Targeting Inflammation Driven by HMGB1 in Bacterial Keratitis—A Review
Abstract
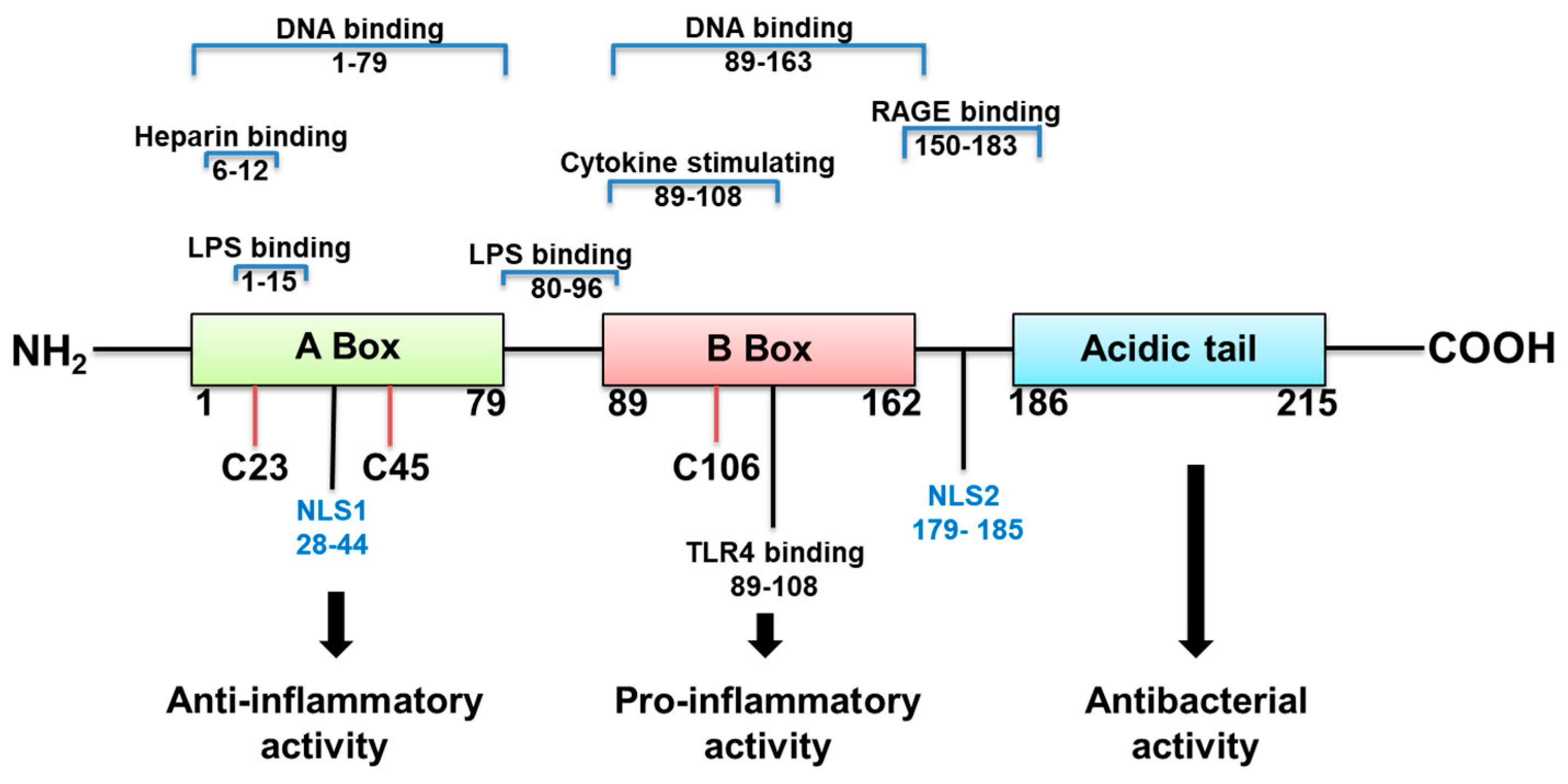
:1. Introduction
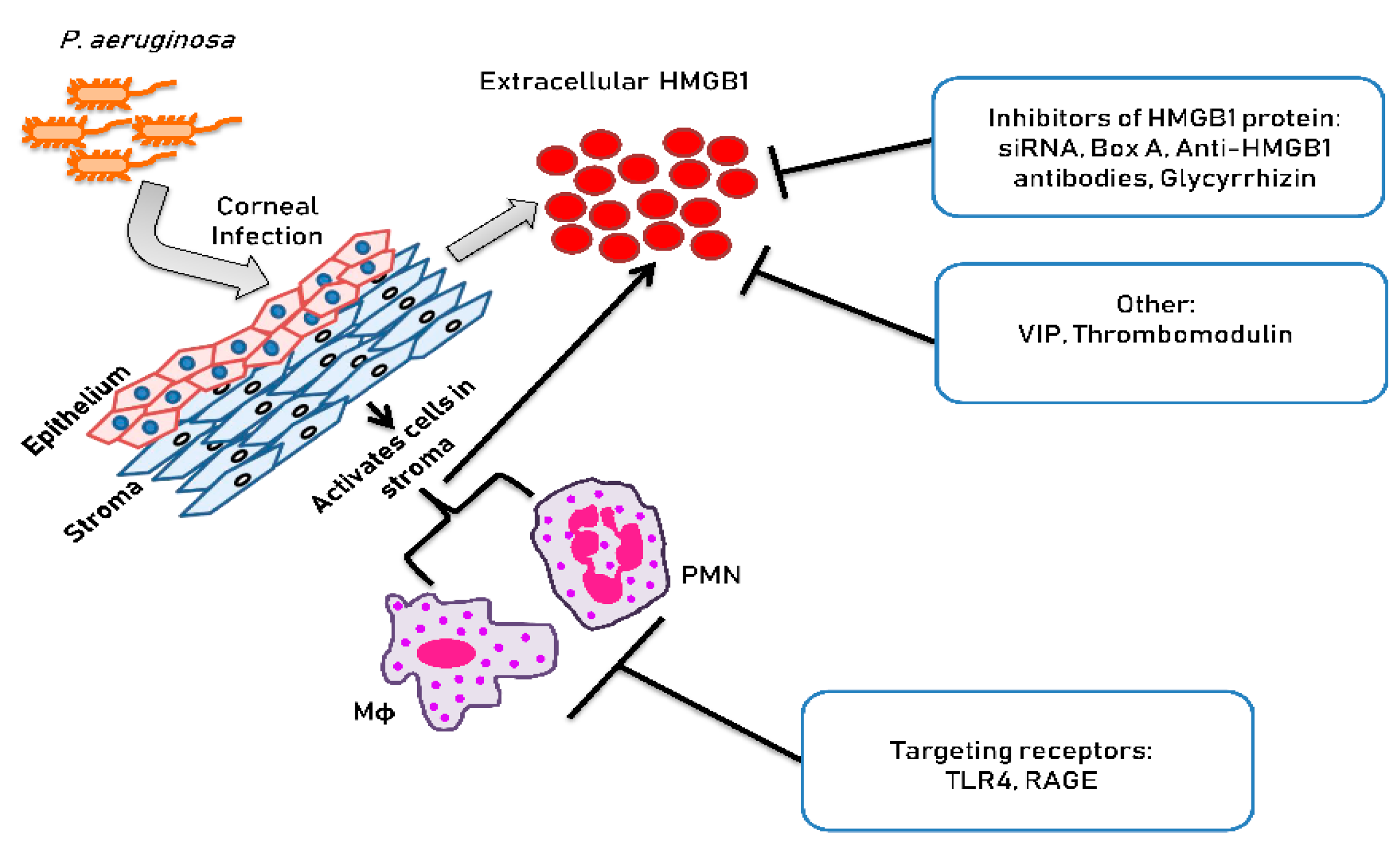
2. P. aeruginosa Keratitis: Role of Immune System in Disease
3. P. aeruginosa Conventional Treatment
Antibiotic Treatment
4. Novel Approaches for Treating P. aeruginosa Keratitis
4.1. Targeting Immune Cell Receptors
Targeting TLR4 Receptor
4.2. Targeting Extracellular HMGB1 Interaction
4.2.1. Vasoactive Intestinal Peptide
4.2.2. Thrombomodulin
4.3. Targeting Extracellular HMGB1 Protein Directly
4.3.1. Silencing HMGB1 by siRNA
4.3.2. Anti-HMGB1 Antibody
4.3.3. HMGB1 Box A Protein
4.3.4. Glycyrrhizin and Carbenoxolone
5. Closing Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Entezar, M.; Weiss, D.J.; Sitapara, R.; Whittaker, L.; Wargo, M.J.; Li, J.; Wang, H.; Yang, H.; Sharma, L.; Phan, B.D.; et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol. Med. 2012, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R. Nuclear functions of the HMG proteins. Biochim. Biophys. Acta. 2010, 1799, 3–14. [Google Scholar] [CrossRef]
- McClellan, S.; Jiang, X.; Barrett, R.; Hazlett, L.D. High-mobility group box 1: A novel target for treatment of Pseudomonas aeruginosa keratitis. J. Immunol. 2015, 194, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, S.A.; McClellan, S.A.; Barrett, R.P.; Kharotia, S.; Hazlett, L.D. Glycyrrhizin reduces HMGB1 and bacterial load in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5799–5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Tang Li, L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 2010, 51, 119–126. [Google Scholar] [CrossRef]
- Czura, C.; Yang, H.; Amella, C.A.; Tracey, K.J. HMGB1 in the immunology of sepsis (Not septic shock) and arthritis. Adv. Immunol. 2004, 84, 181–200. [Google Scholar]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Andersson, E. Landsson-Harris H. HMGB1 is a potent trigger of arthritis. J. Intern. Med. 2004, 255, 344–350. [Google Scholar]
- Zhang, F.; Huang, G.; Hu, B.; Fang, L.P.; Cao, E.; Xin, X.F.; Song, Y.; Shi, Y. Anti-HMGB1 neutralizing antibody ameliorates neutrophilic airway inflammation by suppressing dendritic cell-mediated Th17 polarization. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leuko. Biol. 2007, 81, 741–747. [Google Scholar] [CrossRef]
- Li, G.; Liang, X.; Lotze, M.T. HMGB1: The central cytokine for all lymphoid cells. Front. Immunol. 2013, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, I.E.; Baruah, P.; Valentinis, B.; Voll, R.E.; Herrmann, M.; Nawroth, P.P.; Arnold, B.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Release of high mobility group box 1 by dndritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005, 174, 7506–7515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, H.; Andersson, U. Targeting inflammation driven by HMGB1. Front. Immunol. