Angiostrongylosis in Animals and Humans in Europe
Abstract
1. Introduction
2. Angiostrongylus vasorum (Baillet, 1866)
2.1. Life Cycle and Pathogenesis
2.2. Epidemiology
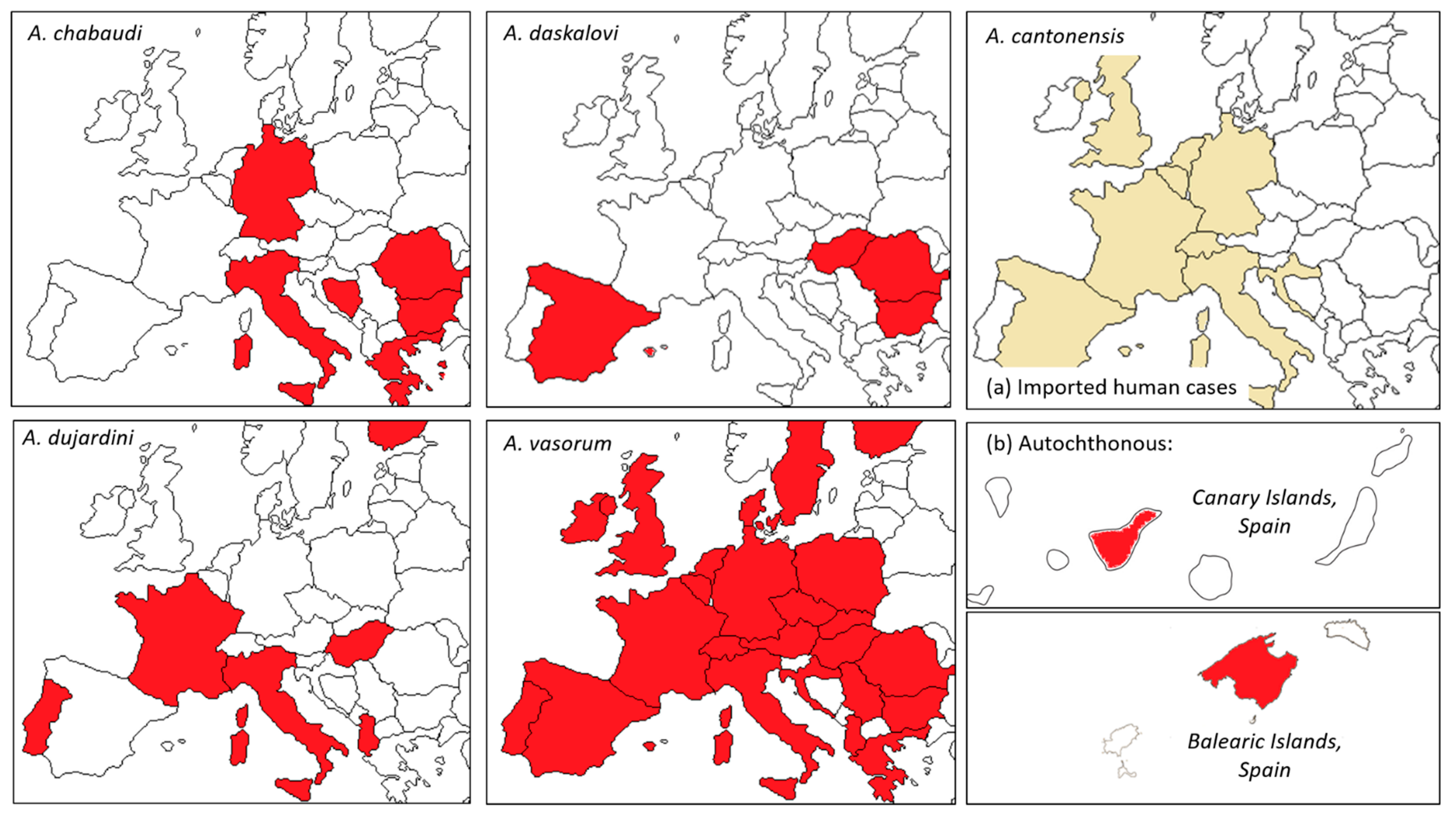
2.2.1. Spread within Europe
2.2.2. Risk Factors for Disease
2.2.3. Prospects for Further Spread
3. Angiostrongylus chabaudi (Biocca, 1957)
3.1. Life Cycle and Pathogenesis
3.2. Epidemiology
4. Angiostrongylus daskalovi (Janchev & Genov, 1988)
4.1. Life Cycle and Pathogenesis
4.2. Epidemiology
5. Angiostrongylus cantonensis (Chen, 1935)
5.1. Life Cycle and Pathogenesis
5.2. Epidemiology
5.2.1. Spread to Europe
5.2.2. Risk Factors for Disease
5.2.3. Chance of Further Spread
6. Angiostrongylus dujardini (Drozdz & Doby, 1970)
6.1. Life Cycle and Pathogenesis
6.2. Epidemiology
7. Gaps in the Understanding Needed for Risk Prediction and Management
7.1. Ecology
7.2. Behaviour
7.3. Genetics
7.4. Climate
7.5. Surveillance
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carreno, R.A.; Nadler, S.A. P hylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J. Parasitol. 2003, 89, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H. Angiostrongylus cantonensis: Agent of a sometimes fatal globally emerging infectious disease (rat lungworm disease). ACS Chem. Neurosci. 2017, 8, 2102–2104. [Google Scholar] [CrossRef]
- Wang, Q.P.; Lai, D.H.; Zhu, X.Q.; Chen, X.G.; Lun, Z.R. Human angiostrongyliasis. Lancet Inf. Dis. 2008, 8, 621–630. [Google Scholar] [CrossRef]
- Barratt, J.; Chan, D.; Sandaradura, I.; Malik, R.; Spielman, D.; Lee, R.; Marriott, D.; Harkness, J.; Ellis, J.; Stark, D. Angiostrongylus cantonensis: A review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 2016, 143, 1087–1118. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.R.; Morgan, E.R.; Jackson, M.W.; Wotton, P.; Bell, R. Canine angiostrongylosis: An emerging disease in Europe. J. Vet. Emerg. Crit. Care 2010, 20, 98–109. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent advances in the epidemiology, clinical and diagnostic features, and control of canine cardio-pulmonary angiostrongylosis. Vet. Res. 2014, 45, 92. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is angiostrongylosis a realistic threat for domestic cats? Front. Vet. Sci. 2020, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Benedek, I.; Zsolnai, A.; Halasz, T.; Csivincsik, A.; Acs, V.; Nagy, G.; Tari, T. Habitat characteristics as potential drivers of the Angiostrongylus daskalovi infection in European badger (Meles meles) populations. Pathogens 2021, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Foronda, P.; López-González, M.; Miquel, J.; Torres, J.; Segovia, M.; Abreu-Acosta, N.; Casanova, J.C.; Valladaresa, B.; Mas-Coma, S.; Bargues, M.D.; et al. Finding of Parastrongylus cantonensis (Chen, 1935) in Rattus rattus in Tenerife, Canary Islands (Spain). Acta Trop. 2010, 114, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Esquivel, C.; Sola, J.; Delgado-Serra, S.; Riera, M.P.; Negre, N.; Miranda, M.A.; Jurado-Rivera, J.A. Angiostrongylus cantonensis in North African hedgehogs as vertebrate hosts, Mallorca, Spain, October 2018. Eurosurveillance 2019, 24, 1900489. [Google Scholar] [CrossRef] [PubMed]
- Eleni, C.; Di Cesare, A.; Cavicchio, P.; Tonnichia, M.C.; Meoli, R.; di Regalbono, A.F.; Paoletti, B.; Pietrobello, M.; De Liberato, C. Fatal Angiostrongylus dujardini infection in callitrichid monkeys and suricates in an Italian zoological garden. Parasitol. Int. 2016, 65, 333–335. [Google Scholar] [CrossRef]
