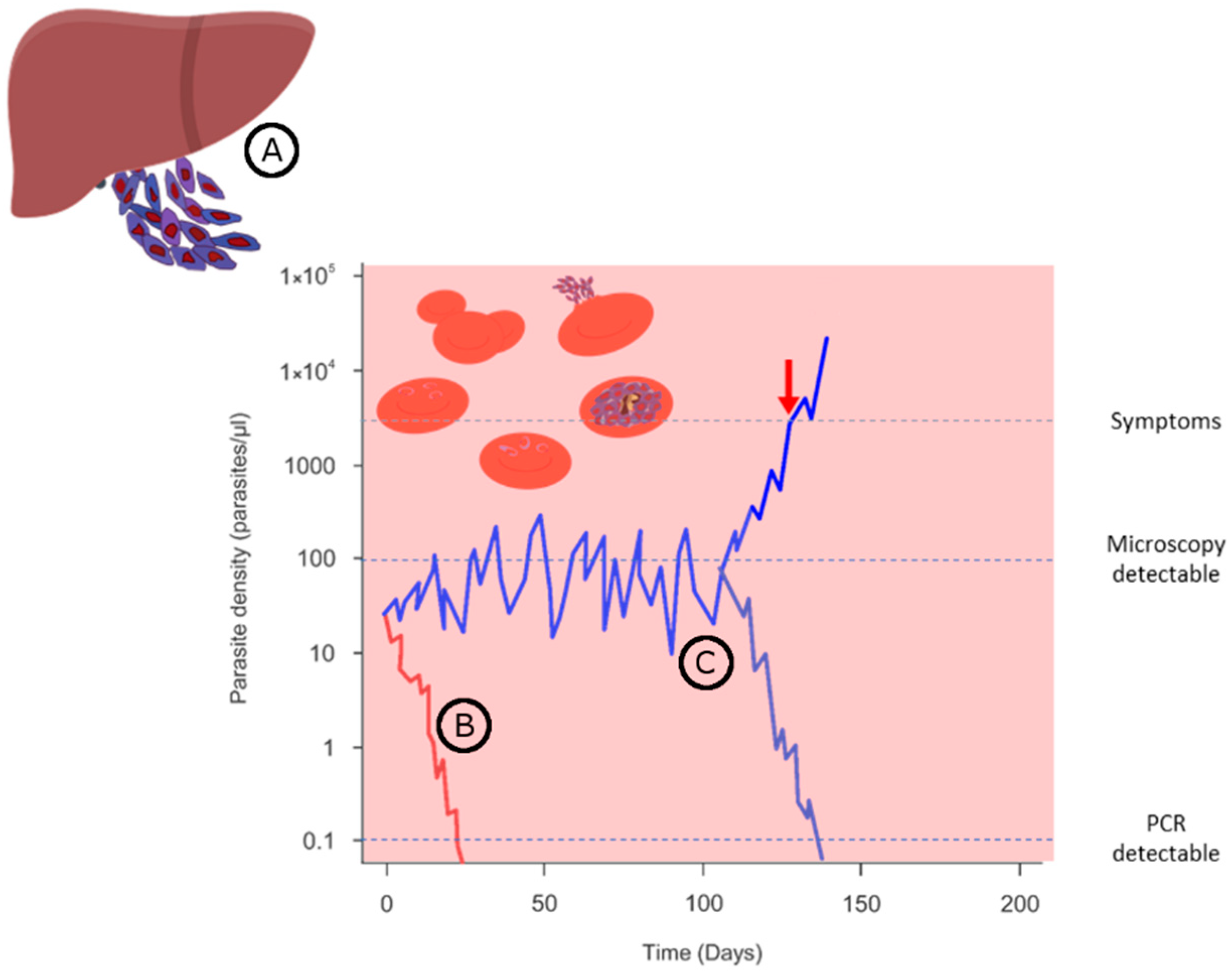
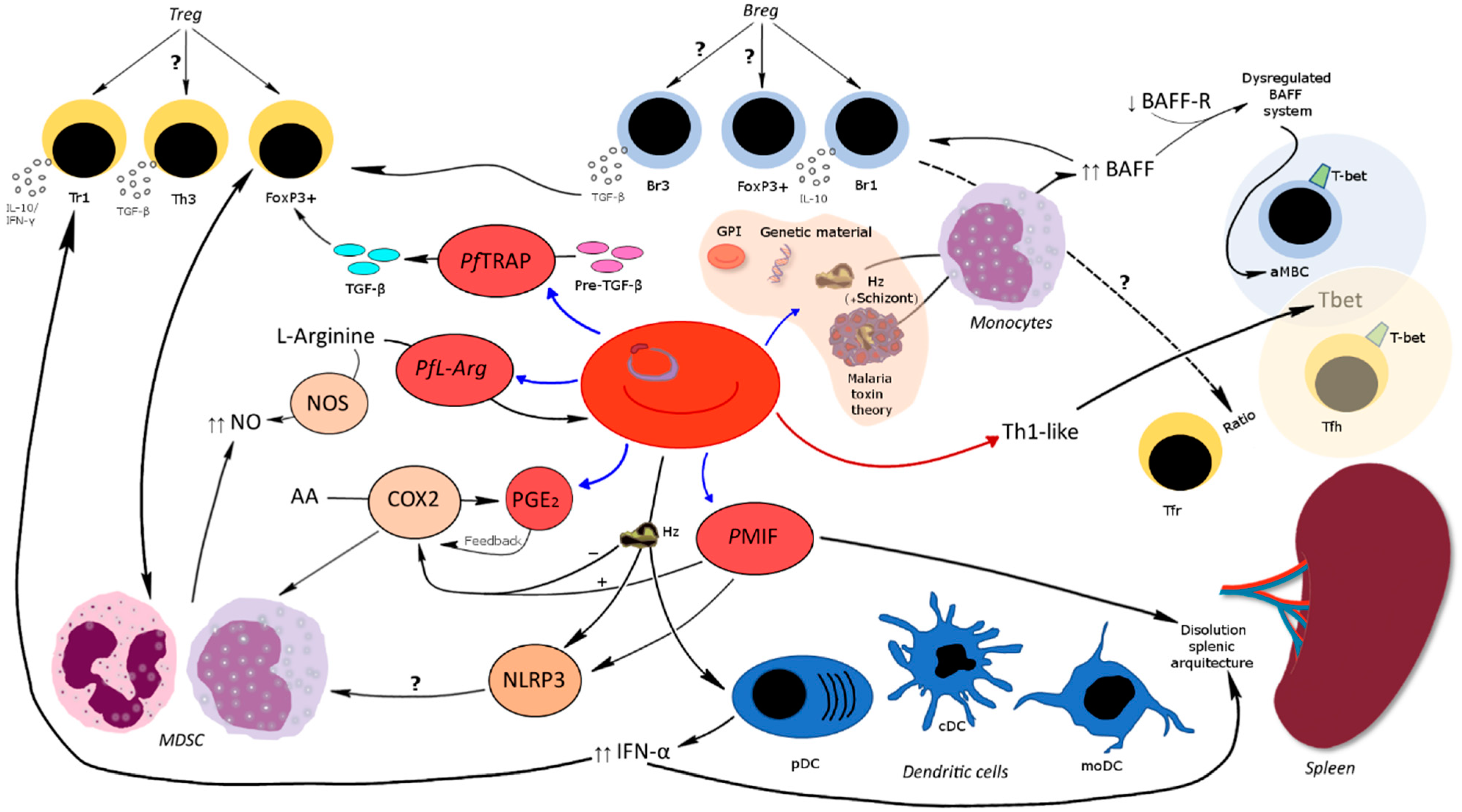
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle, C.L.; Mordmüller, B.; Singh, A. Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host? Pathogens 2021, 10, 1277. https://doi.org/10.3390/pathogens10101277
Calle CL, Mordmüller B, Singh A. Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host? Pathogens. 2021; 10(10):1277. https://doi.org/10.3390/pathogens10101277
Chicago/Turabian StyleCalle, Carlos Lamsfus, Benjamin Mordmüller, and Anurag Singh. 2021. "Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host?" Pathogens 10, no. 10: 1277. https://doi.org/10.3390/pathogens10101277
APA StyleCalle, C. L., Mordmüller, B., & Singh, A. (2021). Immunosuppression in Malaria: Do Plasmodium falciparum Parasites Hijack the Host? Pathogens, 10(10), 1277. https://doi.org/10.3390/pathogens10101277