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazlett, L.D. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 2004, 23, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.H.; Stapleton, F.; Willcox, M.D.; Zhu, H. Comparison of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens-and non-contact lens-related keratitis. J. Med. Microbiol. 2008, 57, 1539–1546. [Google Scholar] [CrossRef]
- Stapleton, F.; Carnt, N. Contact lens-related microbial keratitis: How have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye 2012, 26, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Apel, A.; Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 2008, 27, 22–27. [Google Scholar] [CrossRef]
- Tam, C.; Mun, J.J.; Evans, D.J.; Fleiszig, S.M. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3100–3106. [Google Scholar] [CrossRef]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Kernacki, K.A.; Barrett, R.P.; Hobden, J.A.; Hazlett, L.D. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J. Immunol. 2000, 164, 1037–1045. [Google Scholar] [CrossRef]
- Kernacki, K.A.; Barrett, R.P.; McClellan, S.; Hazlett, L.D. MIP-1alpha regulates CD4+ T cell chemotaxis and indirectly enhances PMN persistence in Pseudomonas aeruginosa corneal infection. J. Leukoc. Biol. 2001, 70, 911–919. [Google Scholar]
- Hazlett, L.D. Pathogenic mechanisms of P. aeruginosa keratitis: A review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol. 2002, 21, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Rudner, X.L.; Kernacki, K.A.; Barrett, R.P.; Hazlett, L.D. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol. 2000, 164, 6576–6582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kernacki, K.A.; Barrett, R.P.; McClellan, S.A.; Hazlett, L.D. Aging and PMN response to P. aeruginosa infection. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3019–3025. [Google Scholar]
- Zhou, Z.; Barrett, R.P.; McClellan, S.A.; Zhang, Y.; Szliter, E.A.; van Rooijen, N.; Hazlett, L.D. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4458–4467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, S.A.; Huang, X.; Barrett, R.P.; van Rooijen, N.; Hazlett, L.D. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 2003, 170, 5219–5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, B.; Hazlett, L.D. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J. Immunol. 1997, 159, 6283–6290. [Google Scholar] [PubMed]
- Hazlett, L.; McClellan, S.; Kwon, B.; Barrett, R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Investig. Ophthalmol. Vis. Sci. 2000, 41, 805–810. [Google Scholar]
- Berk, R.S.; Leon, M.A.; Hazlett, L.D. Genetic control of the murine corneal response to Pseudomonas aeruginosa. Infect. Immun. 1979, 26, 1221–1223. [Google Scholar] [CrossRef] [Green Version]