- Morgan, E.R.; Shaw, S.E. Angiostrongylus vasorum: A real heartbreaker? Trends Parasitol. 2005, 21, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Willesen, J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Lemming, L.; Jorgensen, A.C.; Nielsen, L.B.; Nielsen, S.T.; Mejer, H.; Chriel, M.; Petersen, H.H. Cardiopulmonary nematodes of wild carnivores in Denmark: Do they serve as reservoir hosts for infections in domestic animals? Int. J. Parasitol. Parasites Wildl. 2021, 13, 90–97. [Google Scholar] [CrossRef]
- Bolt, G.; Monrad, J.; Frandsen, F.; Henriksen, P.; Dietz, H.H. The common frog (Rana temporaria) as a potential paratenic and intermediate host for Angiostrongylus vasorum. Parasitol. Res. 1993, 79, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Helm, J.; Roberts, L.; Jefferies, R.; Shaw, S.E.; Morgan, E.R. Epidemiological survey of Angiostrongylus vasorum in dogs and slugs around a new endemic focus in Scotland. Vet. Rec. 2015, 177, 46. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Daly, E.; Allen, S.; Rowson, B.; Greig, C.; Forman, D.; Morgan, E.R. Distribution of Angiostrongylus vasorum and its gastropod intermediate hosts along the rural-urban gradient in two cities in the United Kingdom, using real time PCR. Parasites Vectors 2016, 9, 56. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Hirzmann, J.; Failing, K.; Schaper, R.; Van Bourgonie, Y.R.; Backeljau, T.; Hermosilla, C.; Taubert, A. Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Vet. Parasitol. 2018, 254, 120–130. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Morelli, S.; Bleicher, J.; Brauchart, T.; Edler, M.; Eisschel, N.; Hering, T.; Lercher, S.; Mohab, K.; Reinelt, S.; et al. Detection of Crenosoma spp., Angiostrongylus vasorum and Aelurostrongylus abstrusus in gastropods in Eastern Austria. Pathogens 2020, 9, 1046. [Google Scholar] [CrossRef]
- Segeritz, L.; Cardona, A.; Taubert, A.; Hermosilla, C.; Ruiz, A. Autochthonous Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus infections in native terrestrial gastropods from the Macaronesian Archipelago of Spain. Parasitol. Res. 2021, 120, 2671–2680. [Google Scholar] [CrossRef]
- Mozzer, L.R.; Lima, W.S. Gallus gallus domesticus: Paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015, 207, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Barçante, T.A.; Barçante, J.M.P.; Dias, S.R.C.; Lima, W.S. Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905: Emergence of third-stage larvae from infected Biomphalaria glabrata snails. Parasitol. Res. 2003, 91, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.; Conboy, G.; Greenwood, S.; Schaper, R. Infectivity of gastropod-shed third-stage larvae of Angiostrongylus vasorum and Crenosoma vulpis to dogs. Parasites Vectors 2021, 14, 307. [Google Scholar] [CrossRef]
- Morgan, E.R.; Jefferies, R.; Van Otterdijk, L.; McEniry, R.B.; Allen, F.; Bakewell, M.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Presentation and risk factors. Vet. Parasitol. 2010, 173, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lepri, E.; Veronesi, F.; Traversa, D.; Conti, M.B.; Marchesi, M.C.; Miglio, A.; Mandara, M.T. Disseminated angiostrongylosis with massive cardiac and cerebral involvement in a dog from Italy. Parasitol. Res. 2011, 109, 505–508. [Google Scholar] [CrossRef]
- Colella, V.; Lia, R.P.; Premont, J.; Gilmore, P.; Cervone, M.; Latrofa, M.S.; D’Anna, N.; Williams, D.; Otranto, D. Angiostrongylus vasorum in the eye: New case reports and a review of the literature. Parasites Vectors 2016, 9, 161. [Google Scholar] [CrossRef]
- Morgan, E.R.; Tomlinson, A.; Hunter, S.; Nichols, T.; Roberts, E.; Fox, M.T.; Taylor, M.A. Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008, 154, 48–57. [Google Scholar] [CrossRef]
- Gueldner, E.K.; Schuppisser, C.; Borel, N.; Hilbe, M.; Schnyder, M. First case of a natural infection in a domestic cat (Felis catus) with the canid heart worm Angiostrongylus vasorum. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100342. [Google Scholar] [CrossRef]
- Gillis-Germitsch, N.; Manser, M.B.; Hilbe, M.; Schnyder, M. Meerkats (Suricata suricatta), a new definitive host of the canid nematode Angiostrongylus vasorum. Int. J. Parasitol. Parasites Wildl. 2017, 6, 349–353. [Google Scholar] [CrossRef]
- Morgan, E.R.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Continuing spread and developments in diagnosis and treatment. J. Sm. Anim. Pract. 2010, 51, 616–621. [Google Scholar] [CrossRef]
- Taylor, C.S.; Gato, R.G.; Learmount, J.; Aziz, N.A.; Montgomery, C.; Rose, H.; Coulthwaite, C.L.; McGarry, J.W.; Forman, D.W.; Allen, S.; et al. Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology 2015, 142, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Gillis-Germitsch, N.; Tritten, L.; Hegglin, D.; Deplazes, P.; Schnyder, M. Conquering Switzerland: Emergence of Angiostrongylus vasorum over three decades and rapid regional increase in the fox population contrasts with the stable prevalence of lungworms. Parasitology 2020, 147, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–335. [Google Scholar] [CrossRef] [PubMed]
- Tieri, E.E.; Saletti, M.A.; D’Angelo, A.R.; Parisciani, G.; Pelini, S.; Cocco, A.; Di Teodoro, G.; Di Censo, E.; D’Alterio, N.; Latrofa, M.S. Angiostrongylus vasorum in foxes (Vulpes vulpes) and wolves (Canis lupus italicus) from Abruzzo region, Italy. Int. J. Parasitol. Parasites Wildl. 2021, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Angelou, A.; Gelasakis, A.I.; Schnyder, M.; Schaper, R.; Papadopoulos, E. The ‘French heartworm’ in Greece: A countrywide serological survey of Angiostrongylus vasorum infection by combined detection of circulating antigens and specific antibodies. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100376. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, M.; Schaper, R.; Bilbrough, G.; Morgan, E.R.; Deplazes, P. Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitology 2013, 140, 1442–1450. [Google Scholar] [CrossRef]
- Schnyder, M.; Schaper, R.; Pantchev, N.; Kowalska, D.; Szwedko, A.; Deplazes, P. Serological detection of circulating Angiostrongylus vasorum antigen- and parasite-specific antibodies in dogs from Poland. Parasitol. Res. 2013, 112, 109–117. [Google Scholar] [CrossRef]
- Jeffery, R.A.; Lankester, M.W.; McGrath, M.J.; Whitney, H.G. Angiostrongylus vasorum and Crenosoma vulpis in red foxes (Vulpes vulpes) in Newfoundland, Canada. Can. J. Zool. 2004, 82, 66–74. [Google Scholar] [CrossRef]
- Kistler, W.M.; Brown, J.D.; Allison, A.B.; Nemeth, N.M.; Yabsley, M.J. First report of Angiostrongylus vasorum and Hepatozoon from a red fox (Vulpes vulpes) from West Virginia, USA. Vet. Parasitol. 2014, 200, 216–220. [Google Scholar] [CrossRef]
- Morgan, E.R.; Jefferies, R.; Krajewski, M.; Ward, P.; Shaw, S.E. Canine pulmonary angiostrongylosis: The influence of climate on parasite distribution. Parasitol. Int. 2009, 58, 406–410. [Google Scholar] [CrossRef]