- Berk, R.S.; Beisel, K.; Hazlett, L.D. Genetic studies of the murine corneal response to Pseudomonas aeruginosa. Infect. Immunity 1981, 34, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, A.; Cao, Z.; Thitiprasert, T.; Zaidi, T.S.; Panjwani, N. Galectin-1-mediated suppression of Pseudomonas aeruginosa—Induced corneal immunopathology. J. Immunol. 2013, 190, 6397–6409. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; McClellan, S.A.; Barrett, R.; Hazlett, L.D. Interleukin 17 regulates Mer tyrosine kinase-positive cells in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6886–6900. [Google Scholar] [CrossRef] [Green Version]
- Gadjeva, M.; Nagashima, J.; Zaidi, T.; Mitchell, R.A.; Pier, G.B. Inhibition of macrophage migration inhibitory factor ameliorates ocular Pseudomonas aeruginosa—Induced keratitis. PLoS Pathog. 2010, 6, e1000826. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.P.; Maguire, M.G.; Fink, N.E.; Alfonso, E.; McDonnell, P. Efficacy of ofloxacin vs. cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group. Arch. Ophthalmol. 1995, 113, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Mesaros, N.; Nordmann, P.; Plesiat, P.; Roussel-Delvallez, M.; Van Eldere, J.; Glupczynski, Y.; Van Laethem, Y.; Jacobs, F.; Lebecque, P.; Malfroot, A.; et al. Pseudomonas aeruginosa: Resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infec. 2007, 13, 560–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States; CDC: Atlanta, GA, USA, 2013; pp. 1–114. [Google Scholar]
- Smith, R.; Coast, J. The true cost of antimicrobial resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, H.; Ju, Z.; Ragab, A.A.; Lundback, P.; Long, W.; Valdes-Ferrer, S.I.; He, M.; Pribis, J.P.; Li, J.; et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J. Exp. Med. 2015, 212, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Du, W.; McClellan, S.A.; Barrett, R.P.; Hazlett, L.D. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4910–4916. [Google Scholar] [CrossRef] [Green Version]
- Szliter, E.A.; Lighvani, S.; Barret, R.P.; Hazlett, L.D. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa—Infected cornea and protects against corneal perforation. J. Immunol. 2007, 178, 1105–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; McClellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. Vasoactive intestinal peptide downregulates proinflammatory TLRs while upregulating anti-inflammatory TLRs in the infected cornea. J. Immunol. 2012, 189, 269–278. [Google Scholar] [CrossRef]