- Deplazes, P.; Hegglin, D.; Gloor, S.; Romig, T. Wilderness in the city: The urbanization of Echinococcus multilocularis. Trends Parasitol. 2004, 20, 77–84. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; Iossa, G.; Baker, P.J.; Cole, N.C.; Funk, S.M.; Harris, S. The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mammal Rev. 2007, 4, 278–296. [Google Scholar]
- Engeleke, S.; Kompf, J.; Jordaens, K.; Tomiuk, J.; Parker, E.D. The genetic dynamics of the rapid and recent colonization of Denmark by Arion lusitanicus (Mollusca, Pulmonata, Arionidae). Genetica 2011, 139, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, M.A.; Knop, E.; Heckel, G. Introgressive replacement of natives by invading Arion pest slugs. Sci. Rep. 2017, 7, 14908. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Colombo, M.; Diakou, A.; Traversa, D.; Grillini, M.; di Regalbono, A.F.; Di Cesare, A. The influence of temperature on the larval development of Aelurostrongylus abstrusus in the land snail Cornu aspersum. Pathogens 2021, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Bohan, D.A.; Choi, Y.H.; Conrad, K.F.; Semenov, M.A. Use of in individual-based model to forecast the effect of climate change on the dynamics, abundance and geographical range of the pest slug Deroceras reticulatum in the UK. Glob. Chang. Biol. 2006, 9, 1643–1657. [Google Scholar]
- Gherman, C.M.; Ionic, A.M.; D’Amico, G.; Otranto, D.; Mihalca, A.D. Angiostrongylus chabaudi (Biocca, 1957) in wildcat (Felis silvestris silvestris, S) from Romania. Parasitol. Res. 2016, 115, 2511–2517. [Google Scholar] [CrossRef]
- Diakou, A.; Psalla, D.; Migli, D.; Di Cesare, A.; Youlatos, D.; Marcer, F.; Traversa, D. First evidence of the European wildcat (Felis silvestris silvestris) as definitive host of Angiostrongylus chabaudi. Parasitol. Res. 2016, 115, 1235–1244. [Google Scholar] [CrossRef]
- Diakou, A.; Migli, D.; Dimzas, D.; Morelli, S.; Di Cesare, A.; Youlatos, D.; Lymberakis, P.; Traversa, D. Endoparasites of European wildcats (Felis silvestris) in Greece. Pathogens 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Morelli, S.; Di Cesare, A.; Diakou, A. Felid cardiopulmonary nematodes: Dilemmas solved and new questions posed. Pathogens 2021, 10, 30. [Google Scholar] [CrossRef]
- Colella, V.; Cavalera, M.A.; Deak, G.; Tarallo, V.D.; Gherman, C.M.; Mihalca, A.D.; Otranto, D. Larval development of Angiostrongylus chabaudi, the causative agent of feline angiostrongylosis, in the snail Cornu aspersum. Parasitology 2017, 144, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Dimzas, D.; Morelli, S.; Traversa, D.; Di Cesare, A.; Van Bourgonie, Y.R.; Breugelmans, K.; Backeljau, T.; di Regalbono, A.F.; Diakou, A. Intermediate gastropod hosts of major feline cardiopulmonary nematodes in an area of wildcat and domestic cat sympatry in Greece. Parasites Vectors 2020, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Traversa, D.; Colombo, M.; Raue, K.; Strube, C.; Pollmeier, M.; Di Cesare, A. The effect of the hibernation on the larval development of Troglostrongylus brevior in the land snail Cornu aspersum. Vet. Parasitol. 2020, 282, 109123. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Diakou, A.; Colombo, M.; Di Cesare, A.; Barlaam, A.; Dimzas, D.; Traversa, D. Cat respiratory nematodes: Current knowledge, novel data and warranted studies on clinical features, treatment and control. Pathogens 2021, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, O.; Diakou, A.; Morelli, S.; Paraš, S.; Trbojević, I.; Nedić, D.; Sladojević, Ž.; Kasagić, D.; Di Cesare, A. Severe verminous pneumonia caused by natural mixed infection with Aelurostrongylus abstrusus and Angiostrongylus chabaudi in a European wildcat from western Balkan area. Acta Parasitol. 2019, 64, 411–417. [Google Scholar] [CrossRef]