- Chorny, A.; Delgado, M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am. J. Pathol. 2008, 172, 1297–1307. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Lee, D.; Sung, Y.K. Prospect of vasoactive intestinal peptide therapy for COPD/PAH and asthma: A review. Respir. Res. 2011, 12, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Mori, S.; Takahashi, H.K.; Tomono, Y.; Wake, H.; Kanke, T.; Sato, Y.; Hiraga, N.; Adachi, N.; Yoshino, T.; et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007, 21, 3904–3916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, S.A.; Ekanayaka, S.A.; Li, C.; Barrett, R.P.; Hazlett, L.D. Thrombomodulin protects against bacterial keratitis, is anti- inflammatory, but not angiogenic. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8091–8100. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.L.; Chang, C.F.; Shi, C.S.; Shi, G.Y.; Wu, H.L. Recombinant lectin-like domain of thrombomodulin suppresses vascular inflammation by reducing leukocyte recruitment via interacting with Lewis Y on endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2366–2373. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Yang, X.; Liu, K.; Gu, Q.; Xu, X. Effects of a novel peptide derived from human thrombomodulin on endotoxin-induced uveitis in vitro and in vivo. FEBS Lett. 2011, 585, 3457–3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Ishii, H.; Higuchi, T.; Sato, K.; Hayashi, Y.; Ikeda, K.; Hirabayashi, Y. Localization of thrombomodulin in the anterior segment of the human eye. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3383–3390. [Google Scholar]
- Huang, Y.H.; Kuo, I.C.C.; Ching Chang, I.; Hsu, Y.Y.; Lee, F.T.; Shi, G.-Y.; Tseng, S.-H.; Wu, H.-L. Thrombomodulin promotes corneal epithelial wound healing. PLoS ONE 2015, 10, e0122491. [Google Scholar] [CrossRef] [Green Version]
- Abeyama, K.; Stern, D.; Ito, Y.; Kawahara, K.; Yoshimoto, Y.; Tanaka, M.; Uchimura, T.; Ida, N.; Yamazaki, Y.; Shingo Yamada, S.; et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Investig. 2005, 115, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Kawahara, K.; Okamoto, K.; Yamada, S.; Yasuda, M.; Imaizumi, H.; Nawa, Y.; Meng, X.; Shrestha, B.; Hashiguchi, T.; et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1825–1830. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Hazlett, L.D.; Du, W.; Barrett, R.P. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J. Immunol. 2006, 177, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Du, W.; Barrett, R.P.; Hazlett, L.D. ST2 is essential for TH2 responsiveness and resistance to Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4626–4633. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, K.; Tamura, T.; Sawatsubashi, Y. Sepsis and disseminated intravascular coagulation. J. Intensive. Care. 2016, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Schiraldi, M.; Raucci, A.; Munoz, L.