- Diakou, A.; Dimzas, D.; Astaras, C.; Savvas, I.; Di Cesare, A.; Morelli, S.; Neofitos, Κ.; Migli, D.; Traversa, D. Clinical investigations and treatment outcome in a European wildcat (Felis silvestris silvestris) infected by cardio-pulmonary nematodes. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100357. [Google Scholar] [CrossRef]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Colombo, M.; Traversa, D. Canine and feline parasitology: Analogies, differences, and relevance for human health. Clin. Microbiol. Rev. 2021, 34, e0026620. [Google Scholar]
- Martinez-Rondan, F.J.; de Ybanez, M.R.R.; Tizzani, P.; Lopez-Beceiro, A.M.; Fidalgo, L.E.; Martinez-Carrasco, C. The American mink (Neovison vison) is a competent host for native European parasites. Vet. Parasitol. 2017, 247, 93–99. [Google Scholar] [CrossRef]
- Gerrikagoitia, X.; Barral, M.; Juste, R.A. Angiostrongylus species in wild carnivores in the Iberian Peninsula. Vet. Parasitol. 2010, 174, 175–180. [Google Scholar] [CrossRef]
- Byrne, J.O.C.; Byrne, A.W.; Zintl, A.; Jankowska, K.; Coulange, E.; de Waal, T.; McCarthy, G.; O’Keefe, J.; Hamnes, I.S.; Fogarty, U. Identification and epidemiological analysis of Perostrongylus falciformis infestation in Irish badgers. Ir. Vet. J. 2019, 72, 7. [Google Scholar] [CrossRef]
- Gherman, C.M.; Deak, G.; Matei, I.A.; Ionică, A.M.; D’Amico, G.; Taulescu, M.; Barbu-Tudoran, L.; Sarmaşi, A.; Mihalca, A.D.; Cozma, V. A rare cardiopulmonary parasite of the European badger, Meles meles: First description of the larvae, ultrastructure, pathological changes and molecular identification of Angiostrongylus daskalovi Janchev & Genov 1988. Parasites Vectors 2016, 9, 423. [Google Scholar]
- Yong, H.S.; Eamsobhana, P. Definitive rodent hosts of the rat lungworm Angiostrongylus cantonensis. Raffles Bull. Zool. 2013, 29, 111–115. [Google Scholar]
- Martin-Alonso, A.; Abreu-Yanes, E.; Feliu, C.; Mas-Coma, S.; Bargues, M.D.; Valladares, B.; Foronda, P. Intermediate hosts of Angiostrongylus cantonensis in Tenerife, Spain. PLoS ONE 2015, 10, e0120686. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.H. Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawai′i J. Med. Public Health 2013, 72, 6–9. [Google Scholar]
- Wang, H.; Lu, L.; She, D.; Wen, Z.; Mo, Z.; Li, J.; Li, H. Eating centipedes can result in Angiostrongylus cantonensis infection: Two case reports and pathogen investigation. Am. J. Trop. Med. Hyg. 2018, 99, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Kaluna, L.; Lozano, A.; Torres Fischer, B.; Tagami, Y.; McHugh, R.; Jarvi, S. Water transmission potential of Angiostrongylus cantonensis: Larval viability and effectiveness of rainwater catchment sediment filters. PLoS ONE 2019, 14, e0209813. [Google Scholar] [CrossRef]
- Modrý, D.; Fecková, B.; Putnová, B.; Manalo, S.M.; Otranto, D. Alternative pathways in Angiostrongylus cantonensis (Metastrongyloidea: Angiostrongylidae) transmission. Parasitology 2021, 148, 167–173. [Google Scholar] [CrossRef]
- Bhaibulaya, M. Comparative studies on the life history of Angiostrongylus mackerrasae Bhaibulaya, 1968 and Angiostrongylus cantonensis (Chen, 1935). Int. J. Parasitol. 1975, 5, 7–20. [Google Scholar] [CrossRef]