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; De Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, C.W.; Collier, A.C.; Lederman, M.M.; Schols, D.; Pollard, R.B.; Brown, S.; Jackson, J.B.; Coombs, R.W.; Glesby, M.J.; Flexner, C.W.; et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2004, 37, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, Y.; Wang, J.; Shi, X.; Liu, Q.; Liu, Z.; Li, Y.; Scott, M.J.; Xiao, G.; Li, S.; et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014, 21, 1229–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 2018, 49, 740–753. [Google Scholar] [CrossRef] [Green Version]
- Nishibori, M.; Mori, S.; Takahashi, H.K. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J. Pharmacol. Sci. 2019, 140, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Weir, H.M.; Kraulis, P.J.; Hill, C.S.; Raine, A.R.C.; Laue, E.D.; Thomas, J.O. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993, 12, 1311–1319. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, M.; Wang, W.; Tang, J. The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem. Biophys. Res. Commun. 2007, 360, 14–19. [Google Scholar] [CrossRef]
- Li, J.; Kokkola, R.; Tabibzadeh, S.; Yang, R.; Ochani, M.; Qiang, X.; Harris, H.E.; Czura, J.; Wang, W.; Ulloa, L.; et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003, 9, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Q.; Xu, J.-F.; Yin, H.; Liu, S.-F.; Duan, L.-H.; Bian, Z.-L. Protective effect of antagonist of high-mobility group box 1 on lipopolysaccharide-induced acute lung injury in mice. Scand. J. Immunol. 2009 69, 29–35. [CrossRef]
- Knapp, S.; Muller, S.; Digilio, G.; Bonaldi, T.; Bianchi, M.E.; Musco, G. The long acidic tail of high mobility group box 1 (HMGB1) protein forms an extended and flexible structure that interacts with specific residues within and between the HMG boxes. Biochemistry 2004, 43, 11992–11997. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Tracey, K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekanayaka, S.M.; McClellan, S.A.; Peng, X.; Barrett, R.P.; Francis, R.; Hazlett, L.D. HMGB1 antagonist Box A reduces TLR4, RAGE and inflammatory cytokines in the cornea of P. aeruginosa—Infected mice. J. Ocul. Pharmacol. Ther. 2018, 34, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; He, L.; Long, W.; Zhou, Q.; Zhu, S.; Wang, P.; Fan, S.; Wang, H. Novel mechanisms of herbal therapies for inhibiting hmgb1 secretion or action. Evid. Based Complement Med. 2015, 2015, 456305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollica, L.; De Marchis, F.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, J.; Sama, A.E.; Wang, H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol. Med. 2013, 19, 203–211. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Fang, Y.; Li, X.; Shen, L.; Cao, T.; Zhu, H. Glycyrrhizin protects against porcine endotoxemia through modulation of systemic inflammatory response. Crit. Care 2013, 17, R44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xiang, J.; Liu, M.; Wang, S.; Lee, R.J.; Ding, H. Protective effects of glycyrrhizic acid by rectal treatment on a TNBS-induced rat colitis model. J. Pharm. Pharmacol. 2011, 63, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.F.; Kuai, J.K.; Lu, Z.F.; Yang, G.-D.; Fu, H.-Y.; Wang, J.; Tian, F.; Yan, X.-L.; Zhao, Y.-C.; Wang, Y.-J.; et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J. Surg. Res. 2011, 165, e29–e35. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Xiang, L.; Yuan, L.; Li, X.; Shen, L.; Cao, T.; Zhu, H.; Hu, L.; Wu, W.; Cai, L.; et al. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress and apoptosis in rats. PLoS ONE 2014, 9, e89450. [Google Scholar]
- Arase, Y.; Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Isao Koida, M.; Suzuki, Y.; Satoshi Saitoh, S.; Kobayashi, M.; Hiromitsu Kumada, H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79, 1494–1500. [Google Scholar] [CrossRef]
- Shearman, D.J.; Hetzel, D. The medical management of peptic ulcer. Annu. Rev. Med. 1979, 30, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Matsuda, Y.; Sugawara, T.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Saitoh, S.; Kobayashi, M.; Kumada, H. Effects of carbenoxolone on alveolar fluid clearance and lung inflammation in the rat. Crit. Care Med. 2004, 32, 1910–1915. [Google Scholar] [CrossRef]
- Tamura, K.; Alessandri, B.; Heimann, A.; Kempski, O. The effect of a gap-junction blocker, carbenoxolone, on ischemic brain injury and cortical spreading depression. Neuroscience 2011, 194, 262–271. [Google Scholar] [CrossRef]
- Peng, X.; Ekanayaka, S.A.; McClellan, S.A.; Barrett, R.P.; Vistisen, K.; Hazlett, L.D. Effects of glycyrrhizin on a drug resistant isolate of Pseudomonas aeruginosa. EC Ophthalmol. 2018, 9.5, 265–280. [Google Scholar]
- Hazlett, L.D.; Ekanayaka, S.A.; McClellan, S.A.; Francis, R. Glycyrrhizin use for multi-drug resistant Pseudomonas aeruginosa: In vitro and in vivo studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2978–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazlett, L.D.; McClellan, S.; Somayajulu, M.; Bessert, D. Targeting Inflammation Driven by HMGB1 in Bacterial Keratitis—A Review. Pathogens 2021, 10, 1235. https://doi.org/10.3390/pathogens10101235
Hazlett LD, McClellan S, Somayajulu M, Bessert D. Targeting Inflammation Driven by HMGB1 in Bacterial Keratitis—A Review. Pathogens. 2021; 10(10):1235. https://doi.org/10.3390/pathogens10101235
Chicago/Turabian StyleHazlett, Linda D., Sharon McClellan, Mallika Somayajulu, and Denise Bessert. 2021. "Targeting Inflammation Driven by HMGB1 in Bacterial Keratitis—A Review" Pathogens 10, no. 10: 1235. https://doi.org/10.3390/pathogens10101235