- Graeff-Teixeira, C.; da Silva, A.C.A.; Yoshimura, K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin. Microbiol. Rev. 2009, 22, 322–348. [Google Scholar] [CrossRef]
- Murphy, G.S.; Johnson, S. Clinical aspects of eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis, the rat lungworm. Hawai′i J. Med. Publ. Health 2013, 72, 35. [Google Scholar]
- Spratt, D.M. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: A review. Int. J. Parasitol. Parasites Wildl. 2015, 4, 178–189. [Google Scholar] [CrossRef]
- Valladares-Salmeron, B.; Martiín-Alonso, A.; Valladares-Salmeron, M.; Martin-Carrillo, N.; Garcia-Livia, K.; Valladares, B.; Foronda, P. Pathogens in domestic cats and dogs in the Canary Islands, Spain. Int. J. Inf. Dis. 2016, 53, 65. [Google Scholar] [CrossRef][Green Version]
- McAuliffe, L.; Ensign, S.F.; Larson, D.; Bavaro, M.; Yetto, J.; Cathey, M.; Mukaigawara, M.; Narita, M.; Ohkusu, K.; Quast, T.; et al. Severe CNS angiostrongyliasis in a young marine: A case report and literature review. Lancet Inf. Dis. 2019, 19, e132–e142. [Google Scholar] [CrossRef]
- Walden, H.D.; Slapcinsky, J.; Rosenberg, J.; Wellehan, J.F. Angiostrongylus cantonensis (rat lungworm) in Florida, USA: Current status. Parasitology 2021, 148, 149–152. [Google Scholar] [CrossRef]
- Ibrahim, A.; Yousif, F. The freshwater ampullariid snail Lanistes carinatus as a natural intermediate host of Angiostrongylus cantonensis in Egypt. J. Egypt Soc. Parasitol. 1983, 13, 117–124. [Google Scholar] [PubMed]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Ruffino, L.; Bourgeois, K.; Vidal, E.; Duhem, C.; Paracuellos, M.; Escribano, F.; Sposimo, P.; Baccetti, N.; Pascal, M.; Oro, D. Invasive rats and seabirds after 2000 years of an unwanted coexistence on Mediterranean islands. Biol. Invasions 2009, 11, 1631–1651. [Google Scholar] [CrossRef]
- Martín-Carrillo, N.; Feliu, C.; Abreu-Acosta, N.; Izquierdo-Rodriguez, E.; Dorta-Guerra, R.; Miquel, J.; Abreu-Yanes, E.; Martin-Alonso, A.; García-Livia, K.; Quispe-Ricalde, M.A.; et al. A peculiar distribution of the emerging nematode Angiostrongylus cantonensis in the Canary Islands (Spain): Recent introduction or isolation effect? Animals 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Federspiel, F.; Skovmand, S.; Skarphedinsson, S. Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. Int. J. Inf. Dis. 2020, 93, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Rossi, B.; Argy, N.; Baker, C.; Nickel, B.; Marti, H.; Zarrouk, V.; Houzé, S.; Fantin, B.; Lefort, A. Autochthonous case of eosinophilic meningitis caused by Angiostrongylus cantonensis, France, 2016. Emerg. Infect. Dis. 2017, 23, 1045–1046. [Google Scholar] [CrossRef]
- De Sanidad, M.; de España, C.y.B.S. Nota Informativa Sobre la Detección de Angiostrongylus cantonensis en Erizos en las Islas Baleares; Secretaría General de Sanidad y Consumo: Madrid, Spain, 2019. [Google Scholar]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A potential source of protein and other nutrients for feed and food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef]
- Healy, S.R.; Morgan, E.R.; Prada, J.M.; Betson, M. Brain food: Rethinking food borne toxocariasis. Parasitology 2021, in press. [Google Scholar]
- Radomyos, P.; Tungtrongchitr, A.; Praewanich, R.; Khewwatchan, P.; Kantangkul, T.; Junlananto, P.; Ayudhya, S. Occurrence of the infective stage of Angiostrongylus cantonensis in the yellow tree monitor (Varanus bengalensis) in five provinces of Thailand. Southeast Asian J. Trop. Med. Public Health 1994, 25, 498–500. [Google Scholar] [PubMed]
- Yang, L.; Darasavath, C.; Chang, K.; Vilay, V.; Sengduangphachanh, A.; Adsamouth, A.; Vongsouvath, M.; Keolouangkhot, V.; Robinson, M.T. Cluster of angiostrongyliasis cases following consumption of raw monitor lizard in the Lao People’s Democratic Republic and review of the literature. Trop. Med. Inf. Dis. 2021, 6, 107. [Google Scholar]
- Lee, R.; Pai, T.Y.; Churcher, R.; Davies, S.; Braddock, J.; Linton, M.; Yu, J.; Bell, E.; Wimpole, J.; Dengate, A.; et al. Further studies of neuroangiostrongyliasis (rat lungworm disease) in Australian dogs: 92 new cases (2010–2020) and results for a novel, highly sensitive qPCR assay. Parasitology 2021, 148, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Monks, D.J.; Carlisle, M.S.; Carrigan, M.; Rose, K.; Spratt, D.; Gallagher, A.; Prociv, P. Angiostrongylus cantonensis as a cause of cerebrospinal disease in a yellow-tailed black cockatoo (Calyptorhynchus funereus) and two tawny frogmouths (Podargus strigoides). J. Avian Med. Surg. 2005, 19, 289–293. [Google Scholar] [CrossRef]
- Kim, J.R.; Wong, T.W.; Curry, P.A.; Yeung, N.W.; Hayes, K.A.; Cowie, R.H. Modelling the distribution in Hawaii of Angiostrongylus cantonensis (rat lungworm) in its gastropod hosts. Parasitology 2019, 146, 42–49. [Google Scholar] [CrossRef]
- York, E.M.; Butler, C.J.; Lord, W.D. Global decline in suitable habitat for Angiostrongylus (=Parastrongylus) cantonensis: The role of climate change. PLoS ONE 2014, 9, e103831. [Google Scholar] [CrossRef][Green Version]
- York, E.M.; Creecy, J.P.; Lord, W.D.; Caire, W. Geographic range expansion for the rat lungworm in North America. Emerg. Inf. Dis. 2015, 21, 1234–1236. [Google Scholar] [CrossRef]
- Eira, C.; Torres, J.; Vingada, J.; Miquel, J. Ecological aspects influencing the helminth community of the wood mouse Apodemus sylvaticus in Dunas de Mira, Portugal. Acta Parassitol. 2006, 51, 300–308. [Google Scholar] [CrossRef]
- Graille, M.; Ferte, H.; Petit, T.; Courtois, F.O.; Ollivet, F.; Gauchot, J.Y.; Nougaillon, J.L.; Vitaud, C.; Wardzynski, C.; Lemberger, K. Fatal Parastrongylus dujardini Infection in Captive Callitrichids. Vet. Pathol. 2015, 52, 364–368. [Google Scholar] [CrossRef]
- Lange, M.K.; Penagos-Tabares, F.; Munoz-Caro, T.; Gartner, U.; Mejer, H.; Schaper, R.; Hermosilla, C.; Taubert, A. Gastropod-derived haemocyte extracellular traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasites Vectors 2017, 10, 50. [Google Scholar] [CrossRef]
- Tolhurst, B.A.; Overall, A.D.J.; King, P.J.; Morgan, E.R.; Baker, R.J. Correlates of co-occurrence of domestic dogs and gastropod molluscs in public dog-walking spaces and implications for infection with Angiostrongylus vasorum. Animals 2021, 11, 2577. [Google Scholar] [CrossRef]
- Jefferies, R.; Shaw, S.E.; Willesen, J.; Viney, M.E.; Morgan, E.R. Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palaearctic and Nearctic ecozones. Inf. Genet. Evol. 2010, 10, 561–568. [Google Scholar] [CrossRef]
- Blanch-Lazaro, B.; Mitton, Z.; Tudor, C.; Hindle, J.; Martineau, H.; Fox, M.; Blake, D.P. Genetic diversity and population structure of Angiostrongylus vasorum parasites within and between local urban foxes (Vulpes vulpes). Vet. Parasitol. 2018, 262, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Tayyrov, A.; Schnetzler, M.; Gillis-Germitsch, N.; Schnyder, M. Genetic diversity of the cardiopulmonary canid nematode Angiostrongylus vasorum within and between rural and urban fox populations. Inf. Genet. Evol. 2021, 87, 104618. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, R.; Shaw, S.E.; Viney, M.E.; Morgan, E.R. Angiostrongylus vasorum from South America and Europe represent distinct lineages. Parasitology 2009, 136, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cervena, B.; Modry, D.; Feckova, B.; Hrazdilova, K.; Foronda, P.; Alonso, A.M.; Lee, R.; Walker, J.; Niebuhr, C.N.; Malik, R.; et al. Low diversity of Angiostrongylus cantonensis complete mitochondrial DNA sequences from Australia, Hawaii, French Polynesia and the Canary Islands revealed using whole genome next-generation sequencing. Parasites Vectors 2019, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.C.; Gibbons, L.M.; Jefferies, R.; Morgan, E.R.; Wenzlow, N.; Redrobe, S.P. Pneumonia from Angiostrongylus vasorum infection in a red panda (Ailurus fulgens fulgens). J. Vet. Diag. Investig. 2009, 21, 270–273. [Google Scholar] [CrossRef]
- Bertelsen, M.; Meyland-Smith, F.; Willesen, J.; Jefferies, R.; Morgan, E.R.; Monrad, J. Diversity and prevalence of metastrongyloid nematodes infecting the red panda (Ailurus fulgens) in European zoos. Vet. Parasitol. 2010, 172, 299–304. [Google Scholar] [CrossRef]
- Bagardi, M.; Rabbogliatti, V.; Bassi, J.; Gioeni, D.; Oltolina, M.; Villa, L. Angiostrongylus vasorum in a red panda (Ailurus fulgens): Clinical diagnostic trial and treatment protocol. Acta Parassitol. 2021, 66, 282–286. [Google Scholar] [CrossRef]
| Factor | Evidence for | Evidence Against |
|---|---|---|
| Awareness of veterinary clinicians | ↑ Vigilance and new diagnostic tests lead to more records and apparent spread [30] | Expansion and increased prevalence in foxes over time against robust baseline, not affected by clinical awareness [31,32,33,34] |
| Fox urbanization | Emergence in Western Europe followed rabies eradication and ↑ urban foxes, c.f. Echinococcus multilocularis [41] | In U.K. ‘hotspots’, urban foxes were common long before emergence, which also coincided with relatively low populations due to scabies outbreaks [42] |
| Gastropod invasion | Emergence along with the invasion of Arion vulgaris (syn. lusitanicus), e.g., in Denmark [43] and Switzerland [44] | Infects a very broad gastropod range, including displaced species; emergence in areas without much A. lusitanicus invasion, e.g., United Kingdom |
| Climate change | Predicted increased development rate in IH with temperature, based on other metastrongyloids; climate change can favour gastropod abundance [45,46] | Newly colonised areas are predicted to be historically suitable for the parasite [40]; climate change can inhibit as well as improve conditions for gastropods [45] |
| Dog movement | Longer-range dispersal ahead of spread in foxes, e.g., in the United Kingdom [16] | New records in areas long subject to dog movements [33,34,35,36,37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, 10, 1236. https://doi.org/10.3390/pathogens10101236
Morgan ER, Modry D, Paredes-Esquivel C, Foronda P, Traversa D. Angiostrongylosis in Animals and Humans in Europe. Pathogens. 2021; 10(10):1236. https://doi.org/10.3390/pathogens10101236
Chicago/Turabian StyleMorgan, Eric R., David Modry, Claudia Paredes-Esquivel, Pilar Foronda, and Donato Traversa. 2021. "Angiostrongylosis in Animals and Humans in Europe" Pathogens 10, no. 10: 1236. https://doi.org/10.3390/pathogens10101236
APA StyleMorgan, E. R., Modry, D., Paredes-Esquivel, C., Foronda, P., & Traversa, D. (2021). Angiostrongylosis in Animals and Humans in Europe. Pathogens, 10(10), 1236. https://doi.org/10.3390/pathogens10101236